Distribution and Appearance of Ki-67, IL-1α, IL-10, and PGP 9.5 in Reinke’s Oedema-Affected Larynx Tissue Compared with Control Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Subjects
2.2. Routine Histological Analysis
2.3. Immunohistochemical Analysis
2.4. Quantification of Immunoreactive Cells
2.5. Statistical Analysis
3. Results
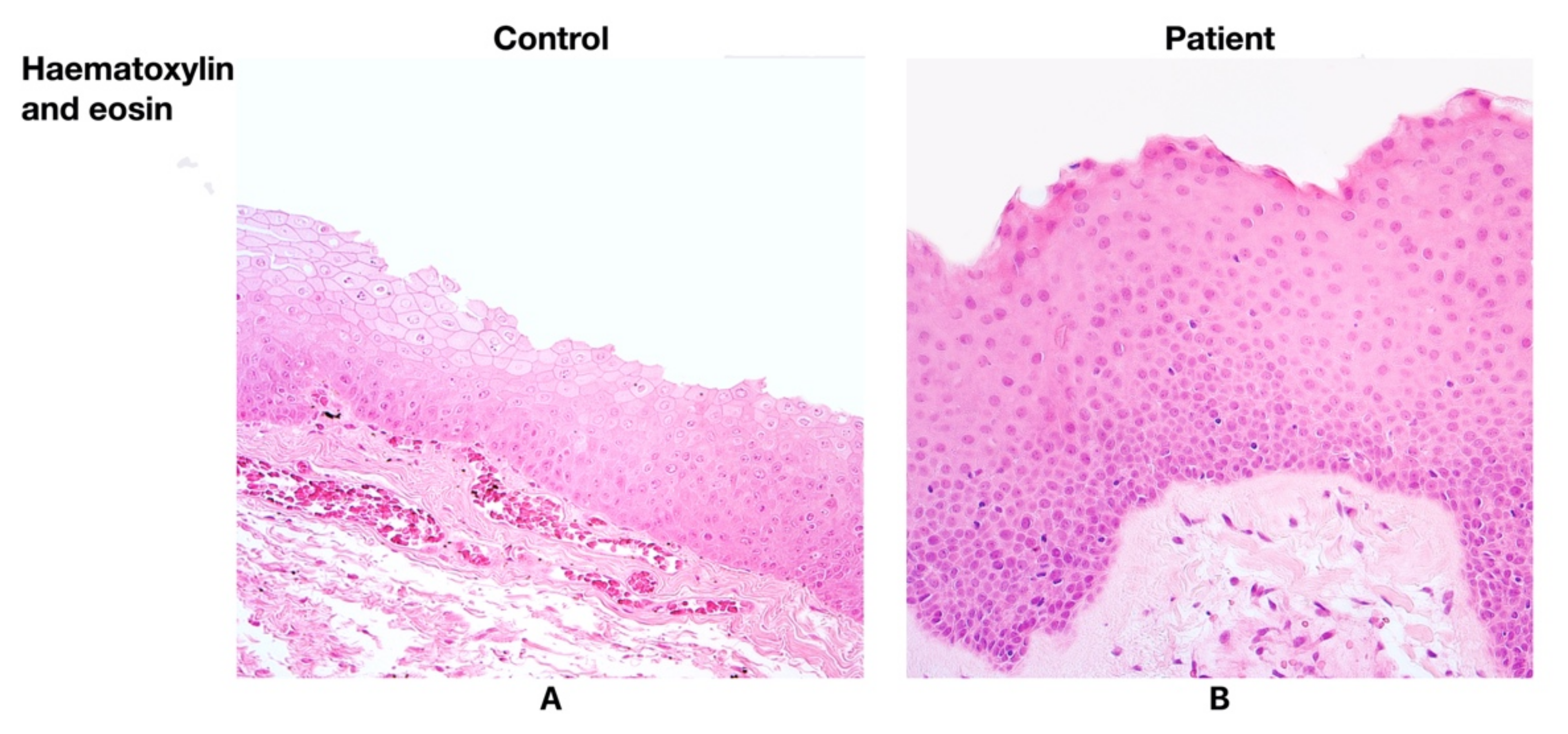
3.1. Findings of Routine Histological Analysis
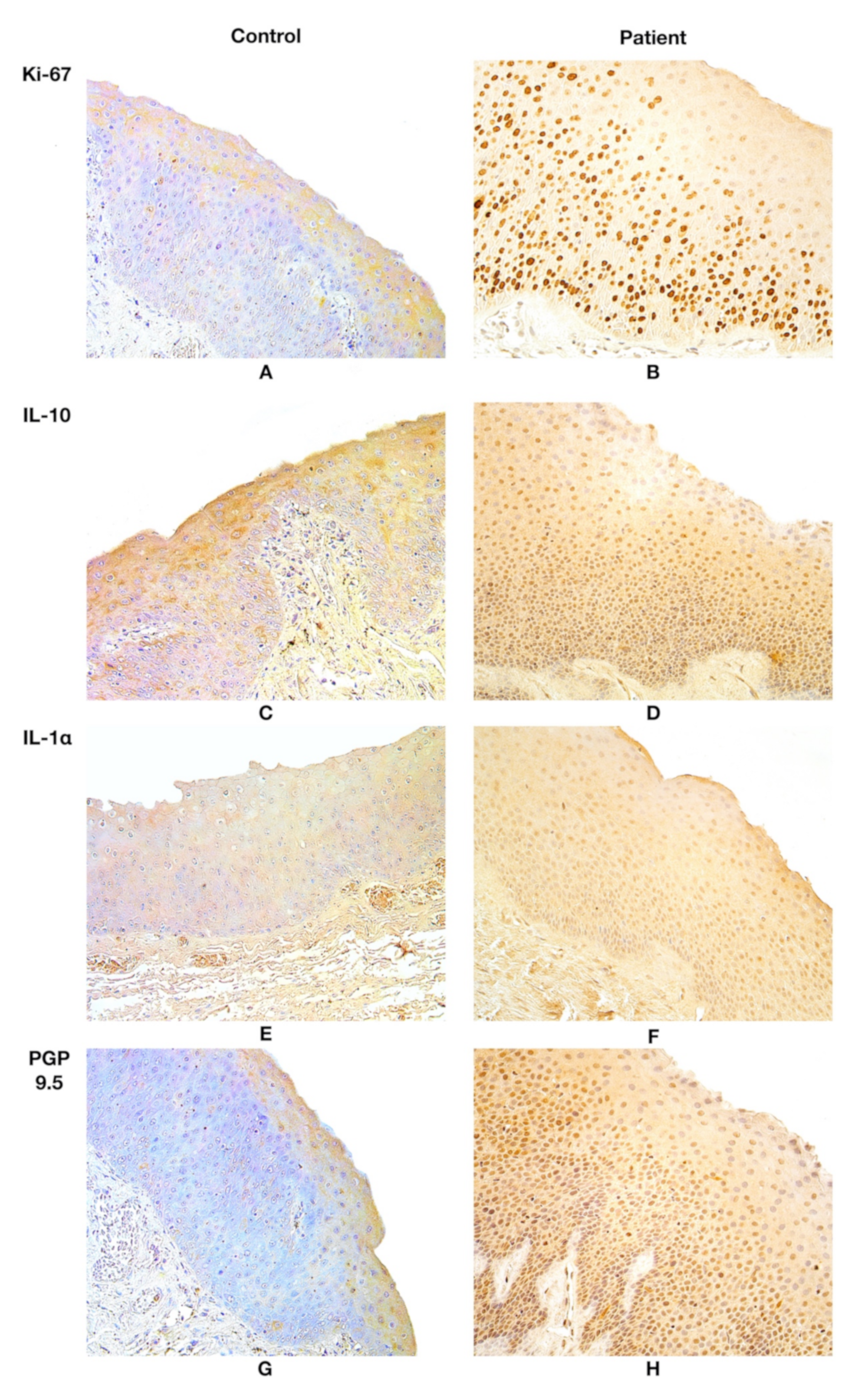
3.2. Immunohistochemistry Findings
3.3. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavaluc, R.; Tan-Geller, M. Reinke’s Edema. Otolaryngol. Clin. North. Am. 2019, 52, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hah, J.H.; Sim, S.; An, S.-Y.; Sung, M.-W.; Choi, H.G. Evaluation of the Prevalence of and Factors Associated with Laryngeal Diseases among the General Population: Prevalence of Laryngeal Diseases. Laryngoscope 2015, 125, 2536–2542. [Google Scholar] [CrossRef] [PubMed]
- Salmen, T.; Ermakova, T.; Schindler, A.; Ko, S.-R.; Göktas, Ö.; Gross, M.; Nawka, T.; Caffier, P.P. Efficacy of Microsurgery in Reinke’s Oedema Evaluated by Traditional Voice Assessment Integrated with the Vocal Extent Measure (VEM). Acta Otorhinolaryngol. Ital. 2018, 38, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Volić, S.V.; Klapan, I.; Seiwerth, S.; Ibrahimpašić, T. Extracellular Matrix of Reinke’s Space in Some Pathological Conditions. Acta Oto-Laryngol. 2004, 124, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Tateya, I.; Omori, K.; Kojima, H.; Hirano, S.; Kaneko, K.; Ito, J. Steroid Injection for Reinke’s Edema Using Fiberoptic Laryngeal Surgery. Acta Oto-Laryngol. 2003, 123, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, D.; Magliulo, G.; Pezone, T. Reinke’s Edema and Risk Factors: Clinical and Histopathologic Aspects. Am. J. Otolaryngol. 