Credibility of the Neutrophil-to-Lymphocyte Count Ratio in Severe Traumatic Brain Injury
Abstract
:1. Introduction
2. Neutrophils in TBI
3. Lymphocytes in Brain Injury
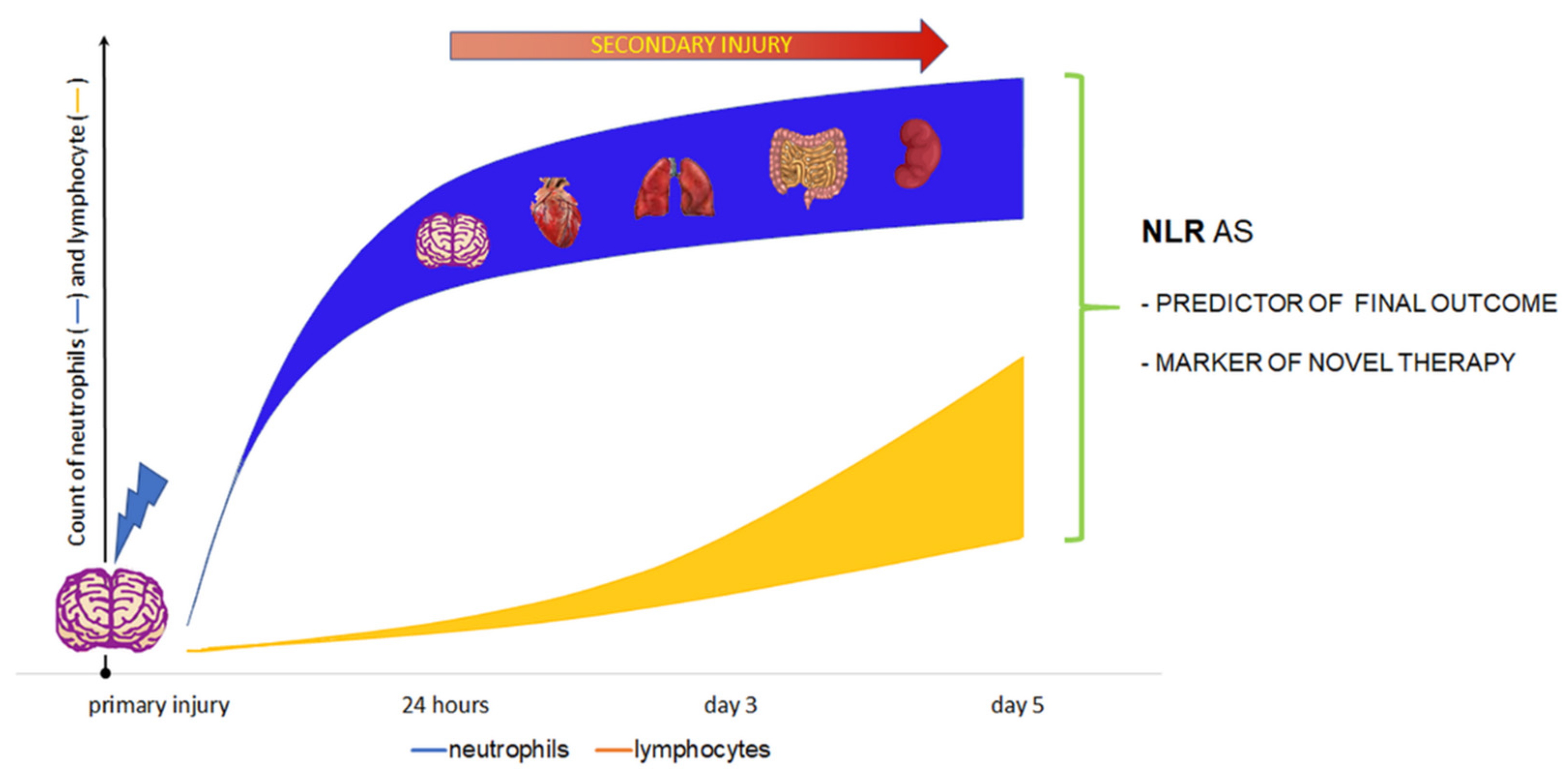
4. NLR in TBI
5. Prognostic Value of the NLR in TBI
5.1. NLR and Mortality
5.2. NLR and Clinical Outcome
5.3. NLR as Not Good Predictor of Mortality and Morbidity
6. Neutrophils as a Target of Future Therapies
7. NLR—A Potential Marker of Future Therapies in Chronic Neuroinflammation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Balu, R. Inflammation and Immune System Activation After Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2014, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; De Simoni, M.G.; Guilfoyle, M.R.; Carpenter, K.L.H.; Hutchinson, P.J. Cytokines and innate inflammation in the patho-genesis of human traumatic brain injury. Prog. Neurobiol. 2011, 95, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.; Davis, T. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Mrass, P.; Weninger, W. Immune cell migration as a means to control immune privilege: Lessons from the CNS and tumors. Immunol. Rev. 2006, 213, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Song, L.; Serwanski, D.R.; Kuziel, W.A.; Pachter, J.S. Transcellular transport of CCL2 across brain microvascular endothelial cells. J. Neurochem. 2008, 104, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Braber, I.D.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Adrover, J.M.; Ávila, J.; Ángel, N.; Hidalgo, A. Aging: A Temporal Dimension for Neutrophils. Trends Immunol. 2016, 37, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; Gonzalez, N.A.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.-E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nat. Cell Biol. 2015, 525, 528–532. [Google Scholar] [CrossRef]
- Hoenderdos, K.; Lodge, K.M.; Hirst, R.A.; Chen, C.; Palazzo, S.G.C.; Emerenciana, A.; Summers, C.; Angyal, A.; Porter, L.; Juss, J.K.; et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 2016, 71, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeldsen, L.; Cowland, J.B.; Johnsen, A.H.; Borregaard, N. SGP28, a novel matrix glycoprotein in specific granules of human neutrophils with similarity to a human testis-specific gene product and to a rodent sperm-coating glycoprotein. FEBS Lett. 1996, 380, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Klopf, J.; Brostjan, C.; Neumayer, C.; Eilenberg, W. Neutrophils as Regulators and Biomarkers of Cardiovascular Inflammation in the Context of Abdominal Aortic Aneurysms. Biomedicines 2021, 9, 1236. [Google Scholar] [CrossRef] [PubMed]
- Vaibhav, K.; Braun, M.; Alverson, K.; Khodadadi, H.; Kutiyanawalla, A.; Ward, A.; Banerjee, C.; Sparks, T.; Malik, A.; Rashid, M.H.; et al. Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury. Sci. Adv. 2020, 6, eaax8847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhu, Y.; Hou, X.; Chen, W.; Qu, X.; Zhang, Y.; Li, Z.; Wang, C.; Chen, J.; Lv, L.; et al. NETs Lead to Sympathetic Hyperactivity After Traumatic Brain Injury Through the LL37-Hippo/MST1 Pathway. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Fortin, C.F.; Ear, T.; McDonald, P.P. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J. 2009, 23, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Kuwar, R.; Rolfe, A.; Di, L.; Xu, H.; He, L.; Jiang, Y.; Zhang, S.; Sun, D. A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J. Neuroinflamm. 2019, 16, 81. [Google Scholar] [CrossRef]
- Battaglini, D.; Siwicka-Gieroba, D.; Rocco, P.R.; Cruz, F.F.; Silva, P.L.; Dabrowski, W.; Brunetti, I.; Patroniti, N.; Pelosi, P.; Robba, C. Novel Synthetic and Natural Therapies for Traumatic Brain Injury. Curr. Neuropharmacol. 2021, 19, 1661–1687. [Google Scholar] [CrossRef]
- Dabrowski, W.; Siwicka-Gieroba, D.; Kotfis, K.; Zaid, S.; Terpilowska, S.; Robba, C.; Siwicki, A.K. The Brain-gut Axis-where are we now and how can we Modulate these Connections? Curr. Neuropharmacol. 2021, 19, 1164–1177. [Google Scholar] [CrossRef]
