The Circadian Rhythms of STAT3 in the Rat Pineal Gland and Its Involvement in Arylalkylamine-N-Acetyltransferase Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.1.1. The Circadian Rhythmicity of Stat3 mRNA
2.1.2. The Circadian Rhythmicity of pSTAT3 Levels in Pineal Glands
2.1.3. The Effect of STAT3 Inhibitors on the Day/Night AANAT Activity and Light-Induced Suppression of AANAT Activity
2.1.4. The Effect of LPS on the Stat3 Expression
2.1.5. The Effect of LPS on STAT3 Phosphorylation
2.1.6. The Effect of LPS and WP1066 on AANAT Activity in Pineal Glands
2.2. Organotypic Cultures
2.3. Primary Cultures
2.4. Isolation of RNA and Quantitative Real-Time PCR
2.5. Immunohistochemistry
2.6. AA-NAT Enzymatic Activity Assay
2.7. Western Blotting
2.8. Data Analysis and Statistical Procedures
3. Results
3.1. Circadian Rhythmicity of Stat3 Expression and the Level of pSTAT3(y) in the Pineal Gland
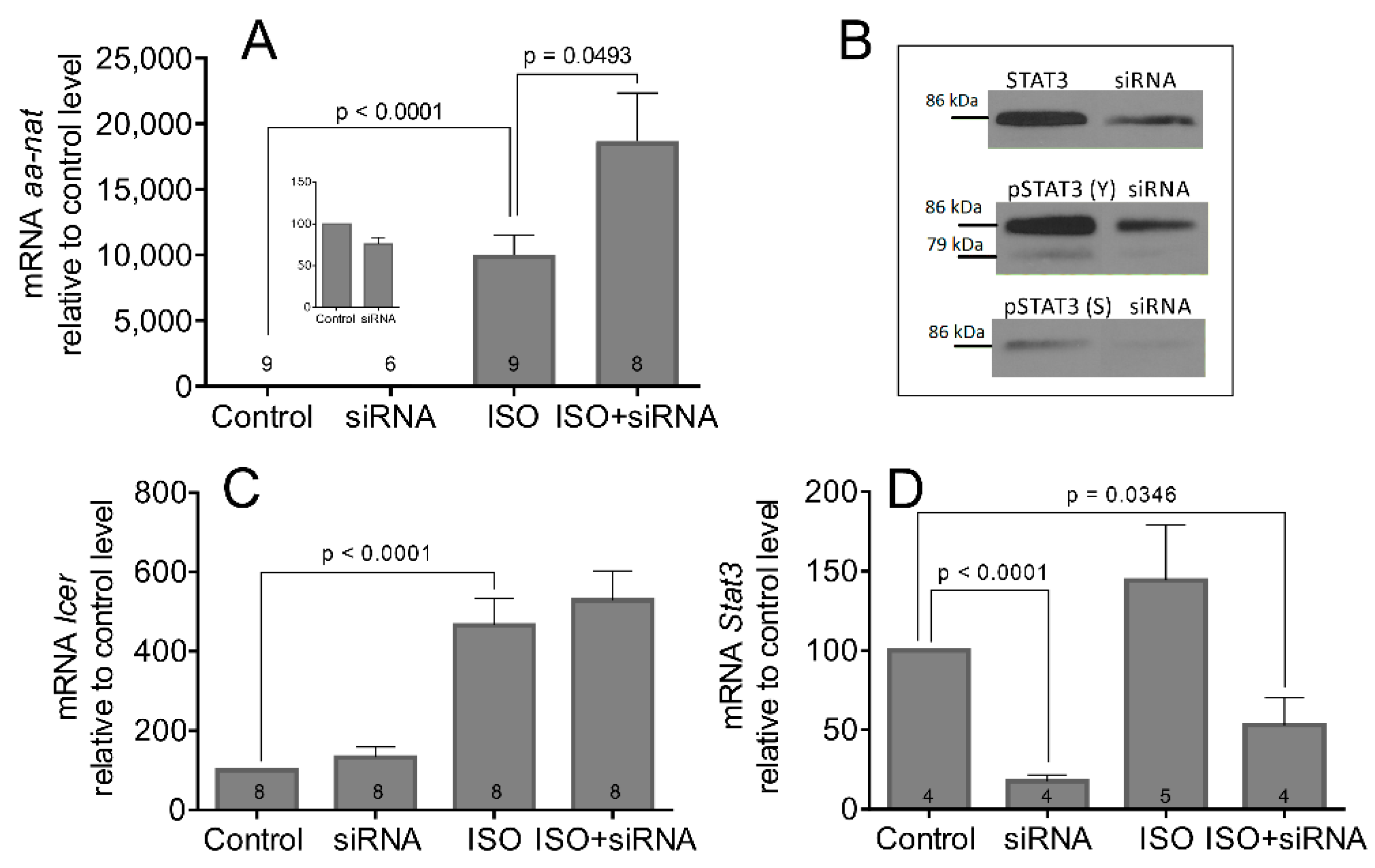
3.2. The STAT3 Inhibition Enhances ISO-Induced Transcription and Activation of AANAT
3.3. The STAT3 Inhibitors Enhances AANAT Activity Only during Subjective Night When the Pineal Gland Receives Adrenergic Stimulation from the SCN but Have No Effect on Light-Induced Downregulation of AANAT Activity
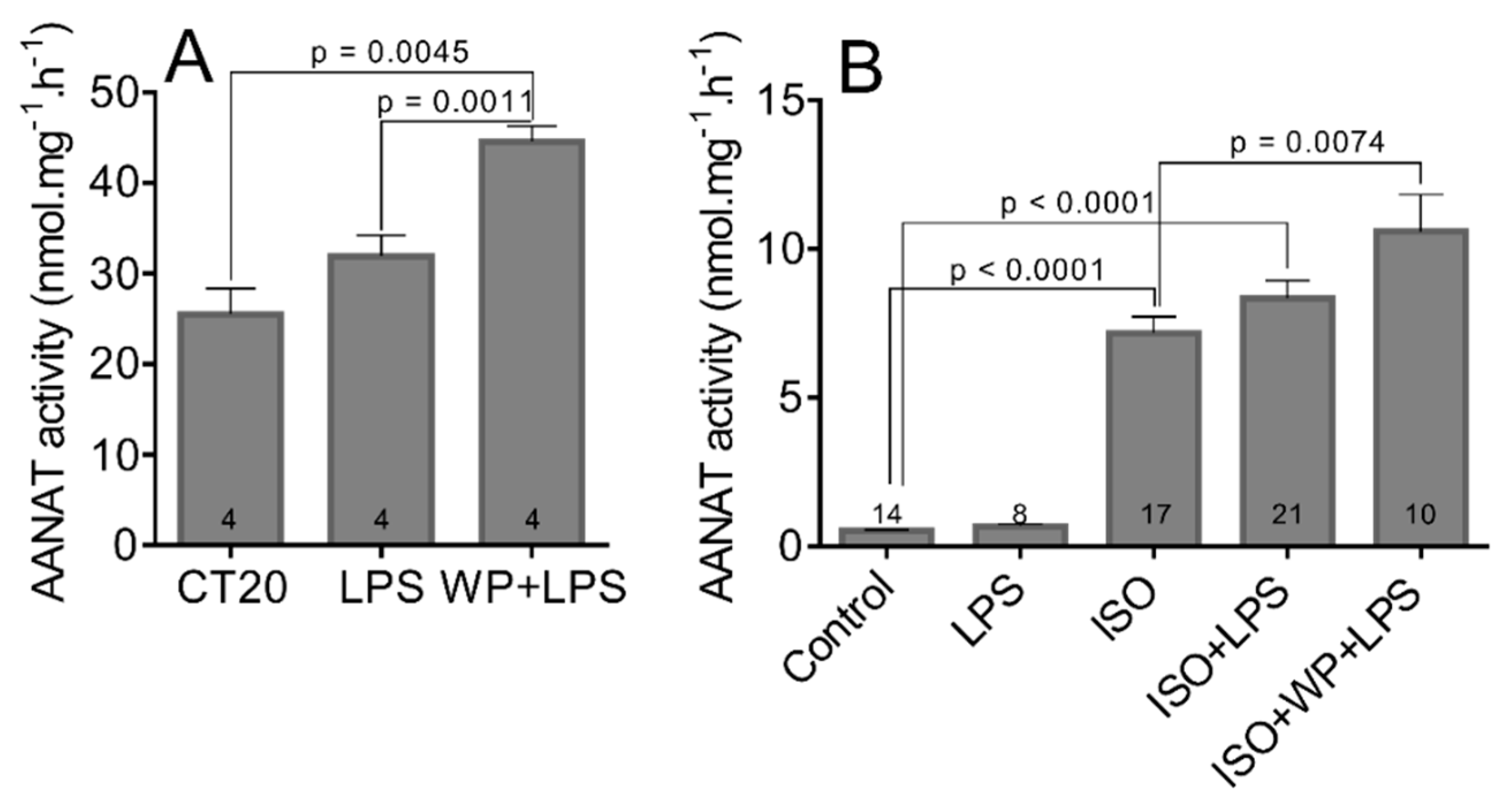
3.4. Bacterial Endotoxin Lipopolysaccharide Induces STAT3 Expression and Phosphorylation
3.5. The LPS and LPS-Induced STAT3 Does Not Affect the Transcription and Activation of AANAT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, D.C. Photoneural Regulation of the Mammalian Pineal Gland. In Novartis Foundation Symposia; Evered, D., Clark, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 38–56. ISBN 978-0-470-72098-1. [Google Scholar]
- Johnston, J.D.; Skene, D.J. 60 Years of Neuroendocrinology: Regulation of Mammalian Neuroendocrine Physiology and Rhythms by Melatonin. J. Endocrinol. 2015, 226, T187–T198. [Google Scholar] [CrossRef] [Green Version]
- Simonneaux, V.; Sinitskaya, N.; Salingre, A.; Garidou, M.L.; Pévet, P. Rat and Syrian Hamster: Two Models for the Regulation of AANAT Gene Expression. Chronobiol. Int. 2006, 23, 351–359. [Google Scholar] [CrossRef]
- Chong, N.W.; Bernard, M.; Klein, D.C. Characterization of the Chicken Serotonin N-Acetyltransferase Gene. Activation via Clock Gene Heterodimer/E Box Interaction. J. Biol. Chem. 2000, 275, 32991–32998. [Google Scholar] [CrossRef] [Green Version]
- Roseboom, P.H.; Coon, S.L.; Baler, R.; McCune, S.K.; Weller, J.L.; Klein, D.C. Melatonin Synthesis: Analysis of the More than 150-Fold Nocturnal Increase in Serotonin N-Acetyltransferase Messenger Ribonucleic Acid in the Rat Pineal Gland. Endocrinology 1996, 137, 3033–3045. [Google Scholar] [CrossRef] [Green Version]
