Coupling/Uncoupling Reversibility in Isolated Mitochondria from Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
3. Results
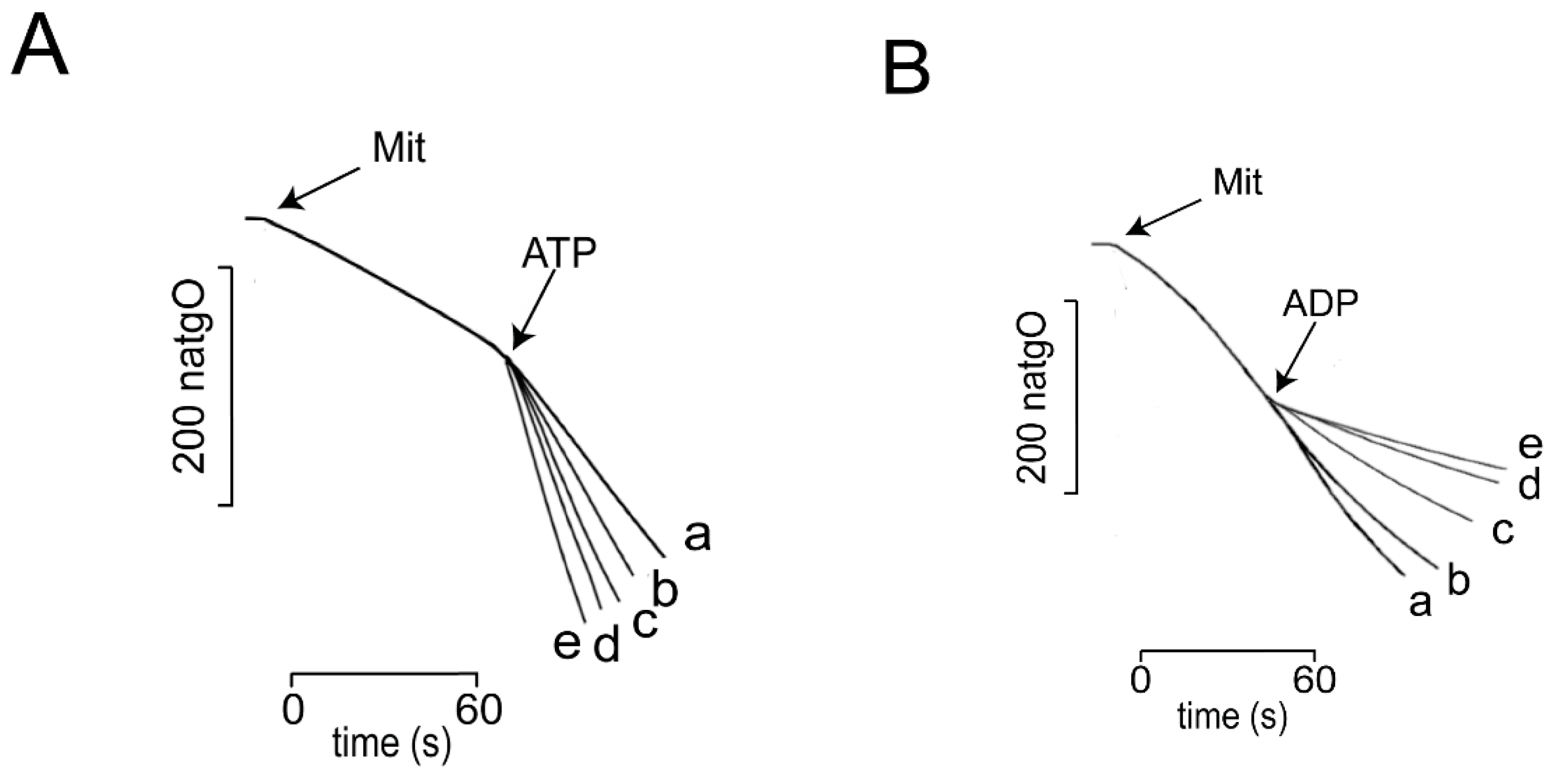
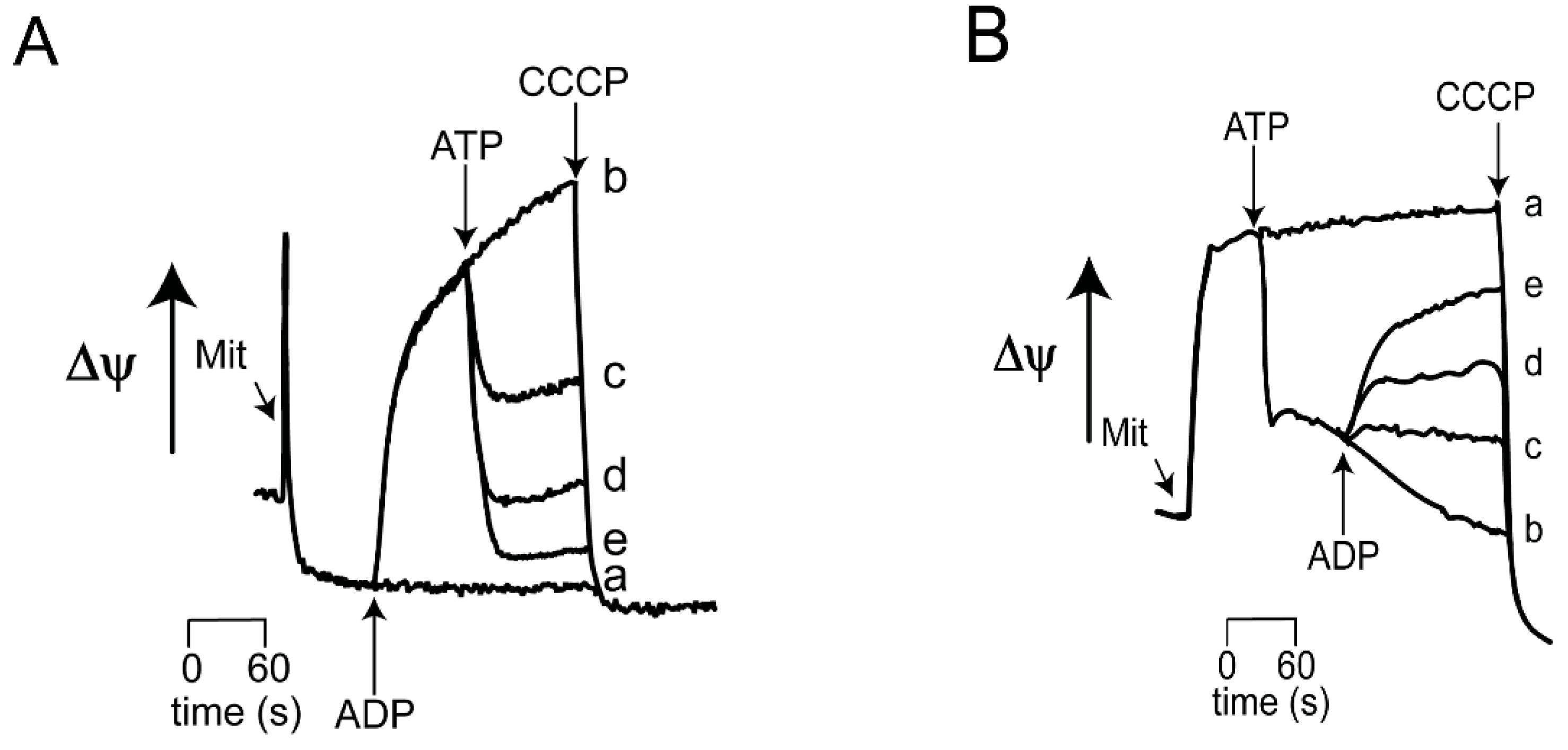
3.1. Effects of ADP/ATP on ScMUC
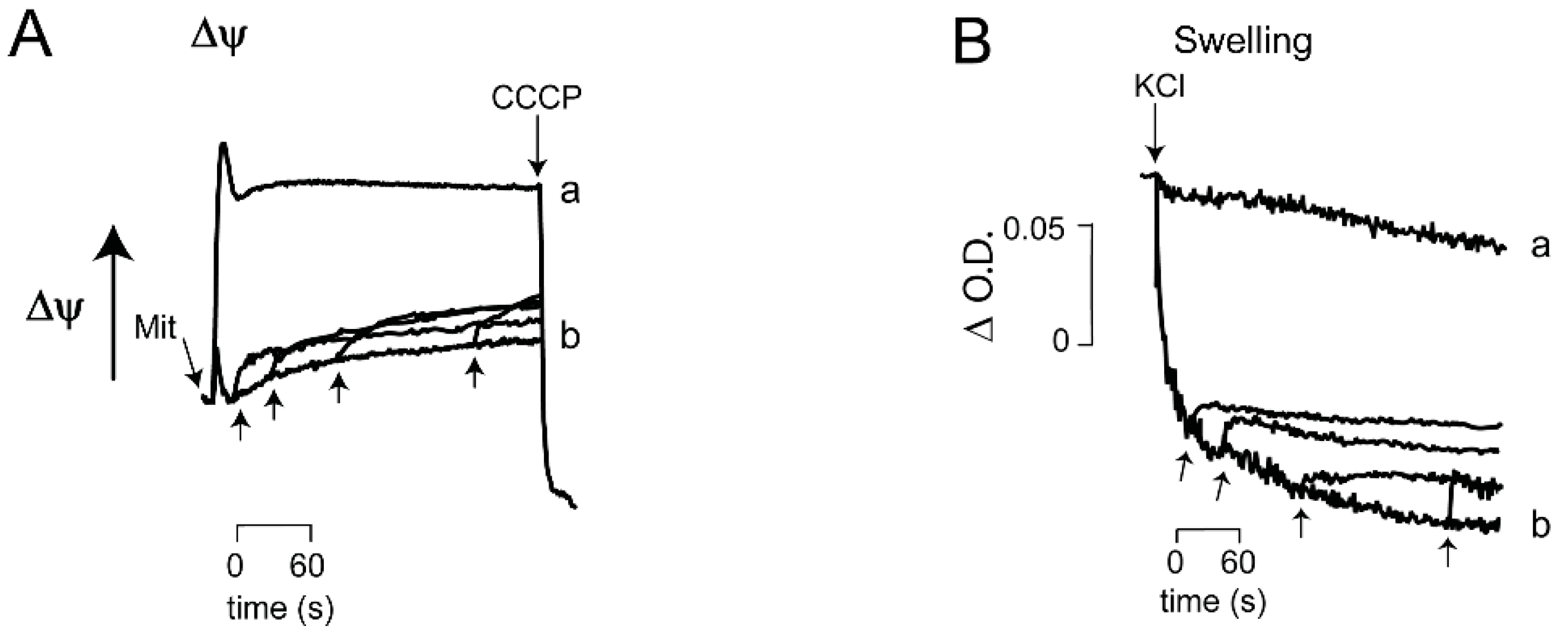
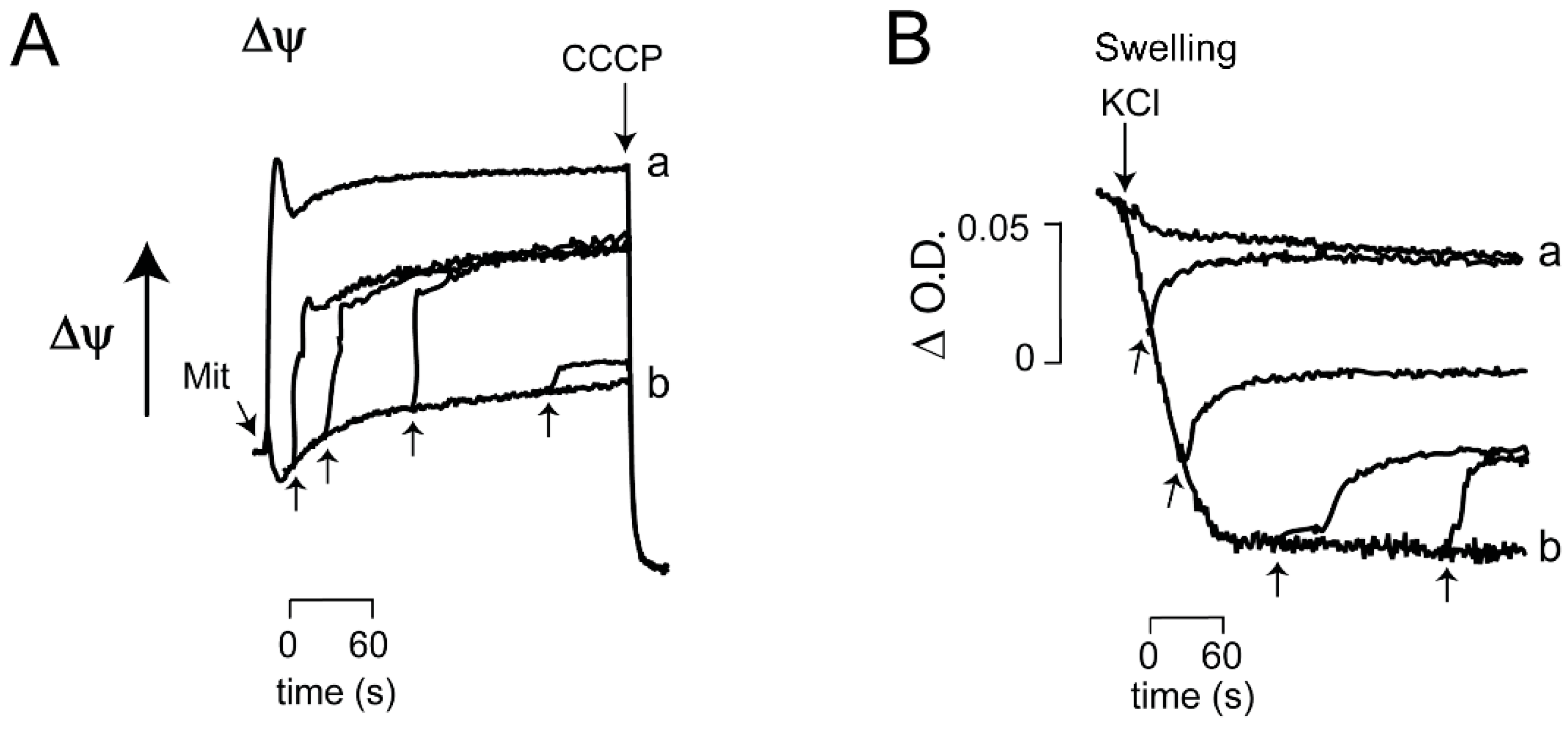
3.2. Ca2+/EGTA Alternating Effects on ScMUC
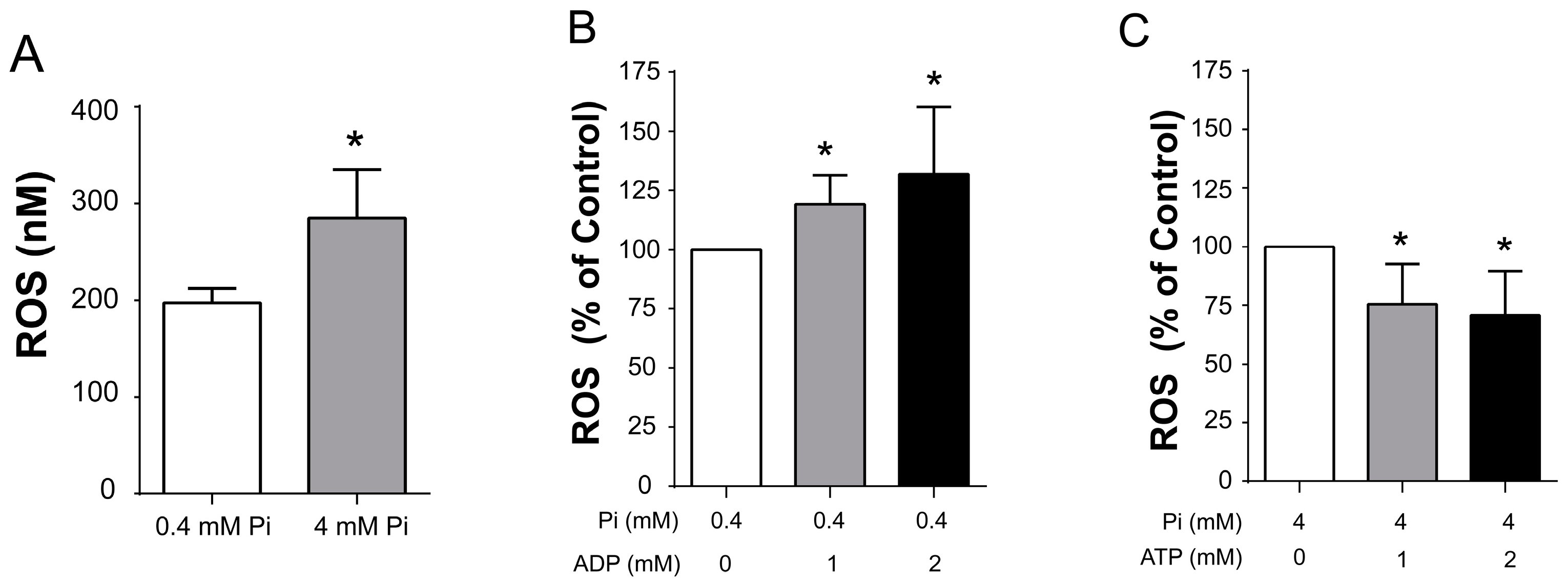
3.3. Reactive Oxygen Species (ROS) Production under Conditions That Open or Close ScMUC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics 4, 4th ed.; Academic Press Inc.: London, UK, 2013; ISBN 9780123884251. [Google Scholar]
- Gena, P.; del Buono, N.; D’Abbicco, M.; Mastrodonato, M.; Berardi, M.; Svelto, M.; Lopez, L.; Calamita, G. Dynamical Modeling of Liver Aquaporin-9 Expression and Glycerol Permeability in Hepatic Glucose Metabolism. Eur. J. Cell Biol. 2017, 96, 61–69. [Google Scholar] [CrossRef]
- Hatefi, Y. The Mitochondrial Electron Transport and Oxidative Phosphorylation System. Annu. Rev. Biochem. 1985, 54, 1015–1069. [Google Scholar] [CrossRef]
- Mazat, J.-P.; Devin, A.; Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Prieto, S.; Bouillaud, F.; Ricquier, D.; Rial, E. Activation by ATP of a Proton-Conducting Pathway in Yeast Mitochondria. Eur. J. Biochem. 1992, 208, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Castrejón, V.; Parra, C.; Moreno, R.; Peña, A.; Uribe, S. Potassium Collapses the ΔP in Yeast Mitochondria While the Rate of ATP Synthesis Is Inhibited Only Partially: Modulation by Phosphate. Arch. Biochem. Biophys. 1997, 346, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Guérin, B.; Bunoust, O.; Rouqueys, V.; Rigoulet, M. ATP-Induced Unspecific Channel in Yeast Mitochondria. J. Biol. Chem. 1994, 269, 25406–25410. [Google Scholar] [CrossRef]
