Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease
Abstract
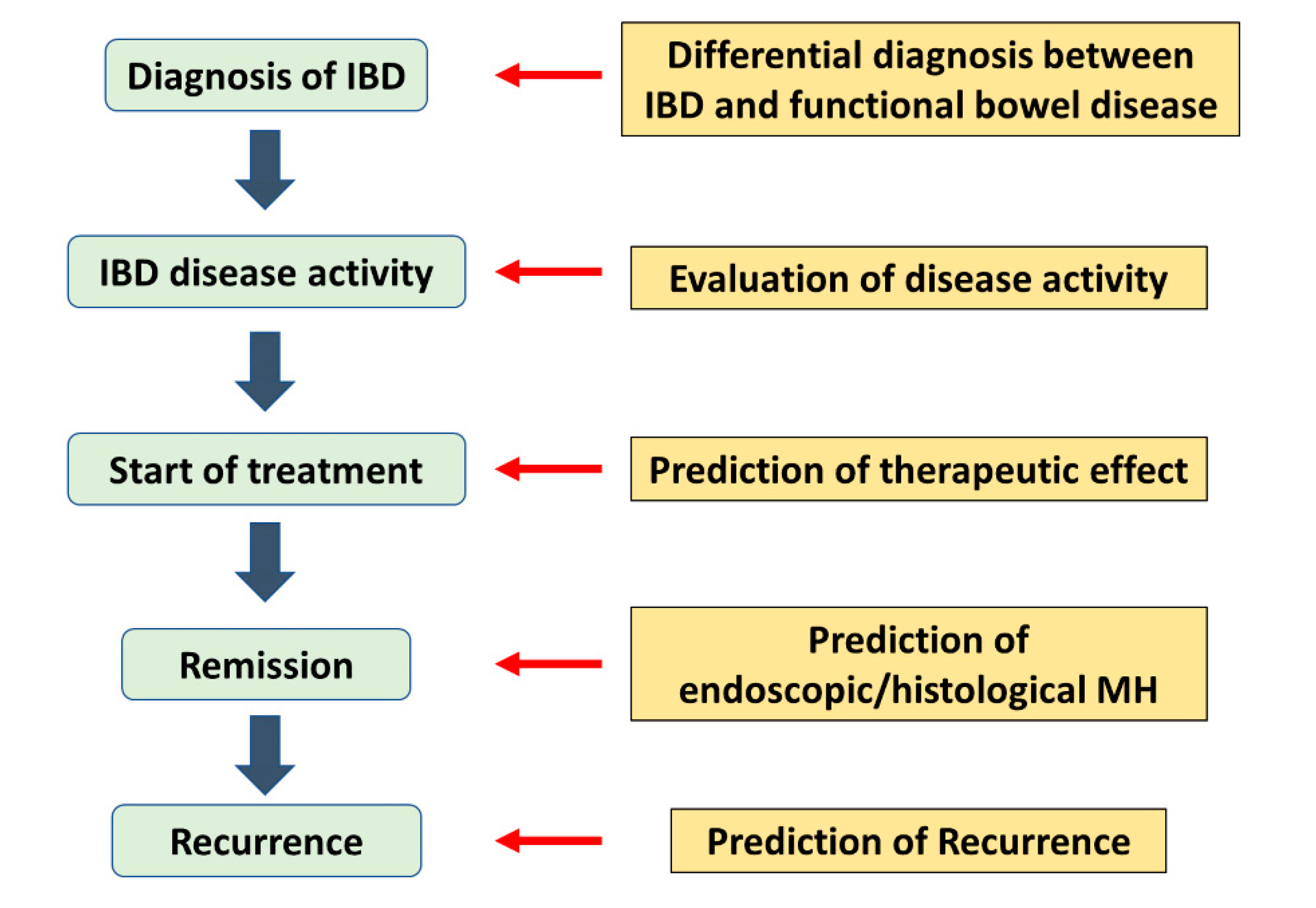
:1. Introduction
2. Treatment Goals for IBD
3. CRP
3.1. Evaluation of Disease Activity by CRP
3.2. Prediction of MH by CRP
3.3. Prediction of Therapeutic Effect by CRP
3.3.1. Prediction of Therapeutic Effect by CRP in CD
3.3.2. Prediction of Therapeutic Effect by CRP in UC
3.4. Prediction of Recurrence by CRP
4. FCP
4.1. Differential Diagnosis between IBD and Functional Bowel Disease
4.2. Evaluation of Disease Activity by FCP
4.2.1. Evaluation of Clinical Disease Activity by FCP
4.2.2. Prediction of Endoscopic MH by FCP
4.2.3. Prediction of Histological MH by FCP
4.3. Prediction of Therapeutic Response by FCP
4.4. Prediction of Recurrence by FCP
4.5. How to Use FCP and Precautions
5. FIT
5.1. Evaluation of Disease Activity by FIT
5.2. Prediction of Recurrence by FIT
5.3. How to Use FIT and Precautions
6. LRG
6.1. Evaluation of Disease Activity by LRG
6.2. Effects of Anti-TNF-α Agents on LRG Levels
7. PGE-MUM
7.1. Evaluation of Recurrence Predictability by PGE-MUM
7.2. Prediction of Recurrence by PGE-MUM
8. Combination of Biomarkers
8.1. Evaluation of Disease Activity by Combined Biomarkers
8.2. Prediction of Therapeutic Effect by Combined Biomarkers
9. Novel Biomarkers
10. Use of Biomarkers Properly
11. Limitation of Biomarkers
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Murakami, Y.; Nishiwaki, Y.; Oba, M.S.; Asakura, K.; Ohfuji, S.; Fukushima, W.; Suzuki, Y.; Nakamura, Y. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: An analysis of a nationwide survey. J. Gastroenterol. 2019, 54, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Colombel, J.F.; Sands, B.E.; Narula, N. Systematic review with meta-analysis: Mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Bouguen, G.; Levesque, B.G.; Pola, S.; Evans, E.; Sandborn, W.J. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2014, 12, 978–985. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Singh, S.; Jairath, V.; Sandborn, W.J.; Dulai, P.S. US Practice Patterns and Impact of Monitoring for Mucosal Inflammation After Biologic Initiation in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1828–1837. [Google Scholar] [CrossRef]
- Buisson, A.; Gonzalez, F.; Poullenot, F.; Nancey, S.; Sollellis, E.; Fumery, M.; Pariente, B.; Flamant, M.; Trang-Poisson, C.; Bonnaud, G.; et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Denters, M.J.; Schreuder, M.; Depla, A.C.; Mallant-Hent, R.C.; van Kouwen, M.C.; Deutekom, M.; Bossuyt, P.M.; Fockens, P.; Dekker, E. Patients’ perception of colonoscopy: Patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur. J. Gastroenterol. Hepatol. 2013, 25, 964–972. [Google Scholar] [CrossRef]
- Navaneethan, U.; Parasa, S.; Venkatesh, P.G.; Trikudanathan, G.; Shen, B. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J. Crohn’s Colitis 2011, 5, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Nakase, H.; Matsumoto, T.; Matsuura, M.; Iijima, H.; Matsuoka, K.; Ohmiya, N.; Ishihara, S.; Hirai, F.; Wagatsuma, K.; Yokoyama, Y.; et al. Expert Opinions on the Current Therapeutic Management of Inflammatory Bowel Disease during the COVID-19 Pandemic: Japan IBD COVID-19 Taskforce, Intractable Diseases, the Health and Labor Sciences Research. Digestion 2021, 102, 814–822. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef]
- Dulai, P.S.; Peyrin-Biroulet, L.; Danese, S.; Sands, B.E.; Dignass, A.; Turner, D.; Mantzaris, G.; Schölmerich, J.; Mary, J.Y.; Reinisch, W.; et al. Approaches to Integrating Biomarkers Into Clinical Trials and Care Pathways as Targets for the Treatment of Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1032–1043.e1. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum Biomarkers for Inflammatory Bowel Disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [Green Version]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285.e2. [Google Scholar] [CrossRef]
- Walsh, A.; Kormilitzin, A.; Hinds, C.; Sexton, V.; Brain, O.; Keshav, S.; Uhlig, H.; Geddes, J.; Goodwin, G.; Peters, M.; et al. Defining Faecal Calprotectin Thresholds as a Surrogate for Endoscopic and Histological Disease Activity in Ulcerative Colitis-a Prospective Analysis. J. Crohn’s Colitis 2019, 13, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, F.; Matsuoka, K.; Motobayashi, M.; Takenaka, K.; Kuno, T.; Tanaka, K.; Tsukui, Y.; Kobayashi, S.; Yoshida, T.; Fujii, T.; et al. Prediction of disease activity of Crohn’s disease through fecal calprotectin evaluated by balloon-assisted endoscopy. J. Gastroenterol. Hepatol. 2018, 33, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Ishihara, S.; Yuki, T.; Fukuba, N.; Sonoyama, H.; Kazumori, H.; Yamashita, N.; Tada, Y.; Kusunoki, R.; Oka, A.; et al. Fecal Calprotectin More Accurately Predicts Endoscopic Remission of Crohn’s Disease than Serological Biomarkers Evaluated Using Balloon-assisted Enteroscopy. Inflamm. Bowel Dis. 2017, 23, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Takeuchi, K.; Miyamura, M.; Ishikawa, R.; Yamada, A.; Katsumata, M.; Igarashi, Y.; Suzuki, Y. Level of Fecal Calprotectin Correlates With Severity of Small Bowel Crohn’s Disease, Measured by Balloon-assisted Enteroscopy and Computed Tomography Enterography. Clin. Gastroenterol. Hepatol. 2017, 15, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819; quiz 820. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Niwa, Y.; Goto, H.; Hirooka, Y.; Hayakawa, T.; Ohmiya, N.; Kobayashi, S. Asymptomatic inflammatory bowel disease with special reference to ulcerative colitis in apparently healthy persons. Am. J. Gastroenterol. 2001, 96, 735–739. [Google Scholar] [CrossRef]
