Sex-Specific Social Effects on Depression-Related Behavioral Phenotypes in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Corticosterone Administration
2.3. Sucrose Preference Test (SPT)
2.4. Forced Swim Test (FST)
2.5. Statistical Analyses
3. Results
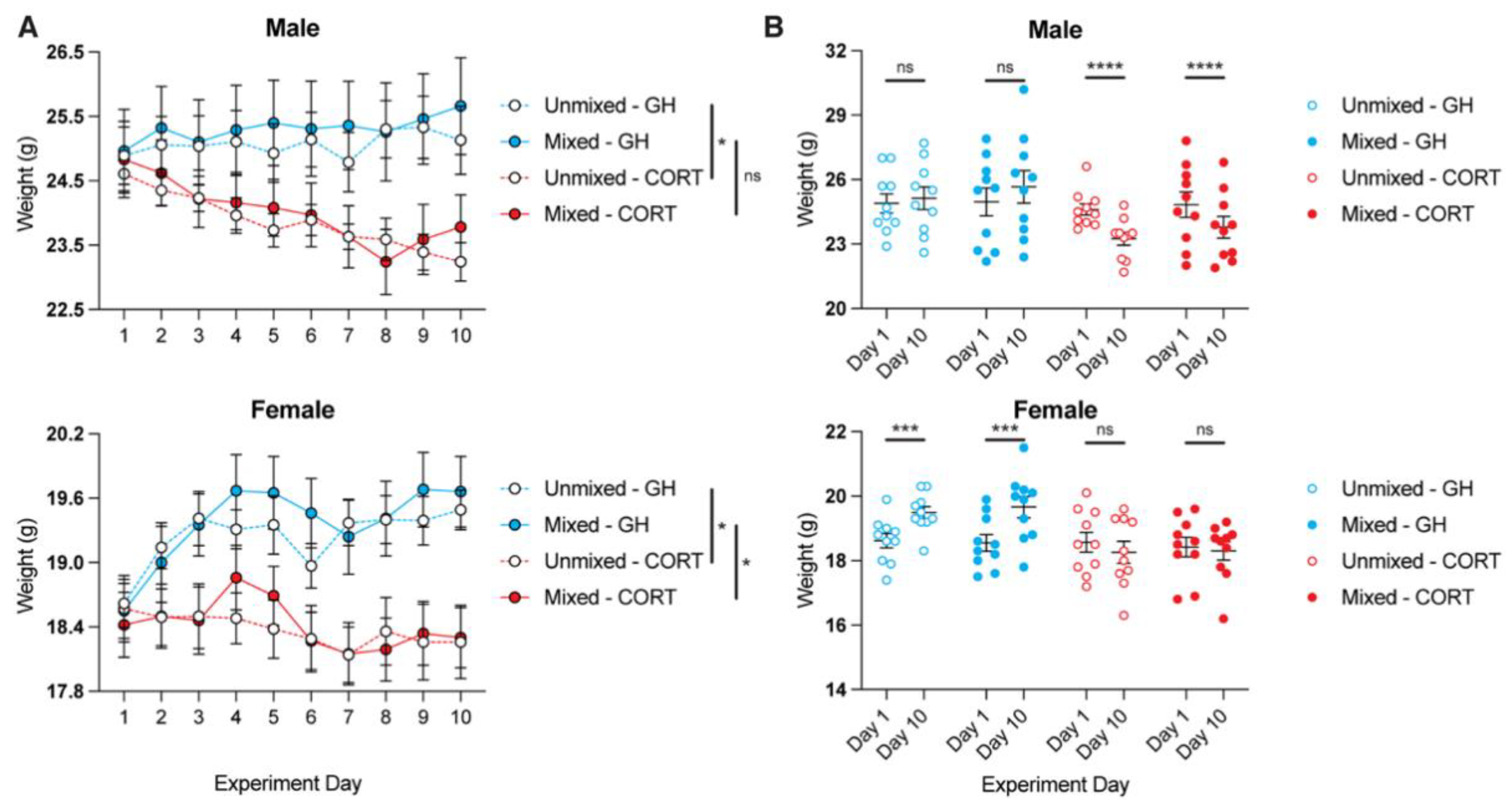
3.1. Chronic CORT Treatment Is Associated with Lower Body Weight
3.2. Mixed Treatment Group Housing Has No Impact on Sucrose Preference
3.3. Mixed Treatment Group Housing Produces Sex-Specific Effects in the Forced Swim Test
3.4. Ratio of CORT/GH Animals in Mixed Housing Does Not Impact FST or SPT Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Khan, R.A. Chronic stress leads to anxiety and depression. Ann. Psychiatry Ment. Health 2017, 5, 1091. [Google Scholar]
- Blackburn-Munro, G.; Blackburn-Munro, R.E. Chronic Pain, Chronic Stress and Depression: Coincidence or Consequence? J. Neuroendocr. 2001, 13, 1009–1023. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Zhang, Y.; Liu, Y.; Yang, L.; Pu, J.; Zhu, D.; Zhou, C.; Xie, P. The identification of metabolic disturbances in the prefrontal cortex of the chronic restraint stress rat model of depression. Behav. Brain Res. 2016, 305, 148–156. [Google Scholar] [CrossRef]
- Venzala, E.; García-García, A.L.; Elizalde, N.; Delagrange, P.; Tordera, R.M. Chronic social defeat stress model: Behavioral features, antidepressant action, and interaction with biological risk factors. Psychopharmacology 2012, 224, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Sterner, E.Y.; Kalynchuk, L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: Relevance to depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 777–790. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef]
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016, 774, 50–54. [Google Scholar] [CrossRef]
- Gourley, S.L.; Taylor, J.R. Recapitulation and Reversal of a Persistent Depression-like Syndrome in Rodents. Curr. Protoc. Neurosci. 2009, 49, 9.32.1–9.32.11. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef]
- Ma, L.; Shen, Q.; Yang, S.; Xie, X.; Xiao, Q.; Yu, C.; Cao, L.; Fu, Z. Effect of chronic corticosterone-induced depression on circadian rhythms and age-related phenotypes in mice. Acta Biochim. Biophys. Sin. 2018, 50, 1236–1246. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Li, Y.; Liu, X.; Liao, X.; Su, Y.-A.; Si, T. Differential Behavioral and Neurobiological Effects of Chronic Corticosterone Treatment in Adolescent and Adult Rats. Front. Mol. Neurosci. 2017, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Demuyser, T.; Deneyer, L.; Bentea, E.; Albertini, G.; Van Liefferinge, J.; Merckx, E.; De Prins, A.; De Bundel, D.; Massie, A.; Smolders, I. In-depth behavioral characterization of the corticosterone mouse model and the critical involvement of housing conditions. Physiol. Behav. 