- Ren, H.; Kong, Y.; Liu, Z.; Zang, D.; Yang, X.; Wood, K.; Li, M.; Liu, Q. Selective NLRP3 (Pyrin Domain–Containing Protein 3)Inflammasome Inhibitor Reduces Brain Injury After Intracerebral Hemorrhage. Stroke 2018, 49, 184–192. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, C.; Zhang, K.; Lan, X.; Chen, X.; Zang, W.; Wang, Z.; Guan, F.; Zhu, C.; Yang, X.; et al. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic. Biol. Med. 2019, 131, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Dai, Q.; Han, K.; Hong, W.; Jia, D.; Mo, Y.; Lv, Y.; Tang, H.; Fu, H.; Geng, W. JNK-IN-8, a c-Jun N-terminal kinase inhibitor, improves functional recovery through suppressing neuroinflammation in ischemic stroke. J. Cell. Physiol. 2020, 235, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, J.; Zinni, M.; Pansiot, J.; Hassan-Abdi, R.; Demene, C.; Colella, M.; Charriaut-Marlangue, C.; Novais, A.R.B.; Tanter, M.; Maccari, S.; et al. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 2018, 67, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-B.; Tu, X.-K.; Song, S.-W.; Liang, R.-S.; Shi, S.-S. Baicalin Reduces Early Brain Injury after Subarachnoid Hemorrhage in Rats. Chin. J. Integr. Med. 2020, 26, 510–518. [Google Scholar] [CrossRef]
- Nishihara, T.; Ochi, M.; Sugimoto, K.; Takahashi, H.; Yano, H.; Kumon, Y.; Ohnishi, T.; Tanaka, J. Subcutaneous injection containing IL-3 and GM-CSF ameliorates stab wound-induced brain injury in rats. Exp. Neurol. 2011, 229, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Chang, C.F.; Goods, B.A.; Hammond, M.D.; Mac Grory, B.; Ai, Y.; Steinschneider, A.F.; Renfroe, S.C.; Askenase, M.H.; McCullough, L.D.; et al. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Investig. 2017, 127, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kumar, A.; Choudhary, A.; Sharma, S.; Khurana, L.; Sharma, N.; Kumar, V.; Bisht, A. Neuroprotective Role of Oral Vitamin D Supplementation on Consciousness and Inflammatory Biomarkers in Determining Severity Outcome in Acute Traumatic Brain Injury Patients: A Double-Blind Randomized Clinical Trial. Clin. Drug Investig. 2020, 40, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Wu, Z.; Bhatti, U.F.; Biesterveld, B.E.; Kemp, M.T.; Wakam, G.K.; Vercruysse, C.A.; Chtraklin, K.; Siddiqui, A.Z.; Pickell, Z.; et al. Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2020, 89, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Szot, P.; Franklin, A.; Figlewicz, D.P.; Beuca, T.P.; Bullock, K.; Hansen, K.; Banks, W.A.; Raskind, M.A.; Peskind, E.R. Multiple lipopolysaccharide (LPS) injections alter interleukin 6 (IL-6), IL-7, IL-10 and IL-6 and IL-7 receptor mRNA in CNS and spleen. Neuroscience 2017, 355, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Shan, Y.; Wang, Y.; Lin, Y.; Liao, S.; Deng, Z.; Zhou, L.; Cai, W.; Zeng, Q.; Zhang, L.; et al. Exacerbation of oxygen–glucose deprivation-induced blood–brain barrier disruption: Potential pathogenic role of interleukin-9 in ischemic stroke. Clin. Sci. 2017, 131, 1499–1513. [Google Scholar] [CrossRef]
- Skundric, D.S.; Dai, R.; Zakarian, V.L.; Bessert, D.; Skoff, R.P.; Cruikshank, W.W.; Kurjakovic, Z. Anti-IL-16 therapy reduces CD4+ T-cell infiltration and improves paralysis and histopathology of relapsing EAE. J. Neurosci. Res. 2005, 79, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Poddubnyy, D.; Miossec, P. The IL-23–IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat. Rev. Rheumatol. 2019, 15, 747–757. [Google Scholar] [CrossRef]
- Bagheri, H.; Pourhanifeh, M.H.; Derakhshan, M.; Mahjoubin-Tehran, M.; Ghasemi, F.; Mousavi, S.; Rafiei, R.; Abbaszadeh-Goudarzi, K.; Mirzaei, H.R.; Mirzaei, H. CXCL-10: A new candidate for melanoma therapy? Cell. Oncol. 2020, 43, 353–365. [Google Scholar] [CrossRef]