- Serada, S.; Fujimoto, M.; Ogata, A.; Terabe, F.; Hirano, T.; Iijima, H.; Shinzaki, S.; Nishikawa, T.; Ohkawara, T.; Iwahori, K.; et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann. Rheum. Dis. 2010, 69, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Arihiro, S.; Matsuura, T.; Kato, T.; Matsuoka, M.; Saruta, M.; Mitsunaga, M.; Matsuura, M.; Fujiwara, M.; Okayasu, I.; et al. Prostaglandin E-major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.D.; Westengard, J.C.; Hay, K.L.; Bull, B.S. Calibration and validation for erythrocyte sedimentation tests. Role of the International Committee on Standardization in Hematology reference procedure. Arch. Pathol. Lab. Med. 1993, 117, 719–723. [Google Scholar] [PubMed]
- Zezos, P.; Papaioannou, G.; Nikolaidis, N.; Patsiaoura, K.; Vassiliadis, T.; Mpoumponaris, A.; Giouleme, O.; Evgenidis, N. Elevated markers of thrombin generation and fibrinolysis in patients with active and quiescent ulcerative colitis. Med. Sci. Monit. 2009, 15, CR563–CR572. [Google Scholar]
- Xu, M.; Cen, M.; Chen, X.; Chen, H.; Liu, X.; Cao, Q. Correlation between Serological Biomarkers and Disease Activity in Patients with Inflammatory Bowel Disease. Biomed. Res. Int. 2019, 2019, 6517549. [Google Scholar] [CrossRef] [Green Version]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef] [Green Version]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenillot, O.; Henny, J.; Steinmetz, J.; Herbeth, B.; Wagner, C.; Siest, G. High sensitivity C-reactive protein: Biological variations and reference limits. Clin. Chem. Lab. Med. 2000, 38, 1003–1011. [Google Scholar] [CrossRef]
- Shine, B.; Berghouse, L.; Jones, J.E.; Landon, J. C-reactive protein as an aid in the differentiation of functional and inflammatory bowel disorders. Clin. Chim. Acta 1985, 148, 105–109. [Google Scholar] [CrossRef]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Suk Danik, J.; Chasman, D.I.; Cannon, C.P.; Miller, D.T.; Zee, R.Y.; Kozlowski, P.; Kwiatkowski, D.J.; Ridker, P.M. Influence of genetic variation in the C-reactive protein gene on the inflammatory response during and after acute coronary ischemia. Ann. Hum. Genet. 2006, 70, 705–716. [Google Scholar] [CrossRef]
- Henderson, P.; Kennedy, N.A.; Van Limbergen, J.E.; Cameron, F.L.; Satsangi, J.; Russell, R.K.; Wilson, D.C. Serum C-reactive protein and CRP genotype in pediatric inflammatory bowel disease: Influence on phenotype, natural history, and response to therapy. Inflamm. Bowel Dis. 2015, 21, 596–605. [Google Scholar] [CrossRef]
- Benitez, J.M.; Meuwis, M.A.; Reenaers, C.; Van Kemseke, C.; Meunier, P.; Louis, E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut 2013, 62, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.A.; Reenaers, C.; Fontaine, F.; Belaïche, J.; Louis, E. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn’s disease with normal C-reactive protein serum level. Inflamm. Bowel Dis. 2007, 13, 1100–1105. [Google Scholar] [CrossRef]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B.; IBSEN Study Group. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Park, S.J.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig. Dis. Sci. 2014, 59, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, H.; Kawashima, K.; Ishihara, S.; Kotani, S.; Fukuba, N.; Oka, A.; Kusunoki, R.; Tada, Y.; Mishima, Y.; Oshima, N.; et al. Capabilities of fecal calprotectin and blood biomarkers as surrogate endoscopic markers according to ulcerative colitis disease type. J. Clin. Biochem. Nutr. 2019, 64, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, N.; Higuchi, T.; Miyazu, T.; Tamura, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Osawa, S.; Furuta, T.; et al. C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Sci. Rep. 2021, 11, 12431. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Vermeire, S.; Rutgeerts, P.; De Vos, M.; Van Gossum, A.; Pescatore, P.; Fiasse, R.; Pelckmans, P.; Reynaert, H.; D’Haens, G.; et al. A positive response to infliximab in Crohn disease: Association with a higher systemic inflammation before treatment but not with -308 TNF gene polymorphism. Scand. J. Gastroenterol. 2002, 37, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Wang, Y.; Oddens, B.J.; Link, R. C-reactive protein; an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: A post-hoc analysis from ACCENT I. Aliment. Pharmacol. Ther. 2012, 35, 568–576. [Google Scholar] [CrossRef]