2016, 156, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Winokur, G.; Eisenstein, A.; Taylor, R.; Sly, M. The effect of group vs. individual housing on behaviour and physiological responses to stress in the albino rat. J. Psychosom. Res. 1960, 4, 185–190. [Google Scholar] [CrossRef]
- Trainor, B.C.; Workman, J.L.; Jessen, R.; Nelson, R.J. Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav. Neurosci. 2007, 121, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatsu-Coleman, A. Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: Mood-congruent memory in male mice? J. Psychiatry Neurosci. 2013, 38, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Berry, A.; Bellisario, V.; Capoccia, S.; Tirassa, P.; Calza, A.; Alleva, E.; Cirulli, F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 2012, 37, 762–772. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Palanza, P.; Sacerdote, P.; Ceresini, G.; Chirieleison, A.; Panerai, A.; Parmigiani, S. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 2003, 28, 540–558. [Google Scholar] [CrossRef]
- Bredy, T.W.; Barad, M. Social modulation of associative fear learning by pheromone communication. Learn. Mem. 2008, 16, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.L.; Hostetler, C.M.; Heinricher, M.M.; Ryabinin, A.E. Social transfer of pain in mice. Sci. Adv. 2016, 2, e1600855. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.L.; Asada, N.; Malenka, R.C. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 2021, 371, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bartal, I.B.-A.; Decety, J.; Mason, P. Empathy and Pro-Social Behavior in Rats. Science 2011, 334, 1427–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, S.L.; Hofford, R.S.; Evert, D.J.; Wellman, P.J.; Eitan, S. Social influences on morphine conditioned place preference in adolescent mice. Addict. Biol. 2013, 18, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, Y.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Partner’s Stress Status Influences Social Buffering Effects in Rats. Behav. Neurosci. 2004, 118, 798–804. [Google Scholar] [CrossRef]
- Yin, C.-Y.; Li, L.-D.; Xu, C.; Du, Z.-W.; Wu, J.-M.; Chen, X.; Xia, T.; Huang, S.-Y.; Meng, F.; Zhang, J.; et al. A novel method for automatic pharmacological evaluation of sucrose preference change in depression mice. Pharmacol. Res. 2021, 168, 105601. [Google Scholar] [CrossRef]
- He, L.-W.; Zeng, L.; Tian, N.; Li, Y.; He, T.; Tan, D.-M.; Zhang, Q.; Tan, Y. Optimization of food deprivation and sucrose preference test in SD rat model undergoing chronic unpredictable mild stress. Anim. Model. Exp. Med. 2020, 3, 69–78. [Google Scholar] [CrossRef]