- Di Bartolomeo, F.; Malina, C.; Campbell, K.; Mormino, M.; Fuchs, J.; Vorontsov, E.; Gustafsson, C.M.; Nielsen, J. Absolute Yeast Mitochondrial Proteome Quantification Reveals Trade-off between Biosynthesis and Energy Generation during Diauxic Shift. Proc. Natl. Acad. Sci. USA. 2020, 117, 7524–7535. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Wang, T.Y.; Tsai, B.S.; Wu, F.T.; Yu, F.J.; Tseng, Y.J.; Sung, H.M.; Li, W.H. Identifying Cis-Regulatory Changes Involved in the Evolution of Aerobic Fermentation in Yeasts. Genome Biol. Evol. 2013, 5, 1065–1078. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Orefice, A.; Ibarra-García-Padilla, R.; Maldonado-Guzmán, R.; Guerrero-Castillo, S.; Luévano-Martínez, L.A.; Pérez-Vázquez, V.; Gutiérrez-Aguilar, M.; Uribe-Carvajal, S. The Saccharomyces cerevisiae Mitochondrial Unselective Channel Behaves as a Physiological Uncoupling System Regulated by Ca2+, Mg2+, Phosphate and ATP. J. Bioenerg. Biomembr. 2015, 47, 477–491. [Google Scholar] [CrossRef]
- Carafoli, E. Intracellular Calcium Homeostasis. Annu. Rev. Biochem. 1987, 56, 395–433. [Google Scholar] [CrossRef]
- Iida, H.; Sakaguchi, S.; Yagawa, Y.; Anraku, Y. Cell Cycle Control by Ca2+ in Saccharomyces cerevisiae. J. Biol. Chem. 1990, 265, 21216–21222. [Google Scholar] [CrossRef]
- Iida, H.; Yagawa, Y.; Anraku, Y. Essential Role for Induced Ca2+ Influx Followed by [Ca2+]i Rise in Maintaining Viability of Yeast Cells Late in the Mating Pheromone Response Pathway. A Study of [Ca2+]i in Single Saccharomyces cerevisiae Cells with Imaging of Fura-2. J. Biol. Chem. 1990, 265, 13391–13399. [Google Scholar] [CrossRef]
- Chalmers, S.; Nicholls, D.G. The Relationship between Free and Total Calcium Concentrations in the Matrix of Liver and Brain Mitochondria. J. Biol. Chem. 2003, 278, 19062–19070. [Google Scholar] [CrossRef] [Green Version]