- Magro, F.; Rodrigues-Pinto, E.; Santos-Antunes, J.; Vilas-Boas, F.; Lopes, S.; Nunes, A.; Camila-Dias, C.; Macedo, G. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J. Crohn’s Colitis 2014, 8, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kamata, N.; Yamada, A.; Endo, K.; Fujii, T.; Yoshino, T.; Sugaya, T.; Yokoyama, Y.; Bamba, S.; Umeno, J.; et al. Long-term retention of adalimumab treatment and associated prognostic factors for 1189 patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2018, 33, 1031–1038. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Iwasa, R.; Yamada, A.; Sono, K.; Furukawa, R.; Takeuchi, K.; Suzuki, Y. C-reactive protein level at 2 weeks following initiation of infliximab induction therapy predicts outcomes in patients with ulcerative colitis: A 3 year follow-up study. BMC Gastroenterol. 2015, 15, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.C.; Bressler, B. Acute Severe Ulcerative Colitis: The Oxford Criteria No Longer Predict In-Hospital Colectomy Rates. Dig. Dis. Sci. 2020, 65, 576–580. [Google Scholar] [CrossRef]
- Consigny, Y.; Modigliani, R.; Colombel, J.F.; Dupas, J.L.; Lémann, M.; Mary, J.Y.; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID). A simple biological score for predicting low risk of short-term relapse in Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 551–557. [Google Scholar] [CrossRef]
- Bitton, A.; Dobkin, P.L.; Edwardes, M.D.; Sewitch, M.J.; Meddings, J.B.; Rawal, S.; Cohen, A.; Vermeire, S.; Dufresne, L.; Franchimont, D.; et al. Predicting relapse in Crohn’s disease: A biopsychosocial model. Gut 2008, 57, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Marotte, H.; Leclerc, M.; Del Tedesco, E.; Phelip, J.M.; Peyrin-Biroulet, L.; Paul, S. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J. Crohn’s Colitis 2015, 9, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Regueiro, M.; Kip, K.E.; Schraut, W.; Baidoo, L.; Sepulveda, A.R.; Pesci, M.; El-Hachem, S.; Harrison, J.; Binion, D. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm. Bowel Dis. 2011, 17, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, G.; Laidet, M.; Moussata, D.; Stefanescu, C.; Roblin, X.; Phelip, G.; Cotte, E.; Passot, G.; Francois, Y.; Drai, J.; et al. Levels of Fecal Calprotectin Are Associated With the Severity of Postoperative Endoscopic Recurrence in Asymptomatic Patients With Crohn’s Disease. Am. J. Gastroenterol. 2015, 110, 865–872. [Google Scholar] [CrossRef]
- Nakashige, T.G.; Zhang, B.; Krebs, C.; Nolan, E.M. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015, 11, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Ayling, R.M.; Kok, K. Fecal Calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. [Google Scholar] [CrossRef]
- Røseth, A.G.; Schmidt, P.N.; Fagerhol, M.K. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1999, 34, 50–54. [Google Scholar] [CrossRef]
- Naess-Andresen, C.F.; Egelandsdal, B.; Fagerhol, M.K. Calcium binding and concomitant changes in the structure and heat stability of calprotectin (L1 protein). Clin. Mol. Pathol. 1995, 48, M278–M284. [Google Scholar] [CrossRef] [Green Version]
- Røseth, A.G.; Aadland, E.; Jahnsen, J.; Raknerud, N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion 1997, 58, 176–180. [Google Scholar] [CrossRef]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef]
- Meucci, G.; D’Incà, R.; Maieron, R.; Orzes, N.; Vecchi, M.; Visentini, D.; Minoli, G.; Dal Pont, E.; Zilli, M.; Benedetti, E.; et al. Diagnostic value of faecal calprotectin in unselected outpatients referred for colonoscopy: A multicenter prospective study. Dig. Liver Dis. 2010, 42, 191–195. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010, 341, c3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Lumb, R.; Walker, E.V.; Foshaug, R.R.; Dang, T.T.; Verma, S.; Huang, V.W.; Kroeker, K.I.; Wong, K.; Dieleman, L.A.; et al. Noninvasive Fecal Immunochemical Testing and Fecal Calprotectin Predict Mucosal Healing in Inflammatory Bowel Disease: A Prospective Cohort Study. Inflamm. Bowel Dis. 2017, 23, 1643–1649. [Google Scholar] [CrossRef]
- Kammerlander, H.; Nielsen, J.; Kjeldsen, J.; Knudsen, T.; Gradel, K.O.; Friedman, S.; Nørgård, B.M. Fecal Calprotectin During Pregnancy in Women With Moderate-Severe Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Chen, J.M.; Zuo, J.H.; Yu, A.; Xiao, Z.J.; Deng, F.H.; Nie, B.; Jiang, B. Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm. Bowel Dis. 2014, 20, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Safroneeva, E.; Romero, Y.; Armstrong, D.; Schmidt, C.; Trummler, M.; Pittet, V.; Vavricka, S.R. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm. Bowel Dis. 2013, 19, 332–341. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Trummler, M.; Vavricka, S.R.; Bruegger, L.E.; Seibold, F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. 2010, 105, 162–169. [Google Scholar] [CrossRef] [Green Version]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, V.; Røseth, A.; Ahmad, T.; Skar, V.; Moum, B. Fecal Calprotectin: A Reliable Predictor of Mucosal Healing after Treatment for Active Ulcerative Colitis. Gastroenterol. Res. Pract. 2017, 2017, 2098293. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, K.; Ishihara, S.; Yuki, T.; Fukuba, N.; Oshima, N.; Kazumori, H.; Sonoyama, H.; Yamashita, N.; Tada, Y.; Kusunoki, R.; et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016, 16, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- State, M.; Negreanu, L.; Voiosu, T.; Voiosu, A.; Balanescu, P.; Mateescu, R.B. Surrogate markers of mucosal healing in inflammatory bowel disease: A systematic review. World J. Gastroenterol. 2021, 27, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Burger, D.C.; Delo, J.; Walsh, A.J.; Thomas, S.; von Herbay, A.; Buchel, O.C.; White, L.; Brain, O.; Keshav, S.; et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016, 65, 408–414. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Stevens, T.W.; Gecse, K.; Turner, J.R.; de Hertogh, G.; Rubin, D.T.; D’Haens, G.R. Diagnostic Accuracy of Fecal Calprotectin Concentration in Evaluating Therapeutic Outcomes of Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2333–2342. [Google Scholar] [CrossRef]
- Hart, L.; Chavannes, M.; Kherad, O.; Maedler, C.; Mourad, N.; Marcus, V.; Afif, W.; Bitton, A.; Lakatos, P.L.; Brassard, P.; et al. Faecal Calprotectin Predicts Endoscopic and Histological Activity in Clinically Quiescent Ulcerative Colitis. J. Crohn’s Colitis 2020, 14, 46–52. [Google Scholar] [CrossRef]
- Magro, F.; Lopes, J.; Borralho, P.; Lopes, S.; Coelho, R.; Cotter, J.; Castro, F.D.; Sousa, H.T.; Salgado, M.; Andrade, P.; et al. Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut 2019, 68, 594–603. [Google Scholar] [CrossRef]
- D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Review article: Faecal calprotectin and histologic remission in ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterman, M.T.; Aberra, F.N.; Cross, R.; Liakos, S.; McCabe, R.; Shafran, I.; Wolf, D.; Hardi, R.; Nessel, L.; Brensinger, C.; et al. Mesalamine dose escalation reduces fecal calprotectin in patients with quiescent ulcerative colitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1887–1893.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vos, M.; Dewit, O.; D’Haens, G.; Baert, F.; Fontaine, F.; Vermeire, S.; Franchimont, D.; Moreels, T.; Staessen, D.; Terriere, L.; et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J. Crohn’s Colitis 2012, 6, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Bertani, L.; Blandizzi, C.; Mumolo, M.G.; Ceccarelli, L.; Albano, E.; Tapete, G.; Baiano Svizzero, G.; Zanzi, F.; Coppini, F.; de Bortoli, N.; et al. Fecal Calprotectin Predicts Mucosal Healing in Patients With Ulcerative Colitis Treated With Biological Therapies: A Prospective Study. Clin. Transl. Gastroenterol. 2020, 11, e00174. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, B.; Iborra, M.; Sáez-González, E.; Marqués-Miñana, M.R.; Moret, I.; Cerrillo, E.; Tortosa, L.; Bastida, G.; Hinojosa, J.; Poveda-Andrés, J.L.; et al. Fecal Calprotectin Pretreatment and Induction Infliximab Levels for Prediction of Primary Nonresponse to Infliximab Therapy in Crohn’s Disease. Dig. Dis. 2019, 37, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, G.; Garnero, P.; Moussata, D.; Cuerq, C.; Préaudat, C.; Duclaux-Loras, R.; Mialon, A.; Drai, J.; Flourié, B.; Nancey, S. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 331–336. [Google Scholar] [CrossRef]
- Plevris, N.; Fulforth, J.; Lyons, M.; Siakavellas, S.I.; Jenkinson, P.W.; Chuah, C.S.; Lucaciu, L.; Pattenden, R.J.; Arnott, I.D.; Jones, G.R.; et al. Normalization of Fecal Calprotectin Within 12 Months of Diagnosis Is Associated With Reduced Risk of Disease Progression in Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 1835–1844.e6. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dulai, P.S.; Marshall, J.K.; Colombel, J.F.; Reinisch, W. Week 6 Calprotectin Best Predicts Likelihood of Long-term Endoscopic Healing in Crohn’s Disease: A Post-hoc Analysis of the UNITI/IM-UNITI Trials. J. Crohn’s Colitis 2021, 15, 462–470. [Google Scholar] [CrossRef]
- Toyonaga, T.; Kobayashi, T.; Nakano, M.; Saito, E.; Umeda, S.; Okabayashi, S.; Ozaki, R.; Hibi, T. Usefulness of fecal calprotectin for the early prediction of short-term outcomes of remission-induction treatments in ulcerative colitis in comparison with two-item patient-reported outcome. PLoS ONE 2017, 12, e0185131. [Google Scholar] [CrossRef] [Green Version]
- Naganuma, M.; Kobayashi, T.; Nasuno, M.; Motoya, S.; Kato, S.; Matsuoka, K.; Hokari, R.; Watanabe, C.; Sakamoto, H.; Yamamoto, H.; et al. Significance of Conducting 2 Types of Fecal Tests in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1102–1111.e5. [Google Scholar] [CrossRef]