- Shan, Y.; Tan, S.; Lin, Y.; Liao, S.; Zhang, B.; Chen, X.; Wang, J.; Deng, Z.; Zeng, Q.; Zhang, L.; et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J. Neuroinflamm. 2019, 16, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semple, B.D.; Trivedi, A.; Gimlin, K.; Noble-Haeusslein, L.J. Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain. Neurobiol. Dis. 2015, 74, 263–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenne, E.; Erlandsson, A.; Lindbom, L.; Hillered, L.; Clausen, F. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J. Neuroinflamm. 2012, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.X.; O’Barr, T.J.; Anderson, A.J. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and TNF-α. J. Neurochem. 2007, 102, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, J.M.; Dutka, A.J.; Tanishima, T.; Kochanek, P.M.; Kumaroo, K.K.; Thompson, C.B.; Obrenovitch, T.P.; Contreras, T.J. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke 1986, 17, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochanek, P.M.; Wallisch, J.S.; Bayır, H.; Clark, R.S.B. Pre-clinical models in pediatric traumatic brain injury—challenges and lessons learned. Child’s Nerv. Syst. 2017, 33, 1693–1701. [Google Scholar] [CrossRef]
- Furze, R.C.; Rankin, S.M. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008, 125, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Dohi, K.; Hansen, K.; Thompson, H.J. Assessing blood granulocyte colony-stimulating factor as a potential bi-omarker of acute traumatic brain injury in mice and humans. Brain Behav. Immun. 2016, 52, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-G.; Cheng, Y.; Yu, X.-C.; Ye, L.-B.; Xia, Q.-H.; Johnson, N.R.; Wei, X.; Chen, D.-Q.; Cao, G.; Fu, X.-B.; et al. bFGF Protects Against Blood-Brain Barrier Damage Through Junction Protein Regulation via PI3K-Akt-Rac1 Pathway Following Traumatic Brain Injury. Mol. Neurobiol. 2016, 53, 7298–7311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, C.A.; Campbell, E.J. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 1999, 65, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Downey, G.P. Leukocyte elastase: Physiological functions and role in acute lung injury. Am. J. Respir. Crit. Care Med. 2001, 164, 896–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, T.-S.; Ji, A.-L.; Ji, X.-Y.; Li, Y.-Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 9671604. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Keeling, K.L.; Hicks, R.R.; Mahesh, J.; Billings, B.B.; Kotwal, G.J. Local neutrophil influx following lateral fluid-percussion brain injury in rats is associated with accumulation of complement activation fragments of the third component (C3) of the com-plement system. J. Neuroimmunol. 2000, 105, 20–30. [Google Scholar] [CrossRef]
- Johnson, E.A.; Dao, T.L.; Guignet, M.; Geddes, C.E.; Koemeter-Cox, A.I.; Kan, R.K. Increased expression of the chemokines CXCL1 and MIP-1α by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J. Neuroinflamm. 2011, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csuka, E.; Morganti-Kossmann, M.C.; Lenzlinger, P.M.; Joller, H.; Trentz, O.; Kossmann, T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: Relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J. Neuroimmunol. 1999, 101, 211–221. [Google Scholar] [CrossRef]
- Yang, L.; Froio, R.M.; Sciuto, T.E.; Dvorak, A.M.; Alon, R.; Luscinskas, F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood 2005, 106, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Emerich, D.F.; Dean, R.L.; Bartus, R.T. The Role of Leukocytes Following Cerebral Ischemia: Pathogenic Variable or Bystander Reaction to Emerging Infarct? Exp. Neurol. 2002, 173, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, R.; Rowe, D.; Sethu, P.; Daugherty, A.; Yu, G.; Shin, H.Y. Shear-Sensitive Regulation of Neutrophil Flow Behavior and Its Potential Impact on Microvascular Blood Flow Dysregulation in Hypercholesterolemia. Arter. Thromb. Vasc. Biol. 2014, 34, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.J.; Brohi, K.; Ganter, M.T.; Manley, G.T.; Mackersie, R.C.; Pittet, J.-F. Early Coagulopathy After Traumatic Brain Injury: The Role of Hypoperfusion and the Protein C Pathway. J. Trauma Inj. Infect. Crit. Care 2007, 63, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Roberts, R.L.; Young, P.I. Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hy-poxic-ischemic brain injury. Pediatr Res. 2004, 55, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernandez-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-positive neutrophil infil-tration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Sun, G.; Zhang, H.; Ting, S.