2002, 23, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Tae, K.; Lee, Y.S.; Jeong, J.H.; Cho, S.H.; Kim, K.R.; Park, C.W.; Han, D.S. The Significance of Laryngopharyngeal Reflux in Benign Vocal Mucosal Lesions. Otolaryngol. Head Neck Surg. 2009, 141, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Zeitels, S.M.; Bunting, G.W.; Hillman, R.E.; Vaughn, T. Reinke’s Edema: Phonatory Mechanisms and Management Strategies. Ann. Otol. Rhinol. Laryngol. 1997, 106, 533–543. [Google Scholar] [CrossRef]
- Kravos, A.; Župevc, A.; Čizmarevič, B.; Hočevar-Boltežar, I. The Role of Allergy in the Etiology of Reinke’s Edema on Vocal Folds. Wien. Klin. Wochenschr. 2010, 122, 44–48. [Google Scholar] [CrossRef]
- Zhukhovitskaya, A.; Battaglia, D.; Khosla, S.M.; Murry, T.; Sulica, L. Gender and Age in Benign Vocal Fold Lesions: Benign VF Lesions: Gender and Age. Laryngoscope 2015, 125, 191–196. [Google Scholar] [CrossRef]
- Sato, K.; Hirano, M.; Nakashima, T. Electron Microscopic and Immunohistochemical Investigation of Reinke’s Edema. Ann. Otol. Rhinol. Laryngol. 1999, 108, 1068–1072. [Google Scholar] [CrossRef]
- Pastuszek, P.; Krecicki, T.; Zalesska-Krecicka, M.; Jeleń, M.; Rak, J.; Krajewska, B. Histological and Electron Microscopic Investigation of Reinke’s Edema. Pol. J. Pathol. 2003, 54, 61–64. [Google Scholar]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a Mouse Monoclonal Antibody Reactive with a Human Nuclear Antigen Associated with Cell Proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef]
- Scholzen, T.; Endl, E.; Wohlenberg, C.; van der Sar, S.; Cowell, I.G.; Gerdes, J.; Singh, P.B. The Ki-67 Protein Interacts with Members of the Heterochromatin Protein 1 (HP1) Family: A Potential Role in the Regulation of Higher-Order Chromatin Structure. J. Pathol. 2002, 196, 135–144. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Phys. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Verdolini Abbott, K.; Li, N.Y.K.; Branski, R.C.; Rosen, C.A.; Grillo, E.; Steinhauer, K.; Hebda, P.A. Vocal Exercise May Attenuate Acute Vocal Fold Inflammation. J. Voice 2012, 26, 814.e1–814.e13. [Google Scholar] [CrossRef] [Green Version]