- Naismith, G.D.; Smith, L.A.; Barry, S.J.; Munro, J.I.; Laird, S.; Rankin, K.; Morris, A.J.; Winter, J.W.; Gaya, D.R. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn’s disease. J. Crohn’s Colitis 2014, 8, 1022–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molander, P.; Färkkilä, M.; Ristimäki, A.; Salminen, K.; Kemppainen, H.; Blomster, T.; Koskela, R.; Jussila, A.; Rautiainen, H.; Nissinen, M.; et al. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J. Crohn’s Colitis 2015, 9, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vos, M.; Louis, E.J.; Jahnsen, J.; Vandervoort, J.G.; Noman, M.; Dewit, O.; D’haens, G.R.; Franchimont, D.; Baert, F.J.; Torp, R.A.; et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm. Bowel Dis. 2013, 19, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Zhulina, Y.; Cao, Y.; Amcoff, K.; Carlson, M.; Tysk, C.; Halfvarson, J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment. Pharmacol. Ther. 2016, 44, 495–504. [Google Scholar] [CrossRef]
- Heida, A.; Park, K.T.; van Rheenen, P.F. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm. Bowel Dis. 2017, 23, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.K.; Kamm, M.A.; De Cruz, P.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Leach, S.; Gorelik, A.; Liew, D.; Prideaux, L.; et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015, 148, 938–947.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Mao, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; Xue, L.; Song, X.M.; Li, Z.P.; Chen, M.H. Fecal calprotectin for evaluating postoperative recurrence of Crohn’s disease: A meta-analysis of prospective studies. Inflamm. Bowel Dis. 2015, 21, 315–322. [Google Scholar] [CrossRef]
- Reenaers, C.; Bossuyt, P.; Hindryckx, P.; Vanpoucke, H.; Cremer, A.; Baert, F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United Eur. Gastroenterol. J. 2018, 6, 1117–1125. [Google Scholar] [CrossRef]
- Amcoff, K.; Stridsberg, M.; Lampinen, M.; Magnuson, A.; Carlson, M.; Halfvarson, J. Clinical implications of assay specific differences in f-calprotectin when monitoring inflammatory bowel disease activity over time. Scand. J. Gastroenterol. 2017, 52, 344–350. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Nancey, S.; Danese, S.; Peyrin-Biroulet, L. A Practical Guide for Faecal Calprotectin Measurement: Myths and Realities. J. Crohn’s Colitis 2021, 15, 152–161. [Google Scholar] [CrossRef]
- Lasson, A.; Stotzer, P.O.; Öhman, L.; Isaksson, S.; Sapnara, M.; Strid, H. The intra-individual variability of faecal calprotectin: A prospective study in patients with active ulcerative colitis. J. Crohn’s Colitis 2015, 9, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremer, A.; Ku, J.; Amininejad, L.; Bouvry, M.R.; Brohet, F.; Liefferinckx, C.; Devière, J.; van Gossum, A.; Smet, J.; Stordeur, P.; et al. Variability of Faecal Calprotectin in Inflammatory Bowel Disease Patients: An Observational Case-control Study. J. Crohn’s Colitis 2019, 13, 1372–1379. [Google Scholar] [CrossRef]
- Kim, E.S.; Tarassishin, L.; Eisele, C.; Barre, A.; Nair, N.; Rendon, A.; Hawkins, K.; Debebe, A.; White, S.; Thjømøe, A.; et al. Longitudinal Changes in Fecal Calprotectin Levels Among Pregnant Women With and Without Inflammatory Bowel Disease and Their Babies. Gastroenterology 2021, 160, 1118–1130.e3. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Ribaldone, D.G.; Rosso, C.; Saracco, G.M.; Astegiano, M.; Pellicano, R. Fecal calprotectin: Beyond intestinal organic diseases. Panminerva Med. 2018, 60, 29–34. [Google Scholar] [CrossRef]
- Osborne, J.M.; Flight, I.; Wilson, C.J.; Chen, G.; Ratcliffe, J.; Young, G.P. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient Prefer. Adherence 2018, 12, 1825–1836. [Google Scholar] [CrossRef] [Green Version]
- Vilkin, A.; Rozen, P.; Levi, Z.; Waked, A.; Maoz, E.; Birkenfeld, S.; Niv, Y. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am. J. Gastroenterol. 2005, 100, 2519–2525. [Google Scholar] [CrossRef]
- Navarro, M.; Hijos, G.; Sostres, C.; Lué, A.; Puente-Lanzarote, J.J.; Carrera-Lasfuentes, P.; Lanas, A. Reducing the Cut-Off Value of the Fecal Immunochemical Test for Symptomatic Patients Does Not Improve Diagnostic Performance. Front. Med. 2020, 7, 410. [Google Scholar] [CrossRef]
- Kato, J.; Hiraoka, S.; Nakarai, A.; Takashima, S.; Inokuchi, T.; Ichinose, M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: Can it rival fecal calprotectin? Intest. Res. 2016, 14, 5–14. [Google Scholar] [CrossRef]
- Nakarai, A.; Kato, J.; Hiraoka, S.; Kuriyama, M.; Akita, M.; Hirakawa, T.; Okada, H.; Yamamoto, K. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am. J. Gastroenterol. 2013, 108, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Kato, J.; Hiraoka, S.; Nakarai, A.; Takei, D.; Inokuchi, T.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; et al. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin Vs. Fecal Immunochemical Test. Am. J. Gastroenterol. 2015, 110, 873–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.Y.; Chan, F.K.L.; Chan, A.W.H.; Higashimori, A.; Kyaw, M.; Ching, J.Y.L.; Luk, A.K.C.; Wong, S.H.; Wu, J.C.Y.; Sung, J.J.Y.; et al. Accuracy of Faecal Immunochemical Test to Predict Endoscopic and Histological Healing in Ulcerative Colitis: A Prospective Study Based on Validated Histological Scores. J. Crohn’s Colitis 2017, 11, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, S.; Inokuchi, T.; Nakarai, A.; Takashima, S.; Takei, D.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; Kato, J. Fecal Immunochemical Test and Fecal Calprotectin Results Show Different Profiles in Disease Monitoring for Ulcerative Colitis. Gut Liver 2018, 12, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, N.; Miyazu, T.; Matsuura, T.; Takano, R.; Tamura, S.; Kagami, T.; Tani, S.; Yamade, M.; Hamaya, Y.; Iwaizumi, M.; et al. Effect of ulcerative colitis duration on the usefulness of immunochemical fecal occult blood test result as a disease activity biomarker. Int. J. Colorectal Dis. 2020, 35, 1729–1739. [Google Scholar] [CrossRef]