-M.; Song, S.; Gonzales, N.; Aronowski, J. Polymorphonuclear Neutrophil in Brain Parenchyma After Experimental Intracerebral Hemorrhage. Transl. Stroke Res. 2014, 5, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.S.B.; Kochanek, P.M.; Schwarz, M.A.; Schiding, J.K.; Turner, D.S.; Chen, M.; Carlos, T.M.; Watkins, S.C. Inducible Nitric Oxide Synthase Expression in Cerebrovascular Smooth Muscle and Neutrophils after Traumatic Brain Injury in Immature Rats. Pediatr. Res. 1996, 39, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlos, T.M.; Clark, R.S.B.; Franicola-Higgins, D.; Schiding, J.K.; Kochanek, P. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 1997, 61, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Steffen, B.J.; Breier, G.; Butcher, E.C.; Schulz, M.; Engelhardt, B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am. J. Pathol. 1996, 148, 1819–1838. [Google Scholar] [PubMed]
- Allport, J.R.; Ding, H.; Collins, T.; Gerritsen, M.E.; Luscinskas, F.W. Endothelial-dependent mechanisms regulate leukocyte transmigration: A process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J. Exp. Med. 1997, 186, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Grossetete, M.; Phelps, J.; Arko, L.; Yonas, H.; Rosenberg, G.A. Elevation of Matrix Metalloproteinases 3 And 9 In Cerebrospinal Fluid And Blood In Patients With Severe Traumatic Brain Injury. Neurosurgery 2009, 65, 702–708. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Matsumoto, Y.; Ikeda, Y.; Kondo, K.; Ohashi, N.; Umemura, K. SM-20220, a Na(+)/H(+) exchanger inhibitor: Effects on ischemic brain damage through edema and neutrophil accumulation in a rat middle cerebral artery occlusion model. Brain Res. 2002, 945, 242–248. [Google Scholar] [CrossRef]
- Shultz, S.R.; Bao, F.; Weaver, L.C.; Cain, D.P.; Brown, A. Treatment with an anti-CD11d integrin antibody reduces neuroin-flammation and improves outcome in a rat model of repeated concussion. J. Neuroinflamm. 2013, 10, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Sivakumar, G.; Goodden, J.R.; Tyagi, A.K.; Chumas, P.D. Prognostic value of leukocytosis in pediatric traumatic brain injury. J. Neurosurg. Pediatr. 2021, 27, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, R.G.; Hulsbergen, A.F.; Gormley, W.B.; Broekman, M.L. Routine Blood Tests for Severe Traumatic Brain Injury: Can They Predict Outcomes? World Neurosurg. 2020, 136, e60–e67. [Google Scholar] [CrossRef]
- Wolach, B.; Sazbon, L.; Gavrieli, R.; Broda, A.; Schlesinger, M. Early immunological defects in comatose patients after acute brain injury. J. Neurosurg. 2001, 94, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Petrone, A.B.; Gionis, V.; Giersch, R.; Barr, T.L. Immune biomarkers for the diagnosis of mild traumatic brain injury. NeuroRehabilitation 2017, 40, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Dreßler, J.; Hanisch, U.; Kuhlisch, E.; Geiger, K.D. Neuronal and glial apoptosis in human traumatic brain injury. Int. J. Leg. Med. 2007, 121, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Daglas, M.; Draxler, D.F.; Ho, H.; McCutcheon, F.; Galle, A.; Au, A.; Larsson, P.; Gregory, J.; Alderuccio, F.; Sashindranath, M.; et al. Activated CD8+ T Cells Cause Long-Term Neurological Impairment after Traumatic Brain Injury in Mice. Cell Rep. 2019, 29, 1178–1191.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fee, D.; Crumbaugh, A.; Jacques, T.; Herdrich, B.; Sewell, D.; Auerbach, D.; Piaskowski, S.; Hart, M.N.; Sandor, M.; Fabry, Z. Activated/effector CD4+ T cells exacerbate acute damage in the central nervous system following traumatic injury. J. Neuroimmunol. 2003, 136, 54–66. [Google Scholar] [CrossRef]
- Fukuzuka, K.; Edwards, C.K.; Clare-Salzler, M.; Copeland, E.M.; Moldawer, L.L.; Mozingo, D.W. Glucocorticoid-induced, caspa-se-dependent organ apoptosis early after burn injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1005–R1018. [Google Scholar] [CrossRef] [PubMed]