- Illnerová, H.; Vanĕcek, J.; Krecek, J.; Wetterberg, L.; Sääf, J. Effect of One Minute Exposure to Light at Night on Rat Pineal Serotonin N-Acetyltransferase and Melatonin. J. Neurochem. 1979, 32, 673–675. [Google Scholar] [CrossRef]
- Illnerová, H.; Trávnícková, Z.; Jác, M.; Sumová, A. Comparison of the Pineal and SCN Rhythmicity. Effect of Photic and Non-Photic Stimuli, Photoperiod, and Age. Adv. Exp. Med. Biol. 1999, 460, 247–260. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Vinogradova, I.A.; Panchenko, A.V.; Popovich, I.G.; Zabezhinski, M.A. Light-at-Night-Induced Circadian Disruption, Cancer and Aging. Curr. Aging Sci. 2012, 5, 170–177. [Google Scholar] [CrossRef]
- Gastel, J.A.; Roseboom, P.H.; Rinaldi, P.A.; Weller, J.L.; Klein, D.C. Melatonin Production: Proteasomal Proteolysis in Serotonin N-Acetyltransferase Regulation. Science 1998, 279, 1358–1360. [Google Scholar] [CrossRef]
- Klein, D.C.; Coon, S.L.; Roseboom, P.H.; Weller, J.L.; Bernard, M.; Gastel, J.A.; Zatz, M.; Iuvone, P.M.; Rodriguez, I.R.; Bégay, V.; et al. The Melatonin Rhythm-Generating Enzyme: Molecular Regulation of Serotonin N-Acetyltransferase in the Pineal Gland. Recent Prog. Horm. Res. 1997, 52, 307–357, discussion 357–358. [Google Scholar]
- Ho, A.K.; Chik, C.L. Modulation of Aanat Gene Transcription in the Rat Pineal Gland. J. Neurochem. 2010, 112, 321–331. [Google Scholar] [CrossRef]
- Schomerus, C.; Korf, H.-W. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The Role of JAK-STAT Signaling within the CNS. JAKSTAT 2013, 2, e22925. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Stark, G.R. Alternative Activation of STAT1 and STAT3 in Response to Interferon-Gamma. J. Biol. Chem. 2004, 279, 41679–41685. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Blenis, J.; Li, H.C.; Schindler, C.; Chen-Kiang, S. Requirement of Serine Phosphorylation for Formation of STAT-Promoter Complexes. Science 1995, 267, 1990–1994. [Google Scholar] [CrossRef]
- Chung, J.; Uchida, E.; Grammer, T.C.; Blenis, J. STAT3 Serine Phosphorylation by ERK-Dependent and -Independent Pathways Negatively Modulates Its Tyrosine Phosphorylation. Mol. Cell. Biol. 1997, 17, 6508–6516. [Google Scholar] [CrossRef] [Green Version]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 Signaling in Immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Moravcová, S.; Červená, K.; Pačesová, D.; Bendová, Z. Identification of STAT3 and STAT5 Proteins in the Rat Suprachiasmatic Nucleus and the Day/Night Difference in Astrocytic STAT3 Phosphorylation in Response to Lipopolysaccharide. J. Neurosci. Res. 2016, 94, 99–108. [Google Scholar] [CrossRef]
