Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definition of GD
2.2. Systematic Review Protocol
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Collection
2.5. Inclusion and Exclusion Criteria
2.6. Statistical Analysis
2.7. Subgroup Analyses
2.7.1. Comparison 1
2.7.2. Comparison 2
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. Clinical Utility of GD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PubMed | (gustat* OR tast* OR dysgeus* OR ageusi* OR parageu* OR “Taste”[Mesh] OR “Taste Perception”[Mesh] OR “Taste Threshold”[Mesh] OR “Taste Disorders”[Mesh] OR “Taste Buds”[Mesh] OR “Dysgeusia”[Mesh] OR “Ageusia”[Mesh]) AND (COVID* OR SARS-CoV-2 OR 2019-nCoV OR coronavirus OR “COVID-19”[Mesh] OR “SARS-CoV-2”[Mesh]) |
| Embase | (gustat* OR tast* OR dysgeus* OR ageusi* OR parageu*) AND (COVID* OR SARS-CoV-2 OR 2019-nCoV OR coronavirus) |
Appendix B

Appendix C
| Alizadehsani [142] |
| Altman [143] |
| Beltrán-Corbellini [176] |
| Bénézit [34] |
| Bidkar [139] |
| Boudjema [140] |
| Carignan [177] |
| Chas [144] |
| Chen [149] |
| Cho [178] |
| Dawson [179] |
| Dixon [180] |
| Dreyer [150] |
| Elimian [181] |
| Fistera [145] |
| Ganz-Lord [146] |
| Gibbons [151] |
| Gurrola [182] |
| Izquierdo-Domínguez [183] |
| Jeyashree (1) [141] |
| Jeyashree (2) [141] |
| Karni [152] |
| Kempker [184] |
| La Torre [185] |
| Leal [186] |
| Lee [187] |
| Martin-Sanz [188] |
| Martinez-Fierro [189] |
| Moeller [147] |
| Moolla [190] |
| Nakanishi [191] |
| Pérula de Torres [192] |
| Raberahona [148] |
| Riestra-Ayora [193] |
| Rojas-Lechuga [194] |
| Sayin [195] |
| Sbrana [153] |
| Sonoda [196] |
| Trachootham [197] |
| Trubiano [198] |
| Tudrej [199] |
| Villerabel [200] |
| Yan [201] |
| Zayet (1) [202] |
| Zayet (2) [203] |
Appendix D
| Gustatory dysfunction | GD |
| Olfactory dysfunction | OD |
| Reverse transcription polymerase chain reaction | RT-PCR |
| Odds ratio | OR |
| Positive likelihood ratio | positive LR |
| Negative likelihood ratio | negative LR |
| Preferred Reporting Items for Systematic reviews and Meta-analyses | PRISMA |
| Upper respiratory tract infection | URTI |
| COVID+ | COVID-19 positive patients |
| COVID− | COVID-19 negative patients |
| COVID+GD+ | COVID-19 positive patients with gustatory dysfunction |
| COVID+GD− | COVID-19 positive patients without gustatory dysfunction |
| COVID−GD+ | COVID-19 negative patients with gustatory dysfunction |
| COVID−GD− | COVID-19 negative patients without gustatory dysfunction |
Appendix E
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 1–2 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 2 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 3 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | 3, 15 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 3 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 3 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 3 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | 3 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | 3 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | NA |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | NA (only studies with complete data were included) | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 3 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 3 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | 4 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | 3 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | NA |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 5 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | 5 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 16, 17 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 16 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 7–10 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | 6 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 6–10 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 7, 10 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | NA |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | NA |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 11 |
| 23b | Discuss any limitations of the evidence included in the review. | 13 | |
| 23c | Discuss any limitations of the review processes used. | 13 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 13 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | 3 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 3 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | NA | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | 14 |
| Competing interests | 26 | Declare any competing interests of review authors. | 14 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | 14 |
References
- Larici, A.R.; Cicchetti, G.; Marano, R.; Merlino, B.; Elia, L.; Calandriello, L.; Del Ciello, A.; Farchione, A.; Savino, G.; Infante, A.; et al. Multimodality imaging of COVID-19 pneumonia: From diagnosis to follow-up. Acomprehensive review. Eur. J. Radiol. 2020, 131, 109217. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Liu, P.; Shi, X.L.; Chu, Y.L.; Zhang, J.; Xia, J.; Gao, X.Z.; Qu, T.; Wang, M.Y. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut 2020, 69, 1143–1144. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.H.; Lui, R.N.; Sung, J.J. Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 2020, 35, 744–748. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Porfidia, A.; Valeriani, E.; Pola, R.; Porreca, E.; Rutjes, A.W.S.; Di Nisio, M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020, 196, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Savarimuthu, S.; Leung, M.S.T.; Harky, A. The need to manage the risk of thromboembolism in COVID-19 patients. J. Vasc. Surg. 2020, 72, 799–804. [Google Scholar] [CrossRef]
- Tan, Y.K.; Goh, C.; Leow, A.S.T.; Tambyah, P.A.; Ang, A.; Yap, E.S.; Tu, T.M.; Sharma, V.K.; Yeo, L.L.L.; Chan, B.P.L.; et al. COVID-19 and ischemic stroke: A systematic review and meta-summary of the literature. J. Thromb. Thrombolysis 2020, 1–9. [Google Scholar] [CrossRef]
- Ahmad, I.; Rathore, F.A. Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 2020, 77, 8–12. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain-Barre Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Mullol, J.; Alobid, I.; Marino-Sanchez, F.; Izquierdo-Dominguez, A.; Marin, C.; Klimek, L.; Wang, D.Y.; Liu, Z. The Loss of Smell and Taste in the COVID-19 Outbreak: A Tale of Many Countries. Curr. Allergy Asthma Rep. 2020, 20, 61. [Google Scholar] [CrossRef]
- Izquierdo-Dominguez, A.; Rojas-Lechuga, M.J.; Mullol, J.; Alobid, I. Olfactory dysfunction in the COVID-19 outbreak. J. Investig. Allergol. Clin. Immunol. 2020, 30, 317–326. [Google Scholar] [CrossRef]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported Olfactory and Taste Disorders in Patients with Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef] [Green Version]
- De Maria, A.; Varese, P.; Dentone, C.; Barisione, E.; Bassetti, M. High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients. J. Med. Virol. 2020, 92, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Whittaker, E. Kawasaki-like disease: Emerging complication during the COVID-19 pandemic. Lancet 2020, 395, 1741–1743. [Google Scholar] [CrossRef]
- Ng, Y.; Li, Z.; Chua, Y.X.; Chaw, W.L.; Zhao, Z.; Er, B.; Pung, R.; Chiew, C.J.; Lye, D.C.; Heng, D.; et al. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 307–311. [Google Scholar] [CrossRef]
- Steinbrook, R. Contact Tracing, Testing, and Control of COVID-19-Learning from Taiwan. JAMA Intern. Med. 2020, 180, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Jian, S.W.; Liu, D.P.; Ng, T.C.; Huang, W.T.; Lin, H.H.; Taiwan, C.-O.I.T. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern. Med. 2020, 180, 1156–1163. [Google Scholar] [CrossRef]
- Salathe, M.; Althaus, C.L.; Neher, R.; Stringhini, S.; Hodcroft, E.; Fellay, J.; Zwahlen, M.; Senti, G.; Battegay, M.; Wilder-Smith, A.; et al. COVID-19 epidemic in Switzerland: On the importance of testing, contact tracing and isolation. Swiss Med. Wkly. 2020, 150, w20225. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, L.; Leung, T. Evaluating the Effectiveness of Social Distancing Interventions to Delay or Flatten the Epidemic Curve of Coronavirus Disease. Emerg Infect. Dis 2020, 26, 1740–1748. [Google Scholar] [CrossRef]
- Teslya, A.; Pham, T.M.; Godijk, N.G.; Kretzschmar, M.E.; Bootsma, M.C.J.; Rozhnova, G. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: A modelling study. PLoS Med. 2020, 17, e1003166. [Google Scholar] [CrossRef]
- Zhang, J.; Litvinova, M.; Liang, Y.; Wang, Y.; Wang, W.; Zhao, S.; Wu, Q.; Merler, S.; Viboud, C.; Vespignani, A.; et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science 2020, 368, 1481–1486. [Google Scholar] [CrossRef]
- Brinati, D.; Campagner, A.; Ferrari, D.; Locatelli, M.; Banfi, G.; Cabitza, F. Detection of COVID-19 Infection from Routine Blood Exams with Machine Learning: A Feasibility Study. J. Med. Syst. 2020, 44, 135. [Google Scholar] [CrossRef]
- Cheng, M.P.; Papenburg, J.; Desjardins, M.; Kanjilal, S.; Quach, C.; Libman, M.; Dittrich, S.; Yansouni, C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 172, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Burki, T.K. Testing for COVID-19. Lancet Respir Med. 2020, 8, e63–e64. [Google Scholar] [CrossRef]
- Smith, K.P.; Cheng, A.; Chopelas, A.; DuBois-Coyne, S.; Mezghani, I.; Rodriguez, S.; Talay, M.; Kirby, J.E. Large-Scale, In-House Production of Viral Transport Media to Support SARS-CoV-2 PCR Testing in a Multihospital Health Care Network during the COVID-19 Pandemic. J. Clin. Microbiol. 2020, 58, e00913-20. [Google Scholar] [CrossRef] [PubMed]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America. Nucleic Acid Amplification Testing (e.g., RT-PCR). Available online: https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing/ (accessed on 25 July 2021).