- Mencl, S.; Hennig, N.; Hopp, S.; Schuhmann, M.K.; Albert-Weissenberger, C.; Sirén, A.-L.; Kleinschnitz, C. FTY720 does not protect from traumatic brain injury in mice despite reducing posttraumatic inflammation. J. Neuroimmunol. 2014, 274, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillhouse, E.E.; Lesage, S. A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. J. Autoimmun. 2013, 40, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zhao, H.; Cao, X.; Hao, J.; Zhang, H.; Liu, Y.; Zhu, M.-S.; Fan, L.; Weng, L.; Qian, L.; et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 5558–5563. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Kostulas, N.; Pelidou, S.H.; Kivisäkk, P.; Kostulas, V.; Link, H. Increased IL-1β, IL-8, and IL-17 mRNA Expression in Blood Mononuclear Cells Observed in a Prospective Ischemic Stroke Study. Stroke 1999, 30, 2174–2179. [Google Scholar] [CrossRef] [Green Version]
- Kostulas, N.; Kivisäkk, P.; Huang, Y.; Matusevicius, D.; Kostulas, V.; Link, H. Ischemic Stroke Is Associated with a Systemic Increase of Blood Mononuclear Cells Expressing Interleukin-8 mRNA. Stroke 1998, 29, 462–466. [Google Scholar] [CrossRef] [Green Version]
- Brait, V.H.; Arumugam, T.; Drummond, G.; Sobey, C.G. Importance of T Lymphocytes in Brain Injury, Immunodeficiency, and Recovery after Cerebral Ischemia. Br. J. Pharmacol. 2012, 32, 598–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wo, J.; Zhang, F.; Li, Z.; Sun, C.; Zhang, W.; Sun, G. The Role of Gamma-Delta T Cells in Diseases of the Central Nervous System. Front. Immunol. 2020, 11, 580304. [Google Scholar] [CrossRef] [PubMed]
- Tobin, R.P.; Mukherjee, S.; Kain, J.M.; Rogers, S.K.; Henderson, S.K.; Motal, H.L.; Rogers, M.K.N.; Shapiro, L.A. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol. Commun. 2014, 2, 143. [Google Scholar] [CrossRef] [Green Version]
- Newell-Rogers, M.K.; Rogers, S.K.; Tobin, R.P.; Mukherjee, S.; Shapiro, L.A. Antagonism of Macrophage Migration Inhibitory Factory (MIF) after Traumatic Brain Injury Ameliorates Astrocytosis and Peripheral Lymphocyte Activation and Expansion. Int. J. Mol. Sci. 2020, 21, 7448. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Kaiser, A.; Lang, C.; Bohnert, M.; Betz, P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int. J. Leg. Med. 1999, 112, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic-Sutic, I.; Tokmadzic, V.S.; Laskarin, G.; Mahmutefendic, H.; Lucin, P.; Zupan, Z.; Sustic, A. Early changes in frequency of peripheral blood lymphocyte subpopulations in severe traumatic brain-injured patients. Scand. J. Immunol. 2010, 72, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Joachim, R.A.; Kuhlmei, A.; Dinh, Q.T.; Handjiski, B.; Fischer, T.; Peters, E.M.J.; Klapp, B.F.; Paus, R.; Arck, P.C. Neuronal plasticity of the “brain–skin connection”: Stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J. Mol. Med. 2007, 85, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain–Immune Interactions and Neuroinflammation After Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Juul, N.; Morris, G.F.; Marshall, S.B.; Marshall, L.F. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. J. Neurosurg. 2000, 92, 1–6. [Google Scholar] [CrossRef]
- Laird, A.M.; Miller, P.R.; Kilgo, P.D.; Meredith, J.W.; Chang, M.C. Relationship of early hyperglycemia to mortality in trauma pa-tients. J. Trauma Acute Care Surg. 2004, 56, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berghe, G.; Schoonheydt, K.; Becx, P.; Bruyninckx, F.; Wouters, P.J. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 2005, 64, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. A Practical Scale. Lancet 1975, 305, 480–484. [Google Scholar] [CrossRef]
- Chen, W.; Yang, J.; Li, B.; Peng, G.; Li, T.; Li, L.; Wang, S. Neutrophil to Lymphocyte Ratio as a Novel Predictor of Outcome in Patients with Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2018, 33, E53–E59. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Han, Z.-J.; Hu, Z. Red blood cell distribution width and neutrophil to lymphocyte ratio are associated with outcomes of adult subarachnoid haemorrhage patients admitted to intensive care unit. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Siwicka-Gieroba, D.; Malodobry, K.; Biernawska, J.; Robba, C.; Bohatyrewicz, R.; Rola, R.; Dabrowski, W. The Neutrophil/Lymphocyte Count Ratio Predicts Mortality in Severe Traumatic Brain Injury Patients. J. Clin. Med. 2019, 8, 1453. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-L.; Du, Z.-Y.; Yuan, Q.; Yu, J.; Sun, Y.-R.; Wu, X.; Li, Z.-Q.; Wu, X.-H.; Hu, J. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Predicting the 6-Month Outcome of Patients with Traumatic Brain Injury: A Retrospective Study. World Neurosurg. 2019, 124, e411–e416. [Google Scholar] [CrossRef] [PubMed]