- O’Garra, A.; Vieira, P. TH1 Cells Control Themselves by Producing Interleukin-10. Nat. Rev. Immunol. 2007, 7, 425–428. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [Green Version]
- Helmark, I.C.; Mikkelsen, U.R.; Børglum, J.; Rothe, A.; Petersen, M.C.; Andersen, O.; Langberg, H.; Kjaer, M. Exercise Increases Interleukin-10 Levels Both Intraarticularly and Peri-Synovially in Patients with Knee Osteoarthritis: A Randomized Controlled Trial. Arthritis Res. Ther. 2010, 12, R126. [Google Scholar] [CrossRef] [Green Version]
- Rider, P.; Carmi, Y.; Guttman, O.; Braiman, A.; Cohen, I.; Voronov, E.; White, M.R.; Dinarello, C.A.; Apte, R.N. IL-1α and IL-1β Recruit Different Myeloid Cells and Promote Different Stages of Sterile Inflammation. J. Immunol. 2011, 187, 4835–4843. [Google Scholar] [CrossRef] [Green Version]
- England, H.; Summersgill, H.R.; Edye, M.E.; Rothwell, N.J.; Brough, D. Release of Interleukin-1α or Interleukin-1β Depends on Mechanism of Cell Death. J. Biol. Chem. 2014, 289, 15942–15950. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [Green Version]
- Voronov, E.; Dinarello, C.A.; Apte, R.N. Interleukin-1α as an Intracellular Alarmin in Cancer Biology. Semin. Immunol. 2018, 38, 3–14. [Google Scholar] [CrossRef]
- Elkon, K.B. IL-1α Responds to Necrotic Cell Death. Nat. Med. 2007, 13, 778–780. [Google Scholar] [CrossRef]
- Otsuki, T.; Yata, K.; Takata-Tomokuni, A.; Hyodoh, F.; Miura, Y.; Sakaguchi, H.; Hatayama, T.; Hatada, S.; Tsujioka, T.; Sato, Y.; et al. Expression of Protein Gene Product 9·5 (PGP9·5)/Ubiquitin-C-Terminal Hydrolase 1 (UCHL-1) in Human Myeloma Cells: Expression of PGP9·5 in Myeloma. Br. J. Haematol. 2004, 127, 292–298. [Google Scholar] [CrossRef]
- Pirim, İ.; Eyerci, N. Assesment of Protein Gene Product (Pgp9.5) Enzyme Activity against Potential Peptide Substrate. Eurasian J. Med. 2009, 41, 102–107. [Google Scholar]
- Wang, A.R.; May, D.; Bourne, P.; Scott, G. PGP9.5: A Marker for Cellular Neurothekeoma. Am. J. Surg. Pathol. 1999, 23, 1401. [Google Scholar] [CrossRef]