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.F.; Duan, Q.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef]
- Costa, K.; Carnauba, A.T.L.; Rocha, K.W.; Andrade, K.C.L.; Ferreira, S.M.S.; Menezes, P.L. Olfactory and taste disorders in COVID-19: A systematic review. Braz. J. Otorhinolaryngol. 2020, 86, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Luers, J.C.; Rokohl, A.C.; Loreck, N.; Wawer Matos, P.A.; Augustin, M.; Dewald, F.; Klein, F.; Lehmann, C.; Heindl, L.M. Olfactory and Gustatory Dysfunction in Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2262–2264. [Google Scholar] [CrossRef] [PubMed]
- Benezit, F.; Le Turnier, P.; Declerck, C.; Paille, C.; Revest, M.; Dubee, V.; Tattevin, P.; Group, R.C.S. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 5, 94. [Google Scholar] [CrossRef]
- Maheswaran, T.; Abikshyeet, P.; Sitra, G.; Gokulanathan, S.; Vaithiyanadane, V.; Jeelani, S. Gustatory dysfunction. J. Pharm. Bioallied Sci. 2014, 6, S30–S33. [Google Scholar] [CrossRef]
- Gibbons, J.R.; Sadiq, N.M. Neuroanatomy, Neural Taste Pathway. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545236/ (accessed on 30 August 2020).
- Hornuss, D.; Lange, B.; Schroter, N.; Rieg, S.; Kern, W.V.; Wagner, D. Anosmia in COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1426–1427. [Google Scholar] [CrossRef]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020, 42, 1560–1569. [Google Scholar] [CrossRef]
- Heckmann, J.G.; Heckmann, S.M.; Lang, C.J.; Hummel, T. Neurological aspects of taste disorders. Arch. Neurol 2003, 60, 667–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dicpinigaitis, P.V. Post-viral Anosmia (Loss of Sensation of Smell) Did Not Begin with COVID-19! Lung 2021, 199, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.T.; Jafek, B.W.; Eller, P.M.; Rowley III, J.C. Ultrastructural histopathology of human olfactory dysfunction. Microsc. Res. Tech. 1992, 23, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Seiden, A.M. Postviral olfactory loss. Otolaryngol Clin. N. Am. 2004, 37, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Welge-Lüssen, A.; Wolfensberger, M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol. 2006, 63, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.P.; Kanjanaumporn, J.; Aeumjaturapat, S.; Chusakul, S.; Seresirikachorn, K.; Snidvongs, K. Olfactory and gustatory dysfunctions in COVID-19 patients: A systematic review and meta-analysis. Asian Pac. J. Allergy Immunol. 2020, 38, 162–169. [Google Scholar] [CrossRef]
- Liou, J.M.; Chen, M.J.; Hong, T.C.; Wu, M.S. Alteration of taste or smell as a predictor of COVID-19. Gut 2021, 70, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.W.; Chee, J.; Subramaniam, S.; Ng, C.L. Frequency and Clinical Utility of Olfactory Dysfunction in COVID-19: A Systematic Review and Meta-analysis. Curr. Allergy Asthma Rep. 2020, 20, 76. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 July 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Doebler, P. mada: Meta-Analysis of Diagnostic Accuracy. R Package Version 0.5.10. Available online: https://CRAN.R-project.org/package=mada (accessed on 25 July 2021).
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. dmetar: Companion R Package for the Guide ’Doing Meta-Analysis in R’. R package version 0.0.9000. Available online: http://dmetar.protectlab.org (accessed on 25 July 2021).
- Akinbami, L.J.; Petersen, L.R.; Sami, S.; Vuong, N.; Lukacs, S.L.; Mackey, L.; Atas, J.; LaFleur, B.J. COVID-19 symptoms and SARS-CoV-2 antibody positivity in a large survey of first responders and healthcare personnel, May–July 2020. Clin. Infect. Dis. 2021, 73, e822–e825. [Google Scholar] [CrossRef]
- Angulo-Bazán, Y.; Solis-Sánchez, G.; Cardenas, F.; Jorge, A.; Acosta, J.; Cabezas, C. Household transmission of SARS-CoV-2 (COVID-19) in Lima, Peru. Cad. Saude Publica 2021, 37, e00238720. [Google Scholar] [CrossRef] [PubMed]
- Anna, F.; Goyard, S.; Lalanne, A.I.; Nevo, F.; Gransagne, M.; Souque, P.; Louis, D.; Gillon, V.; Turbiez, I.; Bidard, F.C.; et al. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. Eur. J. Immunol. 2021, 51, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.S.; Horton, D.B.; Roy, J.; Xia, W.; Greenberg, P.; Andrews, T.; Gennaro, M.L.; Parmar, V.; Russell, W.D.; Reilly, N.; et al. Risk Factors for Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Hospital Workers: Results from a Screening Study in New Jersey, United States in Spring 2020. Open Forum Infect. Dis. 2020, 7, ofaa534. [Google Scholar] [CrossRef] [PubMed]
- Gelbart, B.; Schapkaitz, E.; Kaftel, S.; Peretz, E.; Peretz, A. The Relationship between Serological Testing, Demographics, Clinical Presentation and RT-PCR Testing for COVID-19. Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Lombardi, A.; Mangioni, D.; Consonni, D.; Cariani, L.; Bono, P.; Cantù, A.P.; Tiso, B.; Carugno, M.; Muscatello, A.; Lunghi, G.; et al. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: A cross-sectional study. BMJ Open 2021, 11, e047216. [Google Scholar] [CrossRef] [PubMed]
- Pritsch, M.; Radon, K.; Bakuli, A.; Le Gleut, R.; Olbrich, L.; Guggenbüehl Noller, J.M.; Saathoff, E.; Castelletti, N.; Garí, M.; Pütz, P.; et al. Prevalence and Risk Factors of Infection in the Representative COVID-19 Cohort Munich. Int. J. Environ. Res. Public Health 2021, 18, 3572. [Google Scholar] [CrossRef]
- Barbhaya, D.; Franco, S.; Gandhi, K.; Arya, R.; Neupane, R.; Foroughi, N.; Oluigbo, N.; Fishbein, D.; Tran, J. Characteristics and Outcomes of COVID-19 Infection from an Urban Ambulatory COVID-19 Clinic-Guidance for Outpatient Clinicians in Triaging Patients. J. Prim. Care Community Health 2021, 12, 21501327211017016. [Google Scholar] [CrossRef]
- Cao, A.C.; Nimmo, Z.M.; Mirza, N.; Cohen, N.A.; Brody, R.M.; Doty, R.L. Objective screening for olfactory and gustatory dysfunction during the COVID-19 pandemic: A prospective study in healthcare workers using self-administered testing. World J. Otorhinolaryngol. Head Neck Surg. 2021. [Google Scholar] [CrossRef]
- Huart, C.; Philpott, C.; Konstantinidis, I.; Altundag, A.; Whitcroft, K.L.; Trecca, E.M.C.; Cassano, M.; Rombaux, P.; Hummel, T. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology 2020, 58, 623–625. [Google Scholar] [CrossRef]
- Kronborg, T.M.; Kimer, N.; Junker, A.E.; Werge, M.P.; Gluud, L.L.; Ytting, H. Experience from a COVID-19 first-line referral clinic in Greater Copenhagen. Dan Med. J. 2020, 67, A05200343. [Google Scholar]
- Sudre, C.H.; Keshet, A.; Graham, M.S.; Joshi, A.D.; Shilo, S.; Rossman, H.; Murray, B.; Molteni, E.; Klaser, K.; Canas, L.S.; et al. Anosmia and other SARS-CoV-2 positive test-associated symptoms, across three national, digital surveillance platforms as the COVID-19 pandemic and response unfolded: An observation study. medRxiv 2020. [Google Scholar] [CrossRef]