- Kimball, R.; Shachar, E.; Eyerly-Webb, S.; Patel, D.M.; Spader, H. Using the neutrophil-to-lymphocyte ratio to predict outcomes in pediatric patients with traumatic brain injury. Clin. Neurol. Neurosurg. 2020, 193, 105772. [Google Scholar] [CrossRef] [PubMed]
- Giede-Jeppe, A.; Reichl, J.; Sprügel, M.I.; Lücking, H.; Hoelter, P.; Eyüpoglu, I.Y.; Gerner, S.T. Neutrophil-to-lymphocyte ratio as an in-dependent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2020, 132, 400–407. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Zhang, X.-T.; Wang, J.-Q.; Wang, C.-Y.; Zheng, W.-L.; Pan, Z.-M.; Xu, Z.-B.; Li, X.-Y.; Zhang, Y.-B. Admission Neutrophil–Lymphocyte Ratio Predicts Rebleeding Following Aneurismal Subarachnoid Hemorrhage. World Neurosurg. 2020, 138, e317–e322. [Google Scholar] [CrossRef]
- Ferro, D.; Matias, M.; Neto, J.; Dias, R.; Moreira, G.; Petersen, N.; Azevedo, E.; Castro, P. Neutrophil-to-Lymphocyte Ratio Predicts Cerebral Edema and Clinical Worsening Early After Reperfusion Therapy in Stroke. Stroke 2021, 52, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Świtońska, M.; Piekuś-Słomka, N.; Słomka, A.; Sokal, P.; Żekanowska, E.; Lattanzi, S. Neutrophil-to-Lymphocyte Ratio and Symptomatic Hemorrhagic Transformation in Ischemic Stroke Patients Undergoing Revascularization. Brain Sci. 2020, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Pikija, S.; Sztriha, L.K.; Killer-Oberpfalzer, M.; Weymayr, F.; Hecker, C.; Ramesmayer, C.; Hauer, L.; Sellner, J. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J. Neuroinflamm. 2018, 15, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, S.D.; Spears, C.; Cummings, C.; Vangilder, R.L.; Stinehart, K.R.; Gutmann, L.; Domico, J.; Culp, S.; Carpenter, J.; Rai, A.; et al. Admission neutrophil–lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J. NeuroInterventional Surg. 2014, 6, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.; Abdalla, R.N.; Batra, A.; Shaibani, A.; Hurley, M.C.; Jahromi, B.S.; Potts, M.B.; Ansari, S.A. Follow-up neutrophil-lymphocyte ratio after stroke thrombectomy is an independent biomarker of clinical outcome. J. NeuroInterventional Surg. 2021, 13, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, J.; Xiao, X.; Li, T.; Li, H.; Bai, X.; Li, Z.; Wang, W. Clinical predictors of prognosis in patients with traumatic brain injury combined with extracranial trauma. Int. J. Med. Sci. 2021, 18, 1639–1647. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Lianos, G.D.; Tzima, A.; Sotiropoulos, A.; Nasios, A.; Metaxas, D.; Voulgaris, S. Neutrophil to lymphocyte ratio as a pre-dictive biomarker for computed tomography scan use in mild traumatic brain injury. Biomark. Med. 2020, 14, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, K.; Gopalakrishna, K.N.; Chakrabarti, D.; Rao, G.U. Outcome Prediction of TBI: Are There Parameters That Affect the IMPACT and CRASH Models? World Neurosurg. 2021, 146, e590–e596. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.-M.; Ho, K.M.; Honeybul, S. Prognostic significance of abnormal hematological parameters in severe traumatic brain injury requiring decompressive craniectomy. J. Neurosurg. 2020, 132, 545–551. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Dumoga, S.; Koul, V.; Singh, N. Modulating neutrophil extracellular traps for wound healing. Biomater. Sci. 2020, 8, 3212–3223. [Google Scholar] [CrossRef]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; Berg, T.K.V.D. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Wipke, B.T.; Allen, P.M. Essential Role of Neutrophils in the Initiation and Progression of a Murine Model of Rheumatoid Arthritis. J. Immunol. 