- Madden, K.; Snyder, M. Cell Polarity and Morphogenesis in Budding Yeast. Annu. Rev. Microbiol. 1998, 52, 687–744. [Google Scholar] [CrossRef] [PubMed]
- Shellhammer, J.P.; Pomeroy, A.E.; Li, Y.; Dujmusic, L.; Elston, T.C.; Hao, N.; Dohlman, H.G. Quantitative Analysis of the Yeast Pheromone Pathway. Yeast 2019, 36, 495–518. [Google Scholar] [CrossRef]
- Uribe, S.; Ramirez, J.; Peña, A. Effects of Beta-Pinene on Yeast Membrane Functions. J. Bacteriol. 1985, 161, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Ruiz, R.; Avéret, N.; Araiza, D.; Pinson, B.; Uribe-Carvajal, S.; Devin, A.; Rigoulet, M. Mitochondrial Oxidative Phosphorylation Is Regulated by Fructose 1,6-Bisphosphate. J. Biol. Chem. 2008, 283, 26948–26955. [Google Scholar] [CrossRef] [Green Version]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of Serum Proteins by Means of the Biuret Reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Åkerman, K.E.O.; Wikström, M.K.F. Safranine as a Probe of the Mitochondrial Membrane Potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Castillo, S.; Cabrera-Orefice, A.; Vázquez-Acevedo, M.; González-Halphen, D.; Uribe-Carvajal, S. During the stationary growth phase, Yarrowia lipolytica prevents the overproduction of reactive oxygen species by activating an uncoupled mitochondrial respiratory pathway. Biochim. Biophys. Acta. 2012, 1817, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Manon, S.; Roucou, X.; Guérin, M.; Rigoulet, M.; Guérin, B. Minireview: Characterization of the Yeast Mitochondria Unselective Channel: A Counterpart to the Mammalian Permeability Transition Pore? J. Bioenerg. Biomembr. 1998, 30, 419–429. [Google Scholar] [CrossRef]
- Gutiérrez-Aguilar, M.; Pérez-Martínez, X.; Chávez, E.; Uribe-Carvajal, S. In Saccharomyces cerevisiae, the Phosphate Carrier Is a Component of the Mitochondrial Unselective Channel. Arch. Biochem. Biophys. 2010, 494, 184–191. [Google Scholar] [CrossRef]