- Moravcová, S.; Pačesová, D.; Melkes, B.; Kyclerová, H.; Spišská, V.; Novotný, J.; Bendová, Z. The Day/Night Difference in the Circadian Clock’s Response to Acute Lipopolysaccharide and the Rhythmic Stat3 Expression in the Rat Suprachiasmatic Nucleus. PLoS ONE 2018, 13, e0199405. [Google Scholar] [CrossRef] [Green Version]
- Kubištová, A.; Spišská, V.; Petrželková, L.; Hrubcová, L.; Moravcová, S.; Maierová, L.; Bendová, Z. Constant Light in Critical Postnatal Days Affects Circadian Rhythms in Locomotion and Gene Expression in the Suprachiasmatic Nucleus, Retina, and Pineal Gland Later in Life. Biomedicines 2020, 8, 579. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Q.; Liu, M.; Sun, A.; Li, X. Possible Involvement of the IL-6/JAK2/STAT3 Pathway in the Hypothalamus in Depressive-Like Behavior of Rats Exposed to Chronic Mild Stress. Neuropsychobiology 2021, 80, 279–287. [Google Scholar] [CrossRef]
- Marrero, B.; He, C.; Oh, H.-M.; Ukwu, U.T.; Yu, C.-R.; Dambuza, I.M.; Sun, L.; Egwuagu, C.E. Persistent Activation of STAT3 Pathway in the Retina Induced Vision Impairment and Retinal Degenerative Changes in Ageing Mice. Adv. Exp. Med. Biol. 2019, 1185, 353–358. [Google Scholar] [CrossRef]
- Iwamaru, A.; Szymanski, S.; Iwado, E.; Aoki, H.; Yokoyama, T.; Fokt, I.; Hess, K.; Conrad, C.; Madden, T.; Sawaya, R.; et al. A Novel Inhibitor of the STAT3 Pathway Induces Apoptosis in Malignant Glioma Cells Both in Vitro and in Vivo. Oncogene 2007, 26, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Plimack, E.R.; Lorusso, P.M.; McCoon, P.; Tang, W.; Krebs, A.D.; Curt, G.; Eckhardt, S.G. AZD1480: A Phase I Study of a Novel JAK2 Inhibitor in Solid Tumors. Oncologist 2013, 18, 819–820. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Koibuchi, N.; Ohtake, H.; Yamaoka, S. Circadian Rhythms of Vasopressin Release in Primary Cultures of Rat Suprachiasmatic Nucleus. Brain Res. 1993, 624, 115–120. [Google Scholar] [CrossRef]
- Svobodova, I.; Bhattaracharya, A.; Ivetic, M.; Bendova, Z.; Zemkova, H. Circadian ATP Release in Organotypic Cultures of the Rat Suprachiasmatic Nucleus Is Dependent on P2X7 and P2Y Receptors. Front. Pharmacol. 