- Abalo-Lojo, J.M.; Pouso-Diz, J.M.; Gonzalez, F. Taste and Smell Dysfunction in COVID-19 Patients. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, A.A.; Ghweil, A.A.; Hassan, M.H.; Rashad, A.; Khodeary, A.; Aref, Z.F.; Sayed, M.A.A.; Elsamman, M.K.; Bazeed, S.E.S. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol. Trace Elem. Res. 2021, 199, 1–8. [Google Scholar] [CrossRef]
- Abraha, H.E.; Gessesse, Z.; Gebrecherkos, T.; Kebede, Y.; Weldegiargis, A.W.; Tequare, M.H.; Welderufael, A.L.; Zenebe, D.; Gebremariam, A.G.; Dawit, T.C.; et al. Clinical features and risk factors associated with morbidity and mortality among patients with COVID-19 in northern Ethiopia. Int. J. Infect. Dis. 2021, 105, 776–783. [Google Scholar] [CrossRef]
- Agarwal, P.; Ray, S.; Madan, A.; Tyson, B. Neurological manifestations in 404 COVID-19 patients in Washington State. J. Neurol. 2021, 268, 770–772. [Google Scholar] [CrossRef]
- Aggarwal, S.; Garcia-Telles, N.; Aggarwal, G.; Lavie, C.; Lippi, G.; Henry, B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis 2020, 7, 91–96. [Google Scholar] [CrossRef]
- Agurto, H.S.; Veramendi-Schultz, I.; Vasquez-Elera, L.; Gonzales-Soler, Z.; Lozano, A.; Zavaleta Alva, R.; Marin-Duenas, I.; Vega, J.; Bautista-Altamirano, C. Prevalence and clinical characteristics of gastrointestinal manifestations in covid-19 patients in peru: A multicenter cohort study. Endoscopy 2021, 53 (Suppl. 1), S267. [Google Scholar]
- Agyeman, A.A.; Chin, K.L.; Landersdorfer, C.B.; Liew, D.; Ofori-Asenso, R. Smell and Taste Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2020, 95, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Ahbab, S.; Turker, F.; Turker, B.C.; Kula, A.C.; Ziyadanoglu, F.P.; Acar, P.; Ak, E.C.; Alcelik, R.D.; Ahbab, M.A.; Ataoglu, H.E. Evaluation of the association between gastrointestinal symptoms and laboratory outcomes in hospitalized covid-19 patients. Haseki Tip Bul. 2021, 59, 108–113. [Google Scholar] [CrossRef]
- Ahn, E.J.; Min, H.J. Prevalence of olfactory or gustatory dysfunction in coronavirus disease patients: An analysis based on Korean nationwide claims data. Clin. Exp. Otorhinolaryngol. 2021. [Google Scholar] [CrossRef]
- Al-Ani, R.M.; Acharya, D. Prevalence of Anosmia and Ageusia in Patients with COVID-19 at a Primary Health Center, Doha, Qatar. Indian J. Otolaryngol Head Neck Surg 2020, 1–7. [Google Scholar] [CrossRef]
- Al-Darzi, W.; Aurora, L.; Michaels, A.; Cowger, J.; Grafton, G.; Selektor, Y.; Tita, C.; Hannawi, B.; Lanfear, D.; Nemeh, H.W.; et al. Heart transplant recipients with confirmed 2019 novel coronavirus infection: The Detroit experience. Clin. Transplant. 2020, 34, e14091. [Google Scholar] [CrossRef]
- Al-Swiahb, J.N.; Motiwala, M.A. Upper respiratory tract and otolaryngological manifestations of coronavirus disease 2019 (COVID-19): A systemic review. Sage Open Med. 2021, 9. [Google Scholar] [CrossRef]
- Alam, M.M.; Khokhar, M.; Qasim, A.P.; Jaan, A.; Mehboob, N.U.H.; Qasim, J.A. Association of clinical presentation & comorbidity among covid-19 patients admitted in tertiary care hospital. Med. Forum Mon. 2020, 31, 39–43. [Google Scholar]
- Alessandro, L.; Appiani, F.; Bendersky, M.; Borrego Guerrero, B.; Bruera, G.; Cairola, P.; Calandri, I.; Cardozo Oliver, J.M.; Clement, M.E.; Di Egidio, M.; et al. Registry of neurological manifestations due to coronavirus-19 (COVID-19). Neurol. Argent. 2021, 13, 84–94. [Google Scholar] [CrossRef]
- AlShakhs, A.; Almomen, A.; AlYaeesh, I.; AlOmairin, A.; AlMutairi, A.A.; Alammar, Z.; Almomen, H.; Almomen, Z. The Association of Smell and Taste Dysfunction with COVID19, And Their Functional Impacts. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alshami, A.; Alattas, R.; Anan, H.; Alhalimi, A.; Alfaraj, A.; Qahtani, H.A. Silent disease and loss of taste and smell are common manifestations of SARS-COV-2 infection in a quarantine facility: Saudi Arabia. PLoS ONE 2020, 15, e0241258. [Google Scholar] [CrossRef]
- Barón-Sánchez, J.; Santiago, C.; Goizueta-San Martín, G.; Arca, R.; Fernández, R. Smell and taste disorders in Spanish patients with mild COVID-19. Neurología (Engl. Ed.) 2020, 35, 633–638. [Google Scholar] [CrossRef]
- Begam, N.; Bashar, M.A. Olfactory and Taste Disorders in Patients with SARS-CoV-2 Infection. Int. Arch. Otorhinolaryngol. 2020, 24, e391–e392. [Google Scholar] [CrossRef]
- Bhatta, S.; Gandhi, S.; Saindani, S.J.; Ganesuni, D.; Ghanpur, A.D. Otorhinolaryngological manifestations of coronavirus disease 2019: A prospective review of 600 patients. J. Laryngol. Otol 2021, 135, 206–211. [Google Scholar] [CrossRef]
- Bhatta, S.; Sharma, D.; Sharma, S.; Maharjan, L.; Bhattachan, S.; Shah, M.K.; Singhal, A.; Ghanpur, A.D.; Ganesuni, D.; Saindani, S.J. Smell and Taste Disturbance in COVID-19 Patients: A Prospective Multicenteric Review. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Biadsee, A.; Biadsee, A.; Kassem, F.; Dagan, O.; Masarwa, S.; Ormianer, Z. Olfactory and Oral Manifestations of COVID-19: Sex-Related Symptoms-A Potential Pathway to Early Diagnosis. Otolaryngol. Head Neck Surg. 2020, 163, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Biadsee, A.; Dagan, O.; Ormianer, Z.; Kassem, F.; Masarwa, S.; Biadsee, A. Eight-month follow-up of olfactory and gustatory dysfunctions in recovered COVID-19 patients. Am. J. Otolaryngol. 2021, 42, 103065. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.R.; Modica, D.M.; Drago, G.D.; Azzolina, A.; Mattina, G.; De Natale, M.; Rossi, G.; Amata, M.; Canzoneri, G.; Manganaro, G.; et al. Alteration of Smell and Taste in Asymptomatic and Symptomatic COVID-19 Patients in Sicily, Italy. Ear Nose Throat J. 2021, 100, 182s–185s. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Gotimukul, A.; Wang, F.; Mina, S.A.; Bartels, H.C.; Burns, M.W.; Kole, A.E.; Vikram, H.R.; Gea-Banacloche, J.C.; Seville, M.T.; et al. Mild to moderate COVID-19 illness in adult outpatients: Characteristics, symptoms, and outcomes in the first 4 weeks of illness. Medicine 2021, 100, e26371. [Google Scholar] [CrossRef]
- Borah, H.; Das, S.; Goswami, A. Otorhinolaryngological Manifestations and Its Management in COVID 19 Patients. Indian J. Otolaryngol. Head Neck Surg 2021, 1–4. [Google Scholar] [CrossRef]
- Borsetto, D.; Hopkins, C.; Philips, V.; Obholzer, R.; Tirelli, G.; Polesel, J.; Boscolo-Rizzo, P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinology 2020, 58, 430–436. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Borsetto, D.; Spinato, G.; Fabbris, C.; Menegaldo, A.; Gaudioso, P.; Nicolai, P.; Tirelli, G.; Da Mosto, M.C.; Rigoli, R.; et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur. Arch. Otorhinolaryngol. 2020, 277, 2637–2640. [Google Scholar] [CrossRef]