- Inokuchi, T.; Kato, J.; Hiraoka, S.; Takashima, S.; Nakarai, A.; Takei, D.; Sugihara, Y.; Takahara, M.; Kawano, S.; Harada, K.; et al. Fecal Immunochemical Test Versus Fecal Calprotectin for Prediction of Mucosal Healing in Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 1078–1085. [Google Scholar] [CrossRef]
- Hiraoka, S.; Kato, J.; Nakarai, A.; Takashima, S.; Inokuchi, T.; Takei, D.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H. Consecutive Measurements by Faecal Immunochemical Test in Quiescent Ulcerative Colitis Patients Can Detect Clinical Relapse. J. Crohn’s Colitis 2016, 10, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakarai, A.; Kato, J.; Hiraoka, S.; Takashima, S.; Takei, D.; Inokuchi, T.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H. Ulcerative colitis patients in clinical remission demonstrate correlations between fecal immunochemical test results, mucosal healing, and risk of relapse. World J. Gastroenterol. 2016, 22, 5079–5087. [Google Scholar] [CrossRef]
- Morikawa, T.; Kato, J.; Yamaji, Y.; Wada, R.; Mitsushima, T.; Shiratori, Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005, 129, 422–428. [Google Scholar] [CrossRef]
- Haupt, H.; Baudner, S. Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author’s transl). Hoppe Seyler’s Z. Physiol. Chem. 1977, 358, 639–646. [Google Scholar] [CrossRef]
- Serada, S.; Fujimoto, M.; Terabe, F.; Iijima, H.; Shinzaki, S.; Matsuzaki, S.; Ohkawara, T.; Nezu, R.; Nakajima, S.; Kobayashi, T.; et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 2169–2179. [Google Scholar] [CrossRef]
- Fujimoto, M.; Serada, S.; Suzuki, K.; Nishikawa, A.; Ogata, A.; Nanki, T.; Hattori, K.; Kohsaka, H.; Miyasaka, N.; Takeuchi, T.; et al. Leucine-rich α2 -glycoprotein as a potential biomarker for joint inflammation during anti-interleukin-6 biologic therapy in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 2056–2060. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Toyonaga, A.; Sasaki, E.; Ishida, O.; Ikeda, H.; Tsuruta, O.; Harada, K.; Tateishi, H.; Nishiyama, T.; Tanikawa, K. Soluble interleukin-6 receptors in inflammatory bowel disease: Relation to circulating interleukin-6. Gut 1995, 36, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, T.; Mitsuyama, K.; Sakemi, R.; Takedatsu, H.; Yoshioka, S.; Kuwaki, K.; Mori, A.; Fukunaga, S.; Araki, T.; Morita, M.; et al. Evaluation of Serum Leucine-Rich Alpha-2 Glycoprotein as a New Inflammatory Biomarker of Inflammatory Bowel Disease. Mediat. Inflamm. 2021, 2021, 8825374. [Google Scholar] [CrossRef]
- Nakajima, H.; Serada, S.; Fujimoto, M.; Naka, T.; Sano, S. Leucine-rich α-2 glycoprotein is an innovative biomarker for psoriasis. J. Dermatol. Sci. 2017, 86, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Rainer, T.H.; Leung, L.Y.; Chan, C.; Leung, Y.K.; Cheng, N.M.; Lai, P.; Cheung, Y.S.; Graham, C.A. Circulating human leucine-rich α-2-glycoprotein 1 mRNA and protein levels to detect acute appendicitis in patients with acute abdominal pain. Clin. Biochem. 2017, 50, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, T.; Serada, S.; Sugase, T.; Tanaka, K.; Miyazaki, Y.; Makino, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; et al. Overexpression of leucine-rich α2-glycoprotein-1 is a prognostic marker and enhances tumor migration in gastric cancer. Cancer Sci. 2017, 108, 2052–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinozaki, E.; Tanabe, K.; Akiyoshi, T.; Tsuchida, T.; Miyazaki, Y.; Kojima, N.; Igarashi, M.; Ueno, M.; Suenaga, M.; Mizunuma, N.; et al. Serum leucine-rich alpha-2-glycoprotein-1 with fucosylated triantennary N-glycan: A novel colorectal cancer marker. BMC Cancer 2018, 18, 406. [Google Scholar] [CrossRef]
- Watson, C.J.; Ledwidge, M.T.; Phelan, D.; Collier, P.; Byrne, J.C.; Dunn, M.J.; McDonald, K.M.; Baugh, J.A. Proteomic analysis of coronary sinus serum reveals leucine-rich α2-glycoprotein as a novel biomarker of ventricular dysfunction and heart failure. Circ. Heart Fail. 2011, 4, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Pek, S.L.; Tavintharan, S.; Wang, X.; Lim, S.C.; Woon, K.; Yeoh, L.Y.; Ng, X.; Liu, J.; Sum, C.F. Elevation of a novel angiogenic factor, leucine-rich-α2-glycoprotein (LRG1), is associated with arterial stiffness, endothelial dysfunction, and peripheral arterial disease in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2015, 100, 1586–1593. [Google Scholar] [CrossRef] [Green Version]
- Pek, S.L.T.; Cheng, A.K.S.; Lin, M.X.; Wong, M.S.; Chan, E.Z.L.; Moh, A.M.C.; Sum, C.F.; Lim, S.C.; Tavintharan, S. Association of circulating proinflammatory marker, leucine-rich-α2-glycoprotein (LRG1), following metabolic/bariatric surgery. Diabetes Metab. Res. Rev. 2018, 34, e3029. [Google Scholar] [CrossRef]
