From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial
Abstract
:1. Introduction
2. Marketed Marine Drugs
2.1. Cytarabine
2.2. Vidarabine
2.3. Fludarabine
2.4. Ziconotide
2.5. Omega-3 Acid Ethyl Esters
2.6. Nelarabine
2.7. Trabectedin
2.8. Eribulin
2.9. Brentuximab Vedotin
2.10. Lurbinectedin
2.11. Polatuzumab Vedotin
2.12. Enfortumab Vedotin
2.13. Belantamab Mafodotin