- Lu, X.; Kwong, J.Q.; Molkentin, J.D.; Bers, D.M. Individual Cardiac Mitochondria Undergo Rare Transient Permeability Transition Pore Openings. Circ. Res. 2016, 118, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Boyman, L.; Coleman, A.K.; Zhao, G.; Wescott, A.P.; Joca, H.C.; Greiser, B.M.; Karbowski, M.; Ward, C.W.; Lederer, W.J. Dynamics of the Mitochondrial Permeability Transition Pore: Transient and Permanent Opening Events. Arch. Biochem. Biophys. 2019, 666, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Pérez-Vázquez, V.; Saavedra-Molina, A.; Uribe, S. In Saccharomyces Cerevisiae, Cations Control the Fate of the Energy Derived from Oxidative Metabolism Through the Opening and Closing of the Yeast Mitochondrial Unselective Channel. J. Bioenerg. Biomembr. 2003, 35, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Deed, R.C.; Pilkington, L.I. Lifestyle, Lineage, and Geographical Origin Influence Temperature-Dependent Phenotypic Variation across Yeast Strains during Wine Fermentation. Microorganisms 2020, 8, 1367. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, G.N.; Eiteman, M.A.; McEwen, J.E.; Olsson, L.; Nielsen, J. Increasing NADH Oxidation Reduces Overflow Metabolism in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 2402–2407. [Google Scholar] [CrossRef] [Green Version]
- Kruglov, A.G.; Kharechkina, E.S.; Nikiforova, A.B.; Odinokova, I.V.; Kruglova, S.A. Dynamics of the Permeability Transition Pore Size in Isolated Mitochondria and Mitoplasts. FASEB J. 2021, 35, 1–16. [Google Scholar] [CrossRef]
- Balakirev, M.Y.; Zimmer, G. Gradual Changes in Permeability of Inner Mitochondrial Membrane Precede the Mitochondrial Permeability Transition. Arch. Biochem. Biophys. 1998, 356, 46–54. [Google Scholar] [CrossRef]