- Brandão Neto, D.; Fornazieri, M.A.; Dib, C.; Di Francesco, R.C.; Doty, R.L.; Voegels, R.L.; Pinna, F.R. Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol. Head Neck Surg. 2021, 164, 512–518. [Google Scholar] [CrossRef]
- Dell’Era, V.; Farri, F.; Garzaro, G.; Gatto, M.; Aluffi Valletti, P.; Garzaro, M. Smell and taste disorders during COVID-19 outbreak: Cross-sectional study on 355 patients. Head Neck 2020, 42, 1591–1596. [Google Scholar] [CrossRef]
- Derashri, A.; Padhi, S.; Vaishnav, D.; Jain, A.; Bose, R.; Tyagi, V. Clinical insights into sars-cov-2 infection in rural rajasthan, india. Indian J. Public Health Res. Dev. 2021, 12, 17–26. [Google Scholar]
- Fantozzi, P.J.; Pampena, E.; Di Vanna, D.; Pellegrino, E.; Corbi, D.; Mammucari, S.; Alessi, F.; Pampena, R.; Bertazzoni, G.; Minisola, S.; et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am. J. Otolaryngol. 2020, 41, 102721. [Google Scholar] [CrossRef]
- Niklassen, A.S.; Draf, J.; Huart, C.; Hintschich, C.; Bocksberger, S.; Trecca, E.M.C.; Klimek, L.; Le Bon, S.D.; Altundag, A.; Hummel, T. COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre study on Smell and Taste. Laryngoscope 2021, 131, 1095–1100. [Google Scholar] [CrossRef]
- Ninchritz-Becerra, E.; Soriano-Reixach, M.M.; Mayo-Yánez, M.; Calvo-Henríquez, C.; Martínez-Ruiz de Apodaca, P.; Saga-Gutiérrez, C.; Parente-Arias, P.; Villareal, I.M.; Viera-Artiles, J.; Poletti-Serafini, D.; et al. Subjective evaluation of smell and taste dysfunction in patients with mild COVID-19 in Spain. Med. Clin. 2021, 156, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, M.; Taran, B.; Ketko, I.; Geva, G.; Yosef, R.; Toledo, I.; Twig, G.; Avramovitch, E.; Gordon, B.; Derazne, E.; et al. Self-reported symptoms in healthy young adults to predict potential coronavirus disease 2019. Clin. Microbiol. Infect. 2021, 27, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Printza, A.; Katotomichelakis, M.; Metallidis, S.; Panagopoulos, P.; Sarafidou, A.; Petrakis, V.; Constantinidis, J. The clinical course of smell and taste loss in COVID-19 hospitalized patients. Hippokratia 2020, 24, 66–71. [Google Scholar] [PubMed]
- Alharbi, H.; You, S.; Katz, J. Should anosmia and dysgeusia be a concern for oral and maxillofacial surgeons during the COVID-19 pandemic? Oral Maxillofac. Surg. 2021, 1–7. [Google Scholar] [CrossRef]
- Altin, F.; Cingi, C.; Uzun, T.; Bal, C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur. Arch. Otorhinolaryngol. 2020, 277, 2775–2781. [Google Scholar] [CrossRef]
- Bertlich, M.; Stihl, C.; Lüsebrink, E.; Hellmuth, J.C.; Scherer, C.; Freytag, S.; Spiegel, J.L.; Stoycheva, I.; Canis, M.; Weiss, B.G.; et al. The course of subjective and objective chemosensory dysfunction in hospitalized patients with COVID-19: A 6-month follow-up. Eur. Arch. Otorhinolaryngol. 2021, 1–7. [Google Scholar] [CrossRef]
- Ronan, G.; Kumar, L.; Davey, M.; Catriona, O.L.; McAleer, S.; Lynch, J.; Lavery, R.; Campion, J.; Ryan, J.; O’Donoghue, P.J.; et al. Factors associated with SARS-CoV-2 infection in patients attending an acute hospital ambulatory assessment unit. J. Med. Virol. 2021, 93, 4488–4495. [Google Scholar] [CrossRef]
- Adorni, F.; Prinelli, F.; Bianchi, F.; Giacomelli, A.; Pagani, G.; Bernacchia, D.; Rusconi, S.; Maggi, S.; Trevisan, C.; Noale, M.; et al. Self-Reported Symptoms of SARS-CoV-2 Infection in a Nonhospitalized Population in Italy: Cross-Sectional Study of the EPICOVID19 Web-Based Survey. JMIR Public Health Surveill 2020, 6, e21866. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Capdevila, J.; Chaudhari, A.; Granerod, J.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; Molteni, E.; Murray, B.; et al. Optimal symptom combinations to aid COVID-19 case identification: Analysis from a community-based, prospective, observational cohort. J. Infect. 2021, 82, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.; Aktürk, H.; Duman, M. Clinical Characteristics of Pediatric COVID-19 and Predictors of PCR Positivity. Pediatr. Int. 2021. [Google Scholar] [CrossRef]
- Bastiani, L.; Fortunato, L.; Pieroni, S.; Bianchi, F.; Adorni, F.; Prinelli, F.; Giacomelli, A.; Pagani, G.; Maggi, S.; Trevisan, C.; et al. Rapid COVID-19 Screening Based on Self-Reported Symptoms: Psychometric Assessment and Validation of the EPICOVID19 Short Diagnostic Scale. J. Med. Internet Res. 2021, 23, e23897. [Google Scholar] [CrossRef]
- Boffetta, P.; Violante, F.; Durando, P.; De Palma, G.; Pira, E.; Vimercati, L.; Cristaudo, A.; Icardi, G.; Sala, E.; Coggiola, M.; et al. Determinants of SARS-CoV-2 infection in Italian healthcare workers: A multicenter study. Sci. Rep. 2021, 11, 5788. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Borsetto, D.; Hopkins, C.; Polesel, J. Challenges in interpreting the diagnostic performance of symptoms to predict COVID-19 status: The case of anosmia. Int. Forum Allergy Rhinol. 2020, 10, 1113–1115. [Google Scholar] [CrossRef]
- Clemency, B.M.; Varughese, R.; Scheafer, D.K.; Ludwig, B.; Welch, J.V.; McCormack, R.F.; Ma, C.; Nan, N.; Giambra, T.; Raab, T. Symptom Criteria for COVID-19 Testing of Heath Care Workers. Acad. Emerg. Med. 2020, 27, 469–474. [Google Scholar] [CrossRef]
- Dini, G.; Montecucco, A.; Rahmani, A.; Barletta, C.; Pellegrini, L.; Debarbieri, N.; Orsi, A.; Caligiuri, P.; Varesano, S.; Manca, A.; et al. Clinical and epidemiological characteristics of COVID-19 during the early phase of the SARS-CoV-2 pandemic: A cross-sectional study among medical school physicians and residents employed in a regional reference teaching hospital in Northern Italy. Int. J. Occup Med. Env. Health 2021, 34, 189–201. [Google Scholar] [CrossRef]
- Feehan, A.K.; Fort, D.; Velasco, C.; Burton, J.H.; Garcia-Diaz, J.; Price-Haywood, E.G.; Sapp, E.; Pevey, D.; Seoane, L. The importance of anosmia, ageusia and age in community presentation of symptomatic and asymptomatic SARS-CoV-2 infection in Louisiana, USA; a cross-sectional prevalence study. Clin. Microbiol. Infect. 2021, 27, 633.e9–633.e16. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.A.; Olson, S.M.; Tenforde, M.W.; Self, W.H.; Wu, M.; Lindsell, C.J.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; et al. Symptoms and recovery among adult outpatients with and without COVID-19 at 11 healthcare facilities-July 2020, United States. Influenza Other Respir. Viruses 2021, 15, 345–351. [Google Scholar] [CrossRef]
- Haehner, A.; Draf, J.; Dräger, S.; de With, K.; Hummel, T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. Orl J. Otorhinolaryngol. Relat Spec. 2020, 82, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, E.; Tahir, E.; Bilek, H.C.; Kemal, Ö.; Deveci, A.; Aksakal Tanyel, E. Clinical significance of smell and taste dysfunction and other related factors in COVID-19. Eur. Arch. Otorhinolaryngol. 2021, 278, 2327–2336. [Google Scholar] [CrossRef]