2018, 9, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Frontini, A.; Bertolotti, P.; Tonello, C.; Valerio, A.; Nisoli, E.; Cinti, S.; Giordano, A. Leptin-Dependent STAT3 Phosphorylation in Postnatal Mouse Hypothalamus. Brain Res. 2008, 1215, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Trávnícková, Z.; Illnerová, H. Melatonin Entrainment of the Circadian N-Acetyltransferase Rhythm in the Newborn Rat Pineal Gland. J. Pineal Res. 1997, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bendová, Z.; Sumová, A. Photoperiodic Regulation of PER1 and PER2 Protein Expression in Rat Peripheral Tissues. Physiol. Res. 2006, 55, 623–632. [Google Scholar] [PubMed]
- Wongchitrat, P.; Felder-Schmittbuhl, M.-P.; Govitrapong, P.; Phansuwan-Pujito, P.; Simonneaux, V. A Noradrenergic Sensitive Endogenous Clock Is Present in the Rat Pineal Gland. Neuroendocrinology 2011, 94, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Maronde, E.; Pfeffer, M.; Olcese, J.; Molina, C.A.; Schlotter, F.; Dehghani, F.; Korf, H.W.; Stehle, J.H. Transcription Factors in Neuroendocrine Regulation: Rhythmic Changes in PCREB and ICER Levels Frame Melatonin Synthesis. J. Neurosci. 1999, 19, 3326–3336. [Google Scholar] [CrossRef] [Green Version]
- da Silveira Cruz-Machado, S.; Carvalho-Sousa, C.E.; Tamura, E.K.; Pinato, L.; Cecon, E.; Fernandes, P.A.C.M.; de Avellar, M.C.W.; Ferreira, Z.S.; Markus, R.P. TLR4 and CD14 Receptors Expressed in Rat Pineal Gland Trigger NFKB Pathway. J. Pineal Res. 2010, 49, 183–192. [Google Scholar] [CrossRef]
- Pigazzi, M.; Manara, E.; Baron, E.; Basso, G. MiR-34b Targets Cyclic AMP-Responsive Element Binding Protein in Acute Myeloid Leukemia. Cancer Res. 2009, 69, 2471–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Yao, Z.; Bhuvaneshwar, K.; Gusev, Y.; Kallakury, B.; Yang, S.; Shetty, K.; He, A.R. Transcriptional Regulation of STAT3 by SPTBN1 and SMAD3 in HCC through CAMP-Response Element-Binding Proteins ATF3 and CREB2. Carcinogenesis 2014, 35, 2393–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheaton, K.L.; Hansen, K.F.; Aten, S.; Sullivan, K.A.; Yoon, H.; Hoyt, K.R.; Obrietan, K. The Phosphorylation of CREB at Serine 133 Is a Key Event for Circadian Clock Timing and Entrainment in the Suprachiasmatic Nucleus. J. Biol. Rhythms 2018, 33, 497–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Pardoll, D.; Jove, R. STATs in Cancer Inflammation and Immunity: A Leading Role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Baler, R.; Covington, S.; Klein, D.C. The Rat Arylalkylamine N-Acetyltransferase Gene Promoter. CAMP Activation via a CAMP-Responsive Element-CCAAT Complex. J. Biol. Chem. 1997, 272, 6979–6985. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.A. Rhythms of Cellular Immediate-Early Gene Expression: More than Just an Early Response. Exp. Physiol. 1997, 82, 237–244. [Google Scholar] [CrossRef]