2001, 167, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgin, Y.M.; de Greef, G.E. Plerixafor for stem cell mobilization: The current status. Curr. Opin. Hematol. 2016, 23, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, J.; Martínez-Moreno, M.; Díaz-Martínez, M.; Sevilla-Movilla, S. The good and bad faces of the CXCR4 chemokine receptor. Int. J. Biochem. Cell Biol. 2018, 95, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.K.; Leong, D.; Edwards, K.M.; Rayzman, V.; Ng, M.; Goldberg, G.L.; Wilson, N.J.; Scalzo-Inguanti, K.; Mackenzie-Kludas, C.; Lawlor, K.E.; et al. Therapeutic targeting of the G-CSF receptor reduces neutrophil trafficking and joint inflammation in antibody-mediated inflammatory arthritis. J. Immunol. 2016, 197, 4392–4402. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Alexaki, V.I.; Kourtzelis, I.; Ziogas, A.; Hajishengallis, G.; Chavakis, T. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 2015, 147, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhu, Y.; Liu, Z.; Chang, L.; Bai, X.; Kang, L.; Cao, Y.; Yang, X.; Yu, H.; Shi, M.-J.; et al. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood 2021, 138, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Obregon, D.; Velisetty, R.; Sanberg, P.R.; Borlongan, C.V.; Tan, J. The immunology of traumatic brain injury: A prime target for Alzheimer’s disease prevention. J. Neuroinflamm. 2012, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Kara, S.P.; Altunan, B.; Unal, A. Investigation of the peripheral inflammation (neutrophil-lymphocyte ratio) in two neurodegenerative diseases of the central nervous system. Neurol. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yue, J.; Lei, P.; Lin, T.; Peng, X.; Xie, D.; Gao, L.; Shu, X.; Wu, C. Neutrophil-lymphocyte ratio as a predictor of delirium in older internal medicine patients: A prospective cohort study. BMC Geriatr. 2021, 21, 334. [Google Scholar] [CrossRef]
- Levochkina, M.; McQuillan, L.; Awan, N.; Barton, D.; Maczuzak, J.; Bianchine, C.; Trombley, S.; Kotes, E.; Wiener, J.; Wagner, A.; et al. Neutrophil-to-Lymphocyte Ratios and Infections after Traumatic Brain Injury: Associations with Hospital Resource Utilization and Long-Term Outcome. J. Clin. Med. 2021, 10, 4365. [Google Scholar] [CrossRef]
- Fluiter, K.; Opperhuizen, A.L.; Morgan, B.P.; Baas, F.; Ramaglia, V. Inhibition of the membrane attack complex of the com-plement system reduces secondary neuroaxonal loss and promotes neurologic recovery after traumatic brain injury in mice. J. Immunol. 2014, 192, 2339–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovesdi, E.; Kamnaksh, A.; Wingo, D.; Ahmed, F.; Grunberg, N.E.; Long, J.B.; Kasper, C.E.; Agoston, D.V. Acute Minocycline Treatment Mitigates the Symptoms of Mild Blast-Induced Traumatic Brain Injury. Front. Neurol. 2012, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Homsi, S.; Federico, F.; Croci, N.; Palmier, B.; Plotkine, M.; Marchand-Leroux, C.; Jafarian-Tehrani, M. Minocycline effects on cerebral edema: Relations with inflam-matory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 2009, 1291, 122–132. [Google Scholar] [CrossRef]
- Ali, T.; Hao, Q.; Ullah, N.; Rahman, S.U.; Shah, F.A.; He, K.; Zheng, C.; Li, W.; Murtaza, I.; Li, Y.; et al. Melatonin Act as an Antidepressant via Attenuation of Neuroinflammation by Targeting Sirt1/Nrf2/HO-1 Signaling. Front. Mol. Neurosci. 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, N.; Acosta, S.A.; Shahaduzzaman, M.; Ishikawa, H.; Shinozuka, K.; Pabon, M.; Hernandez-Ontiveros, D.; Kim, D.W.; Metcalf, C.; Staples, M.; et al. Intravenous Transplants of Human Adipose-Derived Stem Cell Protect the Brain from Traumatic Brain Injury-Induced Neurodegeneration and Motor and Cognitive Impairments: Cell Graft Biodistribution and Soluble Factors in Young and Aged Rats. J. Neurosci. 2014, 34, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Mahmood, A.; Lu, D.; Jiang, H.; Xiong, Y.; Zhou, D.; Chopp, M. Attenuation of astrogliosis and modulation of endothelial growth factor receptor in lipid rafts by simvastatin after traumatic brain injury. J. Neurosurg. 