- Lan, F.Y.; Filler, R.; Mathew, S.; Buley, J.; Iliaki, E.; Bruno-Murtha, L.A.; Osgood, R.; Christophi, C.A.; Fernandez-Montero, A.; Kales, S.N. COVID-19 symptoms predictive of healthcare workers’ SARS-CoV-2 PCR results. PLoS ONE 2020, 15, e0235460. [Google Scholar] [CrossRef]
- Lara, B.A.; Torres, F.; Holger, P.; Perales, C.; Basauri, S.; Clausdorff, H.; Escobedo, E.; Saldias, F.; Swadron, S.; Aguilera, P. Clinical Prediction Tool to Assess the Likelihood of a Positive SARS-Cov-2 (COVID-19) Polymerase Chain Reaction Test in Patients with Flu-like Symptoms. West. J. Emerg. Med. 2021, 22, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Counsell, N.; Ta, N.H.; Rocke, J.; Anmolsingh, R.; Eynon-Lewis, N.; Paun, S.; Hopkins, C.; Khwaja, S.; et al. Course of symptoms for loss of sense of smell and taste over time in one thousand forty-one healthcare workers during the Covid-19 pandemic: Our experience. Clin. Otolaryngol. 2021, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lisan, Q.; Fieux, M.; Tran Khai, N.; Nevoux, J.; Papon, J.F. Prevalence and Characteristics of Altered Sense of Smell/Taste during Covid-19 first wave: A French Nationwide Cross-sectional Study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021. [Google Scholar] [CrossRef]
- Lombardi, A.; Consonni, D.; Carugno, M.; Bozzi, G.; Mangioni, D.; Muscatello, A.; Castelli, V.; Palomba, E.; Cantù, A.P.; Ceriotti, F.; et al. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin. Microbiol. Infect. 2020, 26, 1413.e9–1413.e13. [Google Scholar] [CrossRef]
- Maechler, F.; Gertler, M.; Hermes, J.; van Loon, W.; Schwab, F.; Piening, B.; Rojansky, S.; Hommes, F.; Kausch, F.; Lindner, A.K.; et al. Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020-a cross-sectional study. Clin. Microbiol. Infect. 2020, 26, 1685.e7–1685.e12. [Google Scholar] [CrossRef] [PubMed]
- Mutha, A.S.; Beldar, A.S.; Desai, S.; Kumar, N.; Bhartiya, S.; Singh, T. Risk factors for reverse transcriptase polymerase chain reaction positivity for SARS-CoV-2 among health care workers in a group of tertiary care hospitals in Mumbai: A cross-sectional study. J. Clin. Diagn. Res. 2021, 15, FC18–FC21. [Google Scholar] [CrossRef]
- Nakakubo, S.; Suzuki, M.; Kamada, K.; Yamashita, Y.; Nakamura, J.; Horii, H.; Sato, K.; Matsumoto, M.; Abe, Y.; Tsuji, K.; et al. Proposal of COVID-19 Clinical Risk Score for the management of suspected COVID-19 cases: A case control study. BMC Infect. Dis. 2020, 20, 858. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.J.; Vestergaard, J.M.; Schlünssen, V.; Bonde, J.P.; Kaspersen, K.A.; Biering, K.; Carstensen, O.; Greve, T.; Hansen, K.K.; Dalbøge, A.; et al. Day by day symptoms following positive and negative PCR tests for SARS-CoV-2 in non-hospitalised health-care workers: A 90-day follow-up study. Int. J. Infect. Dis. 2021, 108, 382–390. [Google Scholar] [CrossRef]
- O’Reilly, G.M.; Mitchell, R.D.; Mitra, B.; Akhlaghi, H.; Tran, V.; Furyk, J.S.; Buntine, P.; Bannon-Murphy, H.; Amos, T.; Udaya Kumar, M.; et al. Epidemiology and clinical features of emergency department patients with suspected and confirmed COVID-19: A multisite report from the COVID-19 Emergency Department Quality Improvement Project for July 2020 (COVED-3). Ema Emerg. Med. Australas. 2021, 33, 114–124. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, G.; Jacob, S.; Barrett, P.M.; Gallagher, J. Covid-19 presentation among symptomatic healthcare workers in Ireland. Occup. Med. 2021, 71, 95–98. [Google Scholar] [CrossRef]
- Ozcan, E.; Yavuzer, S.; Borku Uysal, B.; Islamoglu, M.S.; Ikitimur, H.; Unal, O.F.; Akpinar, Y.E.; Seyhan, S.; Koc, S.; Yavuzer, H.; et al. The relationship between positivity for COVID-19 RT-PCR and symptoms, clinical findings, and mortality in Turkey. Expert Rev. Mol. Diagn. 2021, 21, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Selhorst, P.; Cochez, C.; Petrillo, M.; Claes, V.; Van der Beken, Y.; Verbeken, G.; Degueldre, J.; T’Sas, F.; Van den Eede, G.; et al. Study of a SARS-CoV-2 Outbreak in a Belgian Military Education and Training Center in Maradi, Niger. Viruses 2020, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Gurrola, J.G., 2nd; Loftus, P.A.; Cheung, S.W.; Chang, J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int. Forum Allergy Rhinol. 2020, 10, 832–838. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, D.; Park, J.Y.; Lee, S. Symptoms and Characteristics Which Require Attention During COVID-19 Screening at a Port of Entry. J. Korean Med. Sci. 2021, 36, e14. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Vogrin, S.; Smibert, O.C.; Marhoon, N.; Alexander, A.A.; Chua, K.Y.L.; James, F.L.; Jones, N.R.L.; Grigg, S.E.; Xu, C.L.H.; et al. COVID-MATCH65-A prospectively derived clinical decision rule for severe acute respiratory syndrome coronavirus 2. PLoS ONE 2020, 15, e0243414. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Chan, Y.F.Z.; Teo, N.W.Y.; Cherng, B.P.Z.; Thien, S.Y.; Wong, H.M.; Wijaya, L.; Toh, S.T.; Tan, T.T. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur. Arch. Otorhinolaryngol. 2020, 277, 2389–2390. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, R.K.; Nowalk, M.P.; Bear, T.; Taber, R.; Clarke, K.S.; Sax, T.M.; Eng, H.; Clarke, L.G.; Balasubramani, G.K. Proposed clinical indicators for efficient screening and testing for COVID-19 infection using Classification and Regression Trees (CART) analysis. Hum. Vaccin Immunother 2021, 17, 1109–1112. [Google Scholar] [CrossRef]
- King, J.A.; Whitten, T.A.; Bakal, J.A.; McAlister, F.A. Symptoms associated with a positive result for a swab for SARS-CoV-2 infection among children in Alberta. Cmaj 2021, 193, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Bidkar, V.; Mishra, M.; Selvaraj, K.; Joshi, P.; Shrikrishna, B.H.; Dabhekar, S.; Prathipati, K.K.; Rathod, B.S.; Shendre, P.; Gondode, P. Testing Olfactory and Gustatory Dysfunctions among Quarantine COVID-19 Suspects. Indian J. Otolaryngol. Head Neck Surg. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, S.; Finance, J.; Coulibaly, F.; Meddeb, L.; Tissot-Dupont, H.; Michel, M.; Lagier, J.C.; Million, M.; Radulesco, T.; Michel, J.; et al. Olfactory and gustative disorders for the diagnosis of COVID-19. Travel Med. Infect. Dis. 2020, 37, 101875. [Google Scholar] [CrossRef]
- Jeyashree, K.; Raju, M.; Ponnaiah, M.; Muthappan, S.; Rozario, A.G.A.; Raichel, R.; Jeris, W.L.; Gangakhedkar, R.R.; Murhekar, M.V. Self-reported and clinically identified loss of smell and taste among persons tested for COVID-19 in Chennai, southern India, July-August 2020: A cross sectional study. Clin. Epidemiol. Glob. Health 2021, 11, 100718. [Google Scholar] [CrossRef] [PubMed]