- Guerrero-Castillo, S.; Araiza-Olivera, D.; Cabrera-Orefice, A.; Espinasa-Jaramillo, J.; Gutiérrez-Aguilar, M.; Luévano-Martínez, L.A.; Zepeda-Bastida, A.; Uribe-Carvajal, S. Physiological Uncoupling of Mitochondrial Oxidative Phosphorylation. Studies in Different Yeast Species. J. Bioenerg. Biomembr. 2011, 43, 323–331. [Google Scholar] [CrossRef]
- Bernardi, P.; Carraro, M.; Lippe, G. The mitochondrial permeability transition: Recent progress and open questions. FEBS J. 2021. [Google Scholar] [CrossRef]
- Bernardi, P. Mechanisms for Ca2+-Dependent Permeability Transition in Mitochondria. Proc. Natl. Acad. Sci. USA 2020, 117, 2743–2744. [Google Scholar] [CrossRef]
- Carraro, M.; Bernardi, P. Measurement of membrane permeability and the mitochondrial permeability transition. Methods Cell Biol. 2020, 155, 369–379. [Google Scholar]
- Burgess, G.M.; Godfrey, P.P.; McKinney, J.S.; Berridge, M.J.; Irvine, R.F.; Putney, J.W. The Second Messenger Linking Receptor Activation to Internal Ca Release in Liver. Nature 1984, 309, 63–66. [Google Scholar] [CrossRef]
- Kwon, E.; Heo, W. Do Optogenetic Tools for Dissecting Complex Intracellular Signaling Pathways. Biochem. Biophys. Res. Commun. 2020, 527, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Naumova, N.; Šachl, R. Regulation of Cell Death by Mitochondrial Transport Systems of Calcium and Bcl-2 Proteins. Membranes 2020, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.R.; Rosoff, P.M. Characterization of the Energy-Dependent, Mating Factor-Activated Ca2+ Influx in Saccharomyces cerevisiae. Cell Calcium 1992, 13, 615–626. [Google Scholar] [CrossRef]
- Carbó, N.; Tarkowski, N.; Ipiña, E.P.; Dawson, S.P.; Aguilar, P.S. Sexual Pheromone Modulates the Frequency of Cytosolic Ca2+ Bursts in Saccharomyces cerevisiae. Mol. Biol. Cell 2017, 28, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Giorgio, V.; Šileikytė, J.; Sartori, G.; Forte, M.; Lippe, G.; Zoratti, M.; Szabò, I.; Bernardi, P. Channel Formation by Yeast F-ATP Synthase and the Role of Dimerization in the Mitochondrial Permeability Transition. J. Biol. Chem. 2014, 289, 15980–15985. [Google Scholar] [CrossRef] [Green Version]