- Guillaumond, F.; Becquet, D.; Bosler, O.; François-Bellan, A.M. Adrenergic Inducibility of AP-1 Binding in the Rat Pineal Gland Depends on Prior Photoperiod. J. Neurochem. 2002, 83, 157–166. [Google Scholar] [CrossRef]
- Davies, J.S.; Klein, D.C.; Carter, D.A. Selective Genomic Targeting by FRA-2/FOSL2 Transcription Factor: Regulation of the Rgs4 Gene Is Mediated by a Variant Activator Protein 1 (AP-1) Promoter Sequence/CREB-Binding Protein (CBP) Mechanism. J. Biol. Chem. 2011, 286, 15227–15239. [Google Scholar] [CrossRef] [Green Version]
- Hulboy, D.L.; Matrisian, L.M.; Crawford, H.C. Loss of JunB Activity Enhances Stromelysin 1 Expression in a Model of the Epithelial-to-Mesenchymal Transition of Mouse Skin Tumors. Mol. Cell. Biol. 2001, 21, 5478–5487. [Google Scholar] [CrossRef] [Green Version]
- Chik, C.L.; Wloka, M.T.; Price, D.M.; Ho, A.K. The Role of Repressor Proteins in the Adrenergic Induction of Type II Iodothyronine Deiodinase in Rat Pinealocytes. Endocrinology 2007, 148, 3523–3531. [Google Scholar] [CrossRef]
- Coffer, P.; Lutticken, C.; van Puijenbroek, A.; Klop-de Jonge, M.; Horn, F.; Kruijer, W. Transcriptional Regulation of the JunB Promoter: Analysis of STAT-Mediated Signal Transduction. Oncogene 1995, 10, 985–994. [Google Scholar] [PubMed]
- Higashi, N.; Kunimoto, H.; Kaneko, S.; Sasaki, T.; Ishii, M.; Kojima, H.; Nakajima, K. Cytoplasmic C-Fos Induced by the YXXQ-Derived STAT3 Signal Requires the Co-Operative MEK/ERK Signal for Its Nuclear Translocation. Genes Cells 2004, 9, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Han, C.; Wei, B.; Qian, L.; Chen, C.; Guo, L.; Hu, M.; Yu, M.; et al. Catecholamine Up-Regulates MMP-7 Expression by Activating AP-1 and STAT3 in Gastric Cancer. Mol. Cancer 2010, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Schuringa, J.J.; Timmer, H.; Luttickhuizen, D.; Vellenga, E.; Kruijer, W. C-Jun and c-Fos Cooperate with STAT3 in IL-6-Induced Transactivation of the IL-6 Respone Element (IRE). Cytokine 2001, 14, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Lo, H.-W. STAT3 Target Genes Relevant to Human Cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stehle, J.H.; Foulkes, N.S.; Molina, C.A.; Simonneaux, V.; Pévet, P.; Sassone-Corsi, P. Adrenergic Signals Direct Rhythmic Expression of Transcriptional Repressor CREM in the Pineal Gland. Nature 1993, 365, 314–320. [Google Scholar] [CrossRef]
- Foulkes, N.S.; Borjigin, J.; Snyder, S.H.; Sassone-Corsi, P. Transcriptional Control of Circadian Hormone Synthesis via the CREM Feedback Loop. Proc. Natl. Acad. Sci. USA 1996, 93, 14140–14145. [Google Scholar] [CrossRef] [Green Version]