- Hayashi, M.; Abe, K.; Fujita, M.; Okai, K.; Takahashi, A.; Ohira, H. Changes in serum levels of leucine-rich α2-glycoprotein predict prognosis in primary biliary cholangitis. Hepatol. Res. 2019, 49, 385–393. [Google Scholar] [CrossRef]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Shinzaki, S.; Matsuoka, K.; Tanaka, H.; Takeshima, F.; Kato, S.; Torisu, T.; Ohta, Y.; Watanabe, K.; Nakamura, S.; Yoshimura, N.; et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J. Gastroenterol. 2021, 56, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Yanai, S.; Shinzaki, S.; Matsuoka, K.; Mizuno, S.; Iijima, H.; Naka, T.; Kanai, T.; Matsumoto, T. Leucine-Rich Alpha-2 Glycoprotein May Be Predictive of the Adalimumab Trough Level and Antidrug Antibody Development for Patients with Inflammatory Bowel Disease: A Sub-Analysis of the PLANET Study. Digestion 2021, 102, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Kourkoulis, P.; Michalopoulos, G.; Katifelis, H.; Giannopoulou, I.; Lazaris, A.C.; Papaconstantinou, I.; Karamanolis, G.; Gazouli, M. Leucine-rich alpha-2 glycoprotein 1, high mobility group box 1, matrix metalloproteinase 3 and annexin A1 as biomarkers of ulcerative colitis endoscopic and histological activity. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1106–1115. [Google Scholar] [CrossRef]
- Yasutomi, E.; Inokuchi, T.; Hiraoka, S.; Takei, K.; Igawa, S.; Yamamoto, S.; Ohmori, M.; Oka, S.; Yamasaki, Y.; Kinugasa, H.; et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021, 11, 11086. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Okayasu, I.; Oritsu, M.; Komatsu, J.; Yoshitsugu, M.; Katoh, Y.; Bandoh, T.; Toyoshima, H.; Kase, Y.; Sugihara, K.; et al. Significant increase in prostaglandin E-main urinary metabolite by laxative administration: Comparison with ulcerative colitis. Digestion 2000, 61, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Matsuura, T.; Matsuura, M.; Fujiwara, M.; Okayasu, I.; Ito, S.; Arihiro, S. Prostaglandin E-Major Urinary Metabolite as a Biomarker for Inflammation in Ulcerative Colitis: Prostaglandins Revisited. Digestion 2016, 93, 32–39. [Google Scholar] [CrossRef]
- Okayasu, I.; Ohnishi, H.; Sarandi, I.; Shojima, J.; Komatsu, J.; Oritsu, M.; Sasabe, M.; Nanami, K.O.; Matsuura, M.; Azumi, J.; et al. Significant increase of prostaglandin E-major urinary metabolite in male smokers: A screening study of age and gender differences using a simple radioimmunoassay. J. Clin. Lab. Anal. 2014, 28, 32–41. [Google Scholar] [CrossRef]
- Horikiri, T.; Hara, H.; Saito, N.; Araya, J.; Takasaka, N.; Utsumi, H.; Yanagisawa, H.; Hashimoto, M.; Yoshii, Y.; Wakui, H.; et al. Increased levels of prostaglandin E-major urinary metabolite (PGE-MUM) in chronic fibrosing interstitial pneumonia. Respir. Med. 2017, 122, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.C.; Schmidt, C.R.; Shrubsole, M.J.; Billheimer, D.D.; Joshi, P.R.; Morrow, J.D.; Heslin, M.J.; Washington, M.K.; Ness, R.M.; Zheng, W.; et al. Urine PGE-M: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin. Gastroenterol. Hepatol. 2006, 4, 1358–1365. [Google Scholar] [CrossRef]
- Kawamoto, H.; Hara, H.; Araya, J.; Ichikawa, A.; Fujita, Y.; Utsumi, H.; Hashimoto, M.; Wakui, H.; Minagawa, S.; Numata, T.; et al. Prostaglandin E-Major Urinary Metabolite (PGE-MUM) as a Tumor Marker for Lung Adenocarcinoma. Cancers 2019, 11, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, S.I.; Okayasu, I.; Fujiwara, M.; Matsuura, M.; Ohnishi, H.; Ito, S.; Kishimoto, H.; Nambu, R.; Kagimoto, S. Prostaglandin E-major Urinary Metabolite as a Biomarker for Pediatric Ulcerative Colitis Activity. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Miyazu, T.; Takano, R.; Tamura, S.; Tani, S.; Kagami, T.; Yamade, M.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; et al. Prostaglandin E-major urinary metabolite versus fecal immunochemical occult blood test as a biomarker for patient with ulcerative colitis. BMC Gastroenterol. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Tamura, S.; Miyazu, T.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Osawa, S.; Furuta, T.; Sugimoto, K. Comparison between Prostaglandin E-major urinary metabolite and C-reactive protein levels to reflect endoscopic scores in patients with ulcerative colitis. Sci. Rep. 2021, 11, 16205. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Sugiura, K.; Miyazu, T.; Tamura, S.; Suzuki, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Osawa, S.; et al. Prostaglandin E-Major Urinary Metabolite Predicts Relapse in Patients With Ulcerative Colitis in Clinical Remission. Clin. Transl. Gastroenterol. 2020, 11, e00289. [Google Scholar] [CrossRef]
- Minderhoud, I.M.; Steyerberg, E.W.; van Bodegraven, A.A.; van der Woude, C.J.; Hommes, D.W.; Dijkstra, G.; Fidder, H.H.; Schwartz, M.P.; Oldenburg, B. Predicting Endoscopic Disease Activity in Crohn’s Disease: A New and Validated Noninvasive Disease Activity Index (The Utrecht Activity Index). Inflamm. Bowel Dis. 2015, 21, 2453–2459. [Google Scholar] [CrossRef] [Green Version]