- Pilmane, M.; Luts, A.; Sundler, F. Changes in Neuroendocrine Elements in Bronchial Mucosa in Chronic Lung Disease in Adults. Thorax 1995, 50, 551–554. [Google Scholar] [CrossRef] [Green Version]
- Vitenberga, Z.; Pilmane, M.; Babjoniševa, A. An Insight into COPD Morphopathogenesis: Chronic Inflammation, Remodelling, and Antimicrobial Defense. Medicina 2019, 55, 496. [Google Scholar] [CrossRef] [Green Version]
- Sumeraga, G.; Pilmane, M. Distribution of Neuropeptides in Nasal and Nasopharyngeal Mucosa in Patients with the Post Nasal Drip Syndrome. Pap. Anthropol. 2012, 20, 389. [Google Scholar] [CrossRef]
- Goida, J.; Pilmane, M. Characterization of Macrophages and TNF-α in Cleft Affected Lip Tissue. Cosmetics 2021, 8, 42. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Statist. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Pilmane, M.; Sidhoma, E.; Akota, I.; Kazoka, D. Characterization of Cytokines and Proliferation Marker Ki67 in Cleft Affected Lip Tissue. Medicina 2019, 55, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the Inflammatory Process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Swanson, E.R.; Ohno, T.; Abdollahian, D.; Rousseau, B. Effects of Raised-Intensity Phonation on Inflammatory Mediator Gene Expression in Normal Rabbit Vocal Fold. Otolaryngol. Head Neck Surg. 2010, 143, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonina, I.S.; Tynan, G.A.; Logue, S.E.; Cullen, S.P.; Bots, M.; Lüthi, A.U.; Reeves, E.P.; McElvaney, N.G.; Medema, J.P.; Lavelle, E.C.; et al. Granzyme B-Dependent Proteolysis Acts as a Switch to Enhance the Proinflammatory Activity of IL-1α. Mol. Cell 2011, 44, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-J.; Kono, H.; Golenbock, D.; Reed, G.; Akira, S.; Rock, K.L. Identification of a Key Pathway Required for the Sterile Inflammatory Response Triggered by Dying Cells. Nat. Med. 2007, 13, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Branchfield, K.; Nantie, L.; Verheyden, J.M.; Sui, P.; Wienhold, M.D.; Sun, X. Pulmonary Neuroendocrine Cells Function as Airway Sensors to Control Lung Immune Response. Science 2016, 351, 707–710. [Google Scholar] [CrossRef] [Green Version]