- Singleton, J.M.; Garland, T. Influence of corticosterone on growth, home-cage activity, wheel running, and aerobic capacity in house mice selectively bred for high voluntary wheel-running behavior. Physiol. Behav. 2019, 198, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, E.L.; Vaessen, K.R.D.; Pawluski, J.; Sierksma, A.S.; Blokland, A.; Cañete, R.; Steinbusch, H.W.M. Long-Term Corticosterone Exposure Decreases Insulin Sensitivity and Induces Depressive-Like Behaviour in the C57BL/6NCrl Mouse. PLoS ONE 2014, 9, e106960. [Google Scholar] [CrossRef]
- Waters, P.; McCormick, C.M. Caveats of chronic exogenous corticosterone treatments in adolescent rats and effects on anxiety-like and depressive behavior and hypothalamic-pituitary-adrenal (HPA) axis function. Biol. Mood Anxiety Disord. 2011, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, S.; Gureczny, S.; Reisinger, S.N.; Horvath, O.; Pollak, D.D. Effect of Chronic Corticosterone Treatment on Depression-Like Behavior and Sociability in Female and Male C57BL/6N Mice. Cells 2019, 8, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, M.; Becker, A.; Schroeder, A.; Bilkei-Gorzo, A.; Zimmer, A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes, Brain Behav. 2015, 14, 292–300. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-X.; Yin, Y.-Y.; Xi, K.; Xing, Z.-K.; Cao, J.-B.; Liu, T.-Y.; Liu, L.; He, X.-X.; Yu, H.-L.; Zhu, X.-J. Nucleus Accumbens Tac1-Expressing Neurons Mediate Stress-Induced Anhedonia-like Behavior in Mice. Cell Rep. 2020, 33, 108343. [Google Scholar] [CrossRef] [PubMed]
- Warden, M.R.; Selimbeyoglu, A.; Mirzabekov, J.J.; Lo, M.; Thompson, K.R.; Kim, S.-Y.; Adhikari, A.; Tye, K.M.; Frank, L.; Deisseroth, K. A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nat. Cell Biol. 2012, 492, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.D.; Cameron, L.P.; Olson, D.E. Sex-Specific Social Effects on Depression-Related Behavioral Phenotypes in Mice. Life 2021, 11, 1327. https://doi.org/10.3390/life11121327
Patel SD, Cameron LP, Olson DE. Sex-Specific Social Effects on Depression-Related Behavioral Phenotypes in Mice. Life. 2021; 11(12):1327. https://doi.org/10.3390/life11121327
Chicago/Turabian StylePatel, Seona D., Lindsay P. Cameron, and David E. Olson. 2021. "Sex-Specific Social Effects on Depression-Related Behavioral Phenotypes in Mice" Life 11, no. 12: 1327. https://doi.org/10.3390/life11121327
APA StylePatel, S. D., Cameron, L. P., & Olson, D. E. (2021). Sex-Specific Social Effects on Depression-Related Behavioral Phenotypes in Mice. Life, 11(12), 1327. https://doi.org/10.3390/life11121327