- Bodelier, A.G.; Jonkers, D.; van den Heuvel, T.; de Boer, E.; Hameeteman, W.; Masclee, A.A.; Pierik, M.J. High Percentage of IBD Patients with Indefinite Fecal Calprotectin Levels: Additional Value of a Combination Score. Dig. Dis. Sci. 2017, 62, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinisch, W.; Panaccione, R.; Bossuyt, P.; Baert, F.; Armuzzi, A.; Hébuterne, X.; Travis, S.; Danese, S.; Sandborn, W.J.; Schreiber, S.; et al. Association of Biomarker Cutoffs and Endoscopic Outcomes in Crohn’s Disease: A Post Hoc Analysis From the CALM Study. Inflamm. Bowel Dis. 2020, 26, 1562–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, B.O.; Guedry, S.E.; Goldenberg, J.Z.; Hanes, D.A.; Beardsley, J.A.; Ryan, J.J. Inflammatory Bowel Disease and Neutrophil-Lymphocyte Ratio: A Systematic Scoping Review. J. Clin. Med. 2021, 10, 4219. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, L.; Feng, S.Y.; Cai, W.M.; Chen, X.F.; Huang, Z.M. The Relationship between C-Reactive Protein/Albumin Ratio and Disease Activity in Patients with Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2020, 2020, 3467419. [Google Scholar] [CrossRef]
- Gao, S.Q.; Huang, L.D.; Dai, R.J.; Chen, D.D.; Hu, W.J.; Shan, Y.F. Neutrophil-lymphocyte ratio: A controversial marker in predicting Crohn’s disease severity. Int. J. Clin. Exp. Pathol. 2015, 8, 14779–14785. [Google Scholar] [PubMed]
- Cherfane, C.E.; Gessel, L.; Cirillo, D.; Zimmerman, M.B.; Polyak, S. Monocytosis and a Low Lymphocyte to Monocyte Ratio Are Effective Biomarkers of Ulcerative Colitis Disease Activity. Inflamm. Bowel Dis. 2015, 21, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollelis, E.; Quinard, R.M.; Bouguen, G.; Goutte, M.; Goutorbe, F.; Bouvier, D.; Pereira, B.; Bommelaer, G.; Buisson, A. Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J. Gastroenterol. 2019, 25, 2354–2364. [Google Scholar] [CrossRef]
- Choy, M.C.; Seah, D.; Gorelik, A.; An, Y.K.; Chen, C.Y.; Macrae, F.A.; Sparrow, M.P.; Connell, W.R.; Moore, G.T.; Radford-Smith, G.; et al. Predicting response after infliximab salvage in acute severe ulcerative colitis. J. Gastroenterol. Hepatol. 2018, 33, 1347–1352. [Google Scholar] [CrossRef]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Dulai, P.S.; Battat, R.; Barsky, M.; Nguyen, N.H.; Ma, C.; Narula, N.; Mosli, M.; Vande Casteele, N.; Boland, B.S.; Prokop, L.; et al. Incorporating Fecal Calprotectin Into Clinical Practice for Patients With Moderate-to-Severely Active Ulcerative Colitis Treated With Biologics or Small-Molecule Inhibitors. Am. J. Gastroenterol. 2020, 115, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, G.; Innocenti, T.; Galli, A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig. Dis. 2021, 39, 190–203. [Google Scholar] [CrossRef]
- Cui, G.; Fan, Q.; Li, Z.; Goll, R.; Florholmen, J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine 2021, 66, 103329. [Google Scholar] [CrossRef]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef]
- Verdier, J.; Breunig, I.R.; Ohse, M.C.; Roubrocks, S.; Kleinfeld, S.; Roy, S.; Streetz, K.; Trautwein, C.; Roderburg, C.; Sellge, G. Faecal Micro-RNAs in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2020, 14, 110–117. [Google Scholar] [CrossRef]
- Clerc, F.; Novokmet, M.; Dotz, V.; Reiding, K.R.; de Haan, N.; Kammeijer, G.S.M.; Dalebout, H.; Bladergroen, M.R.; Vukovic, F.; Rapp, E.; et al. Plasma N-Glycan Signatures Are Associated With Features of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.M.; Kaser, A.; Blumberg, R.S. A role for oncostatin M in inflammatory bowel disease. Nat. Med. 2017, 23, 535–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreou, N.P.; Legaki, E.; Dovrolis, N.; Boyanov, N.; Georgiou, K.; Gkouskou, K.; Gazouli, M. B-cell activating factor (BAFF) expression is associated with Crohn’s disease and can serve as a potential prognostic indicator of disease response to Infliximab treatment. Dig. Liver Dis. 2021, 53, 574–580. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Sample | Invasion | Expensive | Strong Point | Weak Point | Evidence |
|---|---|---|---|---|---|---|
| CRP | blood | + | + |
|
| +++ |
| FCP | stool | - | ++ |
|
| +++ |
| FIT | stool | - | + |
|
| ++ |
| LRG | blood | + | ++ |
|
| + |
| PGE-MUM | urine | - | No data |
|
| + |
| Endoscopy | +++ | +++ |
|
| +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagatsuma, K.; Yokoyama, Y.; Nakase, H. Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease. Life 2021, 11, 1375. https://doi.org/10.3390/life11121375
Wagatsuma K, Yokoyama Y, Nakase H. Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease. Life. 2021; 11(12):1375. https://doi.org/10.3390/life11121375
Chicago/Turabian StyleWagatsuma, Kohei, Yoshihiro Yokoyama, and Hiroshi Nakase. 2021. "Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease" Life 11, no. 12: 1375. https://doi.org/10.3390/life11121375
APA StyleWagatsuma, K., Yokoyama, Y., & Nakase, H. (2021). Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease. Life, 11(12), 1375. https://doi.org/10.3390/life11121375