- Alizadehsani, R.; Alizadeh Sani, Z.; Behjati, M.; Roshanzamir, Z.; Hussain, S.; Abedini, N.; Hasanzadeh, F.; Khosravi, A.; Shoeibi, A.; Roshanzamir, M.; et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J. Med. Virol. 2021, 93, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Padilla, C.; Merchant, A.; Freshwater, K.; Weinsztok, S.; Clugston, J.R.; Fournier, K.; Edenfield, K.M. COVID-19 prevalence and presenting symptoms in a college student population: A retrospective chart review. J. Am. Coll Health 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chas, J.; Nadal, M.; Siguier, M.; Fajac, A.; Denis, M.; Morand-Joubert, L.; Pialoux, G. Broad-based SARS-CoV-2 testing program for healthcare workers in a primary care hospital in France. Infect. Dis. Now 2021, 51, 556–559. [Google Scholar] [CrossRef]
- Fistera, D.; Pabst, D.; Härtl, A.; Schaarschmidt, B.M.; Umutlu, L.; Dolff, S.; Holzner, C.; Kill, C.; Risse, J. Separating the wheat from the chaff-COVID-19 in a German emergency department: A case-control study. Int. J. Emerg. Med. 2020, 13, 44. [Google Scholar] [CrossRef]
- Ganz-Lord, F.A.; Segal, K.R.; Rinke, M.L. COVID-19 symptoms, duration, and prevalence among healthcare workers in the New York metropolitan area. Infect. Control. Hosp. Epidemiol. 2020, 1–7. [Google Scholar] [CrossRef]
- Moeller, A.L.; Mills, E.H.A.; Collatz Christensen, H.; Gnesin, F.; Blomberg, S.; Zylyftari, N.; Jensen, B.; Ringgren, K.B.; Broccia, M.D.; Bøggild, H.; et al. Symptom presentation of SARS-CoV-2-positive and negative patients: A nested case-control study among patients calling the emergency medical service and medical helpline. BMJ Open 2021, 11, e044208. [Google Scholar] [CrossRef]
- Raberahona, M.; Rakotomalala, R.; Rakotomijoro, E.; Rahaingoalidera, T.; Andry, C.E.; Mamilaza, N.; Razafindrabekoto, L.D.E.; Rafanomezantsoa, E.; Andriananja, V.; Andrianasolo, R.L.; et al. Clinical and epidemiological features discriminating confirmed COVID-19 patients from SARS-CoV-2 negative patients at screening centres in Madagascar. Int. J. Infect. Dis. 2021, 103, 6–8. [Google Scholar] [CrossRef]
- Chen, A.; Agarwal, A.; Ravindran, N.; To, C.; Zhang, T.; Thuluvath, P.J. Are Gastrointestinal Symptoms Specific for Coronavirus 2019 Infection? A Prospective Case-Control Study from the United States. Gastroenterology 2020, 159, 1161–1163.e2. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, N.A.; Reynolds, M.; DeFilippo Mack, C.; Brinkley, E.; Petruski-Ivleva, N.; Hawaldar, K.; Toovey, S.; Morris, J. Self-reported symptoms from exposure to Covid-19 provide support to clinical diagnosis, triage and prognosis: An exploratory analysis. Travel Med. Infect. Dis. 2020, 38, 101909. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Hussain, M.; O’Keeffe, D.T.; Simpkin, A.J. An analysis of patient self-reported COVID-19 symptoms during the first wave of the pandemic in Ireland. Ir. J. Med. Sci. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Karni, N.; Klein, H.; Asseo, K.; Benjamini, Y.; Israel, S.; Nammary, M.; Olshtain-Pops, K.; Nir-Paz, R.; Hershko, A.; Muszkat, M.; et al. Self-Rated Smell Ability Enables Highly Specific Predictors of COVID-19 Status: A Case-Control Study in Israel. Open Forum Infect. Dis. 2021, 8, ofaa589. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, M.F.; Fornazieri, M.A.; Bruni-Cardoso, A.; Avelino-Silva, V.I.; Schechtman, D.; Voegels, R.L.; Malnic, B.; Glezer, I.; de Rezende Pinna, F. Olfactory Dysfunction in Frontline Health Care Professionals During COVID-19 Pandemic in Brazil. Front. Physiol. 2021, 12, 622987. [Google Scholar] [CrossRef] [PubMed]
- Struyf, T.; Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Leeflang, M.M.; Spijker, R.; Hooft, L.; Emperador, D.; Domen, J.; et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst. Rev. 2021, 2, Cd013665. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.W.; Stybayeva, G.; Lim, S.Y.; Hwang, S.H. Predictive Value of Olfactory and Taste Symptoms in the Diagnosis of COVID-19: A Systematic Review and Meta-Analysis. Clin. Exp. Otorhinolaryngol. 2021, 14, 312–320. [Google Scholar] [CrossRef]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. Acs Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.; Tsukahara, T.; Weinreb, C. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, J.; Marshall, B.; Rekaya, R.; Ye, K.; Liu, H.X. SARS-CoV-2 Receptor ACE2 Is Enriched in a Subpopulation of Mouse Tongue Epithelial Cells in Nongustatory Papillae but Not in Taste Buds or Embryonic Oral Epithelium. ACS Pharm. Transl. Sci. 2020, 3, 749–758. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Brand, J.; Huang, L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. N. Y. Acad. Sci. 2009, 1170, 596–603. [Google Scholar] [CrossRef]
- Lozada-Nur, F.; Chainani-Wu, N.; Fortuna, G.; Sroussi, H. Dysgeusia in COVID-19: Possible Mechanisms and Implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 344. [Google Scholar] [CrossRef]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. Response to Commentary on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J. Med. Virol. 2020, 92, 707–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikrishnan, P. Gustatory Dysfunction as an Early Symptom in COVID-19 Screening. J. Craniofac. Surg. 2020, 31, e656–e658. [Google Scholar] [CrossRef] [PubMed]
- Singer-Cornelius, T.; Cornelius, J.; Oberle, M.; Metternich, F.U.; Brockmeier, S.J. Objective gustatory and olfactory dysfunction in COVID-19 patients: A prospective cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Illini, O.; Uy, D.; Renner, B.; Mueller, C.A. A new extension to the Taste Strips test. Rhinology 2016, 54, 45–50. [Google Scholar] [CrossRef]
- Luo, H.; Lie, Y.; Prinzen, F.W. Surveillance of COVID-19 in the General Population Using an Online Questionnaire: Report from 18,161 Respondents in China. JMIR Public Health Surveill. 2020, 6, e18576. [Google Scholar] [CrossRef]
- Gostic, K.; Gomez, A.C.; Mummah, R.O.; Kucharski, A.J.; Lloyd-Smith, J.O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. eLife 2020, 9, e55570. [Google Scholar] [CrossRef] [PubMed]
- Geldsetzer, P. Use of Rapid Online Surveys to Assess People’s Perceptions during Infectious Disease Outbreaks: A Cross-sectional Survey on COVID-19. J. Med. Internet Res. 2020, 22, e18790. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Nalleballe, K.; Reddy Onteddu, S.; Sharma, R.; Dandu, V.; Brown, A.; Jasti, M.; Yadala, S.; Veerapaneni, K.; Siddamreddy, S.; Avula, A.; et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020, 88, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mcgregor, G. Highly-Vaccinated, But More Cases Than Ever: Singapore Shows the World What ‘Endemic’ COVID Might Look Like. Available online: https://fortune.com/2021/09/28/singapore-covid-reopening-record-cases-vaccines/ (accessed on 24 October 2021).