2010, 113, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiecien, J.M.; Dabrowski, W.; Kwiecien-Delaney, B.J.; Kwiecien-Delaney, C.J.; Siwicka-Gieroba, D.; Yaron, J.R.; Zhang, L.; Delaney, K.H.; Lucas, A.R. Neuroprotective Effect of Subdural Infusion of Serp-1 in Spinal Cord Trauma. Biomedicines 2020, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Hüner, E.A.; Dai, A.I.; Demiryürek, A.T. Association of Neutrophil/Lymphocyte Ratio with Intravenous Immunoglobulin Treatment in Children with Guillain-Barré Syndrome. J. Child. Neurol. 2018, 33, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Koiwa, M.; Goto, S.; Takahashi, K.; Kamada, T.; Takai, S.; Nakamura, H. Neutrophil/Lymphocyte Ratio in Patients with Rheumatoid Arthritis Treated with Biological Agents. J. Nippon. Med. Sch. 2016, 83, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Hirahara, T.; Arigami, T.; Yanagita, S.; Matsushita, D.; Uchikado, Y.; Kita, Y.; Mori, S.; Sasaki, K.; Omoto, I.; Kurahara, H.; et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer 2019, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Gialdini, G.; Kone, G.; Baradaran, H.; Merkler, A.E.; Mangat, H.S.; Navi, B.; Iadecola, C.; Gupta, A.; Kamel, H.; et al. Neutrophil-Lymphocyte Ratio and Perihematomal Edema Growth in Intracerebral Hemorrhage. Stroke 2017, 48, 2589–2592. [Google Scholar] [CrossRef] [PubMed]


| Name | Effects | Therapeutic METHODS | References |
|---|---|---|---|
| Interleukins | |||
| IL-1α | BBB breakdown; apoptosis angiogenesis | Recombinant human IL-1Ra, NLRP3 inhibitor, Mesenchymal stem/stromal cells therapy, ketamine | [18,19,20] |
| IL-1β | Apoptosis; secretion of IL-6 and IL-8 by astrocytes | Melatonin MT1/MT2 receptor agonist, NLRP3 inhibitor, JNK inhibitor, oxytocin, Baicalin, Xanthohumol, ketamine, Serp-1 | [18,19,20,21,22,23,24,25] |
| IL-3 | Inhibition of secondary degeneration | Interleukin-3 (IL-3) and granulocyte/macrophage colony-stimulating factor (GM-CSF) | [26] |
| IL-4 | Matter integrity promotion; long-term neurological recovery | Melatonin MT1/MT2 receptor agonist, Mesenchymal stem/stromal cells therapy | [20,22] |
| IL-6 | Nerve growth factor production | NLRP3 inhibitor, TGF-β1 infusion, metformin, melatonin, Vitamin D, JNK inhibitor, exosomes, lipopolysaccharide (LPS) injection, mesenchymal stem/stromal cells therapy | [18,19,20,21,22,23,27,28,29] |
| IL-7 | Induction of gliosis | Lipopolysaccharide (LPS) injection | [30] |
| IL-9 | excitotoxic damage; destruction of BBB | [31] | |
| IL-10 | Downregulation of pro-inflammatory cytokines | Melatonin MT1/MT2 receptor agonist, lipopolysaccharide (LPS) injection, mesenchymal stem/stromal cells therapy, statins, formononetin, Serp-1 | [19,20,22,26] |
| IL-16 | Lymphocytes and microglia activation; accumulation in cerebral vessels | anti-IL-16 antibody | [32] |
| IL-17 | Neutrophils encroachment | Monoclonal antibodies | [33] |
| IL-18 | Caspase-1 activation | Exosomes, NLRP3 inhibitor | [18,29] |
| IL-23 | Leads to neurologic deficits | Monoclonal antibodies | [33] |
| CHEMOKINES | CXCL immunotherapy, glucagon-like peptide-1 receptor (GLP-1R) agonist | [34,35] | |
| CXCL1 | Neutrophil circulation into the brain | ||
| CXCL3 | Migration of neutrophils across epithelial barriers | ||
| CXCL5 | Microglia activation; BBB damage; astrogliosis | ||
| CXCL8 | Neutrophil infiltration into brain parenchyma | ||
| CXCL9 | Inhibition of selected T cells | ||
| CXCL10 | Blood-derived monocytes promotion (to accumulate around perivascular vessels) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwicka-Gieroba, D.; Dabrowski, W. Credibility of the Neutrophil-to-Lymphocyte Count Ratio in Severe Traumatic Brain Injury. Life 2021, 11, 1352. https://doi.org/10.3390/life11121352
Siwicka-Gieroba D, Dabrowski W. Credibility of the Neutrophil-to-Lymphocyte Count Ratio in Severe Traumatic Brain Injury. Life. 2021; 11(12):1352. https://doi.org/10.3390/life11121352
Chicago/Turabian StyleSiwicka-Gieroba, Dorota, and Wojciech Dabrowski. 2021. "Credibility of the Neutrophil-to-Lymphocyte Count Ratio in Severe Traumatic Brain Injury" Life 11, no. 12: 1352. https://doi.org/10.3390/life11121352