- Lund, I.V.; Hu, Y.; Raol, Y.H.; Benham, R.S.; Faris, R.; Russek, S.J.; Brooks-Kayal, A.R. BDNF Selectively Regulates GABAA Receptor Transcription by Activation of the JAK/STAT Pathway. Sci. Signal. 2008, 1, ra9. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, M.; Maronde, E.; Molina, C.A.; Korf, H.W.; Stehle, J.H. Inducible Cyclic AMP Early Repressor Protein in Rat Pinealocytes: A Highly Sensitive Natural Reporter for Regulated Gene Transcription. Mol. Pharmacol. 1999, 56, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yuan, X.; Liu, Y.; Zhang, K.; Wang, J.; Zhang, H.; Liu, F. Delayed Administration of WP1066, an STAT3 Inhibitor, Ameliorates Radiation-Induced Lung Injury in Mice. Lung 2016, 194, 67–74. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, W.; Wang, Y.; Chen, S.; Han, J.; Wang, L.; Gui, P.; Wu, Q. JAK2/STAT1-Mediated HMGB1 Translocation Increases Inflammation and Cell Death in a Ventilator-Induced Lung Injury Model. Lab. Investig. 2019, 99, 1810–1821. [Google Scholar] [CrossRef]
- Huang, H.; Constante, M.; Layoun, A.; Santos, M.M. Contribution of STAT3 and SMAD4 Pathways to the Regulation of Hepcidin by Opposing Stimuli. Blood 2009, 113, 3593–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, M.D.; Sparkman, N.L.; Johnson, R.W. Inhibition of Interleukin-6 Trans-Signaling in the Brain Facilitates Recovery from Lipopolysaccharide-Induced Sickness Behavior. J. Neuroinflamm. 2011, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.P.; Bochenek, J.; Skipor, J.; Król, K.; Krawczyńska, A.; Antushevich, H.; Pawlina, B.; Marciniak, E.; Tomaszewska-Zaremba, D. Interleukin-1 β Modulates Melatonin Secretion in Ovine Pineal Gland: Ex Vivo Study. BioMed Res. Int. 2015, 2015, e526464. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.A.C.M.; Cecon, E.; Markus, R.P.; Ferreira, Z.S. Effect of TNF-Alpha on the Melatonin Synthetic Pathway in the Rat Pineal Gland: Basis for a “feedback” of the Immune Response on Circadian Timing. J. Pineal Res. 2006, 41, 344–350. [Google Scholar] [CrossRef]
- Barbosa Lima, L.E.; Muxel, S.M.; Kinker, G.S.; Carvalho-Sousa, C.E.; da Silveira Cruz-Machado, S.; Markus, R.P.; Fernandes, P.A.C.M. STAT1-NFκB Crosstalk Triggered by Interferon Gamma Regulates Noradrenaline-Induced Pineal Hormonal Production. J. Pineal Res. 2019, 67, e12599. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Bochenek, J.; Król, K.; Krawczyńska, A.; Antushevich, H.; Pawlina, B.; Herman, A.