- Chong, C. Home Recovery the Default Covid-19 Care Arrangement, Except for Certain Groups. Available online: https://www.straitstimes.com/singapore/home-recovery-the-default-covid-19-care-arrangement-for-everyone-from-sunday (accessed on 24 October 2021).
- Shafeeq, S. Buddy System, ART Tests: What Does Home Recovery Mean for Covid-19 Patients in Singapore? Available online: https://www.straitstimes.com/singapore/what-does-home-recovery-mean-for-covid-19-patients-in-singapore (accessed on 24 October 2021).
- Ministry of Health Singapore. Eligible for Home Recovery Programme. Available online: https://www.covid.gov.sg/unwell/hrp (accessed on 24 October 2021).
- Lechien, J.R.; Diallo, A.O.; Dachy, B.; Le Bon, S.D.; Maniaci, A.; Vaira, L.A.; Saussez, S. COVID-19: Post-vaccine Smell and Taste Disorders: Report of 6 Cases. Ear. Nose Throat J. 2021, 01455613211033125. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Corbellini, Á.; Chico-García, J.L.; Martínez-Poles, J.; Rodríguez-Jorge, F.; Natera-Villalba, E.; Gómez-Corral, J.; Gómez-López, A.; Monreal, E.; Parra-Díaz, P.; Cortés-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case-control study. Eur. J. Neurol. 2020, 27, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Carignan, A.; Valiquette, L.; Grenier, C.; Musonera, J.B.; Nkengurutse, D.; Marcil-Héguy, A.; Vettese, K.; Marcoux, D.; Valiquette, C.; Xiong, W.T.; et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: An age-matched case-control study. Cmaj 2020, 192, E702–E707. [Google Scholar] [CrossRef]
- Cho, R.H.W.; To, Z.W.H.; Yeung, Z.W.C.; Tso, E.Y.K.; Fung, K.S.C.; Chau, S.K.Y.; Leung, E.Y.L.; Hui, T.S.C.; Tsang, S.W.C.; Kung, K.N.; et al. COVID-19 Viral Load in the Severity of and Recovery From Olfactory and Gustatory Dysfunction. Laryngoscope 2020, 130, 2680–2685. [Google Scholar] [CrossRef]
- Dawson, P.; Rabold, E.M.; Laws, R.L.; Conners, E.E.; Gharpure, R.; Yin, S.; Buono, S.A.; Dasu, T.; Bhattacharyya, S.; Westergaard, R.P.; et al. Loss of Taste and Smell as Distinguishing Symptoms of Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 72, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.E.; Wools-Kaloustian, K.K.; Fadel, W.F.; Duszynski, T.J.; Yiannoutsos, C.; Halverson, P.K.; Menachemi, N. Symptoms and symptom clusters associated with SARS-CoV-2 infection in communitybased populations: Results from a statewide epidemiological study. PLoS ONE 2021, 16, e0241875. [Google Scholar] [CrossRef]
- Elimian, K.O.; Ochu, C.L.; Ebhodaghe, B.; Myles, P.; Crawford, E.E.; Igumbor, E.; Ukponu, W.; Olayinka, A.; Aruna, O.; Dan-Nwafor, C.; et al. Patient characteristics associated with COVID-19 positivity and fatality in Nigeria: Retrospective cohort study. BMJ Open 2020, 10, e044079. [Google Scholar] [CrossRef]
- Gurrola, J.G., 2nd; Chang, J.L.; Roland, L.T.; Loftus, P.A.; Cheung, S.W. Short-term chemosensory distortions and phantoms in COVID-19. Laryngoscope Investig. Otolaryngol. 2021, 6, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Ninchritz-Becerra, E.; Soriano-Reixach, M.; Poletti-Serafini, D.; Villarreal, I.M.; Maza-Solano, J.M.; Moreno-Luna, R.; et al. Smell and Taste Dysfunction in COVID-19 Is Associated with Younger Age in Ambulatory Settings: A Multicenter Cross-Sectional Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 346–357. [Google Scholar] [CrossRef]
- Kempker, R.R.; Kempker, J.A.; Peters, M.; Rebolledo, P.A.; Carroll, K.; Toomer, L.; Wang, Y.F.W.; Ray, S.M.; Hunter, M. Loss of Smell and Taste Among Healthcare Personnel Screened for Coronavirus 2019. Clin. Infect. Dis. 2021, 72, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- La Torre, G.; Massetti, A.P.; Antonelli, G.; Fimiani, C.; Fantini, M.; Marte, M.; Faticoni, A.; Previte, C.M.; Turriziani, O.; Pugliese, F.; et al. Anosmia and Ageusia as Predictive Signs of COVID-19 in Healthcare Workers in Italy: A Prospective Case-Control Study. J. Clin. Med. 2020, 9, 2870. [Google Scholar] [CrossRef] [PubMed]
- Leal, F.E.; Mendes-Correa, M.C.; Buss, L.F.; Costa, S.F.; Bizario, J.C.S.; de Souza, S.R.P.; Thomaz, O.; Tozetto-Mendoza, T.R.; Villas-Boas, L.S.; de Oliveira-da Silva, L.C.; et al. Clinical features and natural history of the first 2073 suspected COVID-19 cases in the Corona São Caetano primary care programme: A prospective cohort study. BMJ Open 2021, 11, e042745. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lockwood, J.; Das, P.; Wang, R.; Grinspun, E.; Lee, J.M. Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. Cjem 2020, 22, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanz, E.; Riestra, J.; Yebra, L.; Larran, A.; Mancino, F.; Yanes-Diaz, J.; Garrote, M.; Colmenero, M.; Montiel, E.; Molina, C.; et al. Prospective Study in 355 Patients with Suspected COVID-19 Infection: Value of Cough, Subjective Hyposmia, and Hypogeusia. Laryngoscope 2020, 130, 2674–2679. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Diaz-Lozano, M.; Alvarez-Zuñiga, C.; Ramirez-Hernandez, L.A.; Araujo-Espino, R.; Trejo-Ortiz, P.M.; Mollinedo-Montaño, F.E.; Ortiz-Castro, Y.; Vazquez-Reyes, S.; Velasco-Elizondo, P.; et al. Population-Based COVID-19 Screening in Mexico: Assessment of Symptoms and Their Weighting in Predicting SARS-CoV-2 Infection. Medicine 2021, 57, 363. [Google Scholar] [CrossRef] [PubMed]