- Haworth, R.A.; Hunter, D.R. Control of the Mitochondrial Permeability Transition Pore by High-Affinity ADP Binding at the ADP/ATP Translocase in Permeabilized Mitochondria. J. Bioenerg. Biomembranes. 2000, 32, 91–96. [Google Scholar] [CrossRef]
- Kim, J.-S.; He, L.; Lemasters, J.J. Mitochondrial Permeability Transition: A Common Pathway to Necrosis and Apoptosis. Biochem. Biophys. Res. Commun. 2003, 304, 463–470. [Google Scholar] [CrossRef]
- Gutiérrez-Aguilar, M.; Douglas, D.L.; Gibson, A.K.; Domeier, T.L.; Molkentin, J.D.; Baines, C.P. Genetic Manipulation of the Cardiac Mitochondrial Phosphate Carrier Does Not Affect Permeability Transition. J. Mol. Cell. Cardiol. 2014, 72, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Baines, C.P.; Gutiérrez-Aguilar, M. The Still Uncertain Identity of the Channel-Forming Unit(s) of the Mitochondrial Permeability Transition Pore. Cell Calcium 2018, 73, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Shanmughapriya, S.; Rajan, S.; Hoffman, N.E.; Higgins, A.M.; Tomar, D.; Nemani, N.; Hines, K.J.; Smith, D.J.; Eguchi, A.; Vallem, S.; et al. SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Mol. Cell 2015, 60, 47–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, S.; Hoek, J.; Sheu, S.S. Mitochondrial Ca2+ and Regulation of the Permeability Transition Pore. J. Bioenerg. Biomembr. 2017, 49, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Uribe, S.; Rangel, P.; Pardo, J.P. Interactions of Calcium with Yeast Mitochondria. Cell Calcium 1992, 13, 211–217. [Google Scholar] [CrossRef]