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central Interleukin-1β Suppresses the Nocturnal Secretion of Melatonin. Mediat. Inflamm. 2016, 2016, 2589483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, S.; Li, J.; Qiu, X.; Li, W.; Zhang, C.; Zhang, Z.-N.; Luan, B. A Negative Feedback Loop of ICER and NF-ΚB Regulates TLR Signaling in Innate Immune Responses. Cell Death Differ. 2017, 24, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Takamiya, A.; Takeda, M.; Yoshida, A.; Kiyama, H. Inflammation Induces Serine Protease Inhibitor 3 Expression in the Rat Pineal Gland. Neuroscience 2002, 113, 387–394. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Peineau, S.; Amici, M.; Csaba, Z.; Fafouri, A.; Javalet, C.; Collett, V.J.; Hildebrandt, L.; Seaton, G.; Choi, S.-L.; et al. The Jak/STAT Pathway Is Involved in Synaptic Plasticity. Neuron 2012, 73, 374–390. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Xu, T.; Li, Y.; Fei, W.; Yang, G.; Hong, Y. Inhibition of CRY2 by STAT3/MiRNA-7-5p Promotes Osteoblast Differentiation through Upregulation of CLOCK/BMAL1/P300 Expression. Mol. Ther. Nucleic Acids 2020, 19, 865–876. [Google Scholar] [CrossRef]
- Luo, W.; Sehgal, A. Regulation of Circadian Behavioral Output via a MicroRNA-JAK/STAT Circuit. Cell 2012, 148, 765–779. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jeong, A.J.; Ye, S.-K. Highlighted STAT3 as a Potential Drug Target for Cancer Therapy. BMB Rep 2019, 52, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moravcová, S.; Filipovská, E.; Spišská, V.; Svobodová, I.; Novotný, J.; Bendová, Z. The Circadian Rhythms of STAT3 in the Rat Pineal Gland and Its Involvement in Arylalkylamine-N-Acetyltransferase Regulation. Life 2021, 11, 1105. https://doi.org/10.3390/life11101105
Moravcová S, Filipovská E, Spišská V, Svobodová I, Novotný J, Bendová Z. The Circadian Rhythms of STAT3 in the Rat Pineal Gland and Its Involvement in Arylalkylamine-N-Acetyltransferase Regulation. Life. 2021; 11(10):1105. https://doi.org/10.3390/life11101105
Chicago/Turabian StyleMoravcová, Simona, Eva Filipovská, Veronika Spišská, Irena Svobodová, Jiří Novotný, and Zdeňka Bendová. 2021. "The Circadian Rhythms of STAT3 in the Rat Pineal Gland and Its Involvement in Arylalkylamine-N-Acetyltransferase Regulation" Life 11, no. 10: 1105. https://doi.org/10.3390/life11101105
APA StyleMoravcová, S., Filipovská, E., Spišská, V., Svobodová, I., Novotný, J., & Bendová, Z. (2021). The Circadian Rhythms of STAT3 in the Rat Pineal Gland and Its Involvement in Arylalkylamine-N-Acetyltransferase Regulation. Life, 11(10), 1105. https://doi.org/10.3390/life11101105