- Moolla, M.S.; Parker, A.; Parker, M.A.; Sithole, S.; Amien, L.; Chiecktey, R.; Bawa, T.; Mowlana, A. Staff testing for COVID-19 via an online pre-registration form. S. Afr. J. Infect. Dis. 2021, 36, 1–5. [Google Scholar] [CrossRef]
- Nakanishi, H.; Suzuki, M.; Maeda, H.; Nakamura, Y.; Ikegami, Y.; Takenaka, Y.; Mori, Y.; Hasuo, T.; Hasegawa, C. Differential Diagnosis of COVID-19: Importance of Measuring Blood Lymphocytes, Serum Electrolytes, and Olfactory and Taste Functions. Tohoku J. Exp. Med. 2020, 252, 109–119. [Google Scholar] [CrossRef]
- Pérula de Torres, L.; González-Lama, J.; Jiménez García, C.; Sánchez Montero, R.; Rider Garrido, F.; Ortega López, Y.; Pajares Conde, D.; Ramírez Baena, M.; Párraga Martínez, I.; Romero-Rodríguez, E. Frequency and predictive validity of olfactory and taste dysfunction in patients with SARS-CoV-2 infection. Med. Clin. 2021, 156, 595–601. [Google Scholar] [CrossRef]
- Riestra-Ayora, J.; Yanes-Diaz, J.; Esteban-Sanchez, J.; Vaduva, C.; Molina-Quiros, C.; Larran-Jimenez, A.; Martin-Sanz, E. Long-term follow-up of olfactory and gustatory dysfunction in COVID-19: 6 months case-control study of health workers. Eur Arch. Otorhinolaryngol 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Lechuga, M.J.; Izquierdo-Domínguez, A.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Villarreal, I.M.; Cuesta-Chasco, G.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I. Chemosensory dysfunction in COVID-19 out-patients. Eur Arch. Otorhinolaryngol. 2021, 278, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sayin, İ.; Yaşar, K.K.; Yazici, Z.M. Taste and Smell Impairment in COVID-19: An AAO-HNS Anosmia Reporting Tool-Based Comparative Study. Otolaryngol. Head Neck Surg. 2020, 163, 473–479. [Google Scholar] [CrossRef]
- Sonoda, S.; Kuramochi, J.; Matsuyama, Y.; Miyazaki, Y.; Fujiwara, T. Validity of clinical symptoms score to discriminate patients with COVID-19 from common cold out-patients in general practitioner clinics in Japan. J. Clin. Med. 2021, 10, 854. [Google Scholar] [CrossRef]
- Trachootham, D.; Thongyen, S.; Lam-Ubol, A.; Chotechuang, N.; Pongpirul, W.; Prasithsirikul, W. Simultaneously complete but not partial taste and smell losses were associated with SARS-CoV-2 infection. Int. J. Infect. Dis. 2021, 106, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Trubiano, J.A.; Vogrin, S.; Kwong, J.C.; Homes, N. Alterations in Smell or Taste-Classic Coronavirus Disease 2019? Clin. Infect. Dis. 2020, 71, 2307–2309. [Google Scholar] [CrossRef] [PubMed]
- Tudrej, B.; Sebo, P.; Lourdaux, J.; Cuzin, C.; Floquet, M.; Haller, D.M.; Maisonneuve, H. Self-Reported Loss of Smell and Taste in SARS-CoV-2 Patients: Primary Care Data to Guide Future Early Detection Strategies. J. Gen. Intern. Med. 2020, 35, 2502–2504. [Google Scholar] [CrossRef] [PubMed]
- Villerabel, C.; Makinson, A.; Jaussent, A.; Picot, M.C.; Nègre-Pagès, L.; Rouvière, J.A.; Favier, V.; Crampette, L.; Morquin, D.; Reynes, J.; et al. Diagnostic Value of Patient-Reported and Clinically Tested Olfactory Dysfunction in a Population Screened for COVID-19. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 271–279. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayet, S.; Kadiane-Oussou, N.J.; Lepiller, Q.; Zahra, H.; Royer, P.Y.; Toko, L.; Gendrin, V.; Klopfenstein, T. Clinical features of COVID-19 and influenza: A comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020, 22, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zayet, S.; Klopfenstein, T.; Mercier, J.; Kadiane-Oussou, N.J.; Lan Cheong Wah, L.; Royer, P.Y.; Toko, L.; Gendrin, V. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2021, 49, 361–365. [Google Scholar] [CrossRef] [PubMed]


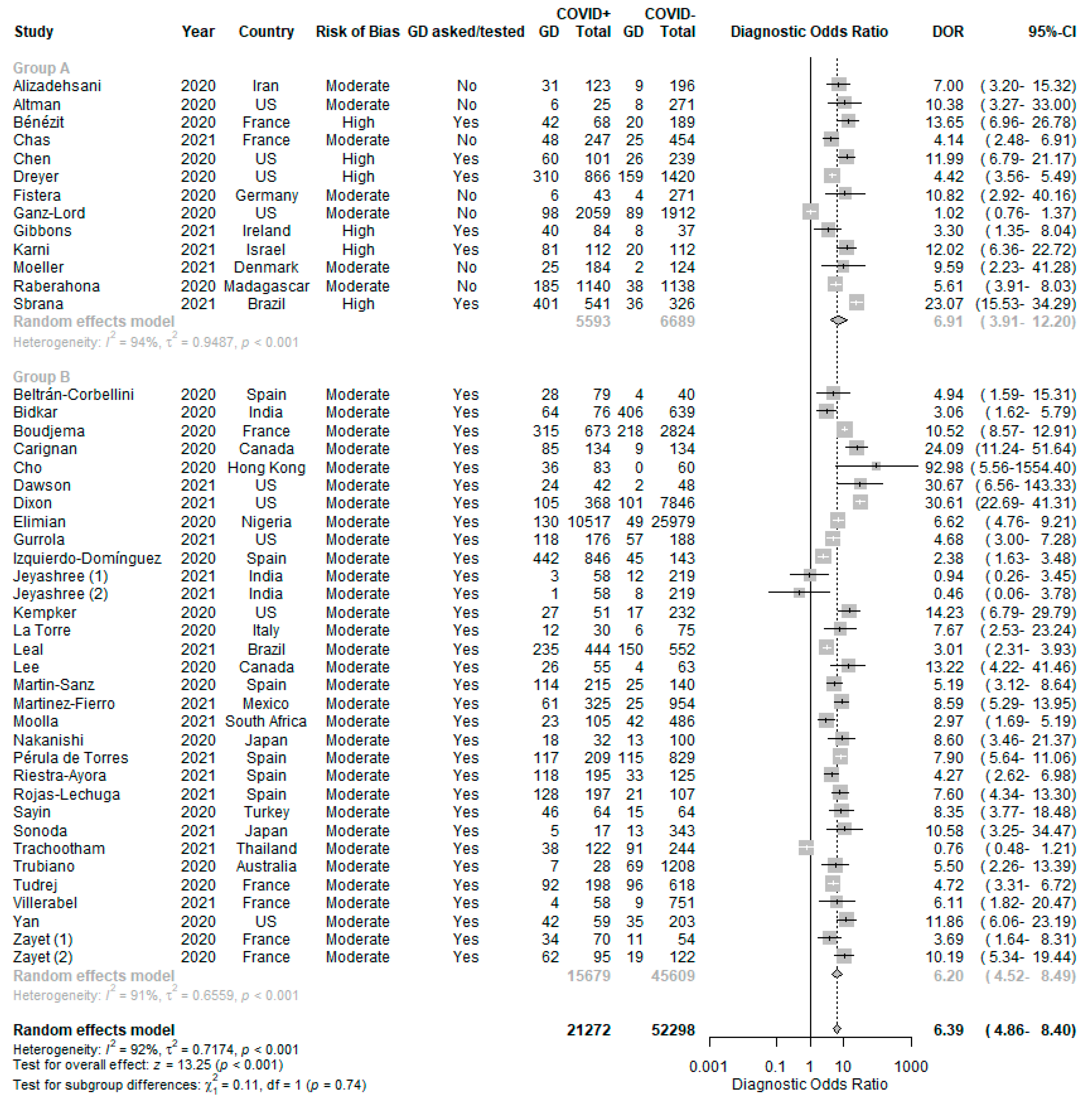




| DOR | Sensitivity | Specificity | Positive LR | Negative LR | |
|---|---|---|---|---|---|
| GD (with or without OD) | 6.39 (4.86–8.40) | 0.37 (0.29–0.47) | 0.92 (0.89–0.94) | 3.84 (3.04–4.84) | 0.67 (0.64–0.70) |
| OD (with or without GD) [49] | 11.5 (8.01–16.5) | 0.48 (0.40–0.56) | 0.93 (0.90–0.96) | 6.05 (4.52–8.11) | 0.60 (0.54–0.67) |
| GD and/or OD [155] | 10.20 (8.43–12.34) | 0.57 (0.47–0.66) | 0.91 (0.83–0.96) | Not reported | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.W.; Tham, S.-L.; Ng, L.S. Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review. Life 2021, 11, 1315. https://doi.org/10.3390/life11121315
Pang KW, Tham S-L, Ng LS. Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review. Life. 2021; 11(12):1315. https://doi.org/10.3390/life11121315
Chicago/Turabian StylePang, Khang Wen, Sher-Lyn Tham, and Li Shia Ng. 2021. "Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review" Life 11, no. 12: 1315. https://doi.org/10.3390/life11121315
APA StylePang, K. W., Tham, S.-L., & Ng, L. S. (2021). Exploring the Clinical Utility of Gustatory Dysfunction (GD) as a Triage Symptom Prior to Reverse Transcription Polymerase Chain Reaction (RT-PCR) in the Diagnosis of COVID-19: A Meta-Analysis and Systematic Review. Life, 11(12), 1315. https://doi.org/10.3390/life11121315