- Carafoli, E.; Lehninger, A.L. A Survey of the Interaction of Calcium Ions with Mitochondria from Different Tissues and Species. Biochem. J. 1971, 122, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Parks, R.J.; Murphy, E.; Liu, J.C. Mitochondrial Permeability Transition Pore and Calcium Handling. Methods Mol. Biol. 2018, 1782, 187–196. [Google Scholar] [CrossRef]
- Rigoulet, M.; Bouchez, C.L.; Paumard, P.; Ransac, S.; Cuvellier, S.; Duvezin-Caubet, S.; Mazat, J.P.; Devin, A. Cell Energy Metabolism: An Update. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148276. [Google Scholar] [CrossRef]
- Trendeleva, T.; Sukhanova, E.; Ural’skaya, L.; Saris, N.-E.; Zvyagilskaya, R. Mitochondria from Dipodascus (Endomyces) magnusii and Yarrowia lipolytica Yeasts Did Not Undergo a Ca2+-Dependent Permeability Transition Even under Anaerobic Conditions. J. Bioenerg. Biomembr. 2011, 43, 623–631. [Google Scholar] [CrossRef]
- Deryabina, Y.; Isakova, E.; Antipov, A.; Saris, N.-E.L. The Inhibitors of Antioxidant Cell Enzymes Induce Permeability Transition in Yeast Mitochondria. J. Bioenerg. Biomembr. 2013, 45, 491–504. [Google Scholar] [CrossRef]
- Cabrera-Orefice, A.; Guerrero-Castillo, S.; Luévano-Martínez, L.A.; Peña, A.; Uribe-Carvajal, S. Mitochondria from the Salt-Tolerant Yeast Debaryomyces hansenii (Halophilic Organelles?). J. Bioenerg. Biomembr. 2010, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J. Mechanism of Homologous Recombination and Implications for Aging-Related Deletions in Mitochondrial DNA. Microbiol. Mol. Biol. Rev. 2013, 77, 476–496. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Yoshida, M. Rolling-Circle Replication in Mitochondrial Dna Inheritance: Scientific Evidence and Significance from Yeast to Human Cells. Genes 2020, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Aliyari Rad, S.; Dehghanian, Z.; Asgari Lajayer, B.; Nobaharan, K.; Astatkie, T. Mitochondrial Respiration and Energy Production Under Some Abiotic Stresses. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Jayawardhane, J.; Cochrane, D.W.; Vyas, P.; Bykova, N.V.; Vanlerberghe, G.C.; Igamberdiev, A.U. Roles for Plant Mitochondrial Alternative Oxidase Under Normoxia, Hypoxia, and Reoxygenation Conditions. Front. Plant Sci. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]



| Low or High Pi without Additions | Effector Additions |
|---|---|
| ScMUC is OPEN at 0.4 mM Pi | and CLOSED by ADP 0.5 to 2 mM Ca2+ 100 to 600 μM |
| ScMUC is CLOSED at 4.0 mM Pi | and OPENED by ATP 0.5 to 2.0 mM |
| At both Low and high Pi, alternating ADP/ATP close/open ScMUC respectively | |
| At low Pi, Ca2+ addition/chelation close/open ScMUC respectively | |
| Ca2+ is highly efficient to revert opening of ScMUC, even after 4 min of incubation. | |
| DATA | |
| OPEN ScMUC High Rate of Oxygen consumption Low ΔΨ Extensive mitochondrial swelling Low ROS generation | CLOSED ScMUC Low rate of oxygen consumption High ΔΨ Low to nil mitochondrial swelling High ROS production |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-García, L.; Ricardez-García, C.; Castañeda-Tamez, P.; Chiquete-Félix, N.; Uribe-Carvajal, S. Coupling/Uncoupling Reversibility in Isolated Mitochondria from Saccharomyces cerevisiae. Life 2021, 11, 1307. https://doi.org/10.3390/life11121307
Morales-García L, Ricardez-García C, Castañeda-Tamez P, Chiquete-Félix N, Uribe-Carvajal S. Coupling/Uncoupling Reversibility in Isolated Mitochondria from Saccharomyces cerevisiae. Life. 2021; 11(12):1307. https://doi.org/10.3390/life11121307
Chicago/Turabian StyleMorales-García, Lilia, Carolina Ricardez-García, Paulina Castañeda-Tamez, Natalia Chiquete-Félix, and Salvador Uribe-Carvajal. 2021. "Coupling/Uncoupling Reversibility in Isolated Mitochondria from Saccharomyces cerevisiae" Life 11, no. 12: 1307. https://doi.org/10.3390/life11121307