| Factors/Subjects | Ki-67 | IL-10 | IL-1α | PGP 9.5 | |||
|---|---|---|---|---|---|---|---|
| Epithelial | Epithelial | Subepithelial | Epithelial | Subepithelial | Epithelial NEC | Subepithelial Nerves | |
| P1 | 229.0 | ++/+++ | ++/+++ | ++ | ++/+++ | +/++ | + |
| P2 | 140.5 | ++/+++ | ++/+++ | ++/+++ | ++/+++ | +/++ | +/++ |
| P3 | 139.0 | ++/+++/+++ | ++/+++ | ++/+++ | ++/+++ | +/++ | + |
| P4 | 204.5 | ++/+++ | ++/+++ | ++/+++ | ++/++/+++ | +/+/++ | + |
| P5 | 126.0 | ++/+++ | ++/+++ | ++/+++ | ++/+++ | +/++ | +/++ |
| Median (P) | 140.5 | ++/+++ | ++/+++ | ++/+++ | ++/+++ | +/++ | + |
| IQR (P) | 84.25 | 0.125 | 0.00 | 0.25 | 0.125 | 0.125 | 0.5 |
| C1 | 0.00 | 0/+ | 0/+ | 0/+ | 0/+ | 0 | 0/+/+ |
| C2 | 0.00 | 0/+ | 0/+ | 0/0/+ | 0/+ | 0/+ | 0/+ |
| C3 | 0.00 | 0/+ | 0/+ | 0/+ | 0/+ | 0 | 0/+ |
| C4 | 0.00 | 0/+ | 0/+ | 0 | 0/+ | 0/+ | 0/+ |
| C5 | 0.00 | 0/0/+ | 0/+ | 0/+ | 0/+ | 0/0/+ | 0/+ |
| C6 | 0.00 | 0/+ | 0/+ | 0/+ | 0/+ | 0/+ | 0/+ |
| C7 | 0.00 | + | 0/+ | 0/+ | 0/+ | 0/+ | 0/+ |
| Median (C) | 0.00 | 0/+ | 0/+ | 0/+ | 0/+ | 0/+ | 0/+ |
| IQR (C) | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.5 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopecka, V.; Pilmane, M.; Sumerags, D.; Sumeraga, G. Distribution and Appearance of Ki-67, IL-1α, IL-10, and PGP 9.5 in Reinke’s Oedema-Affected Larynx Tissue Compared with Control Tissue. Life 2021, 11, 1379. https://doi.org/10.3390/life11121379
Konopecka V, Pilmane M, Sumerags D, Sumeraga G. Distribution and Appearance of Ki-67, IL-1α, IL-10, and PGP 9.5 in Reinke’s Oedema-Affected Larynx Tissue Compared with Control Tissue. Life. 2021; 11(12):1379. https://doi.org/10.3390/life11121379
Chicago/Turabian StyleKonopecka, Vita, Mara Pilmane, Dins Sumerags, and Gunta Sumeraga. 2021. "Distribution and Appearance of Ki-67, IL-1α, IL-10, and PGP 9.5 in Reinke’s Oedema-Affected Larynx Tissue Compared with Control Tissue" Life 11, no. 12: 1379. https://doi.org/10.3390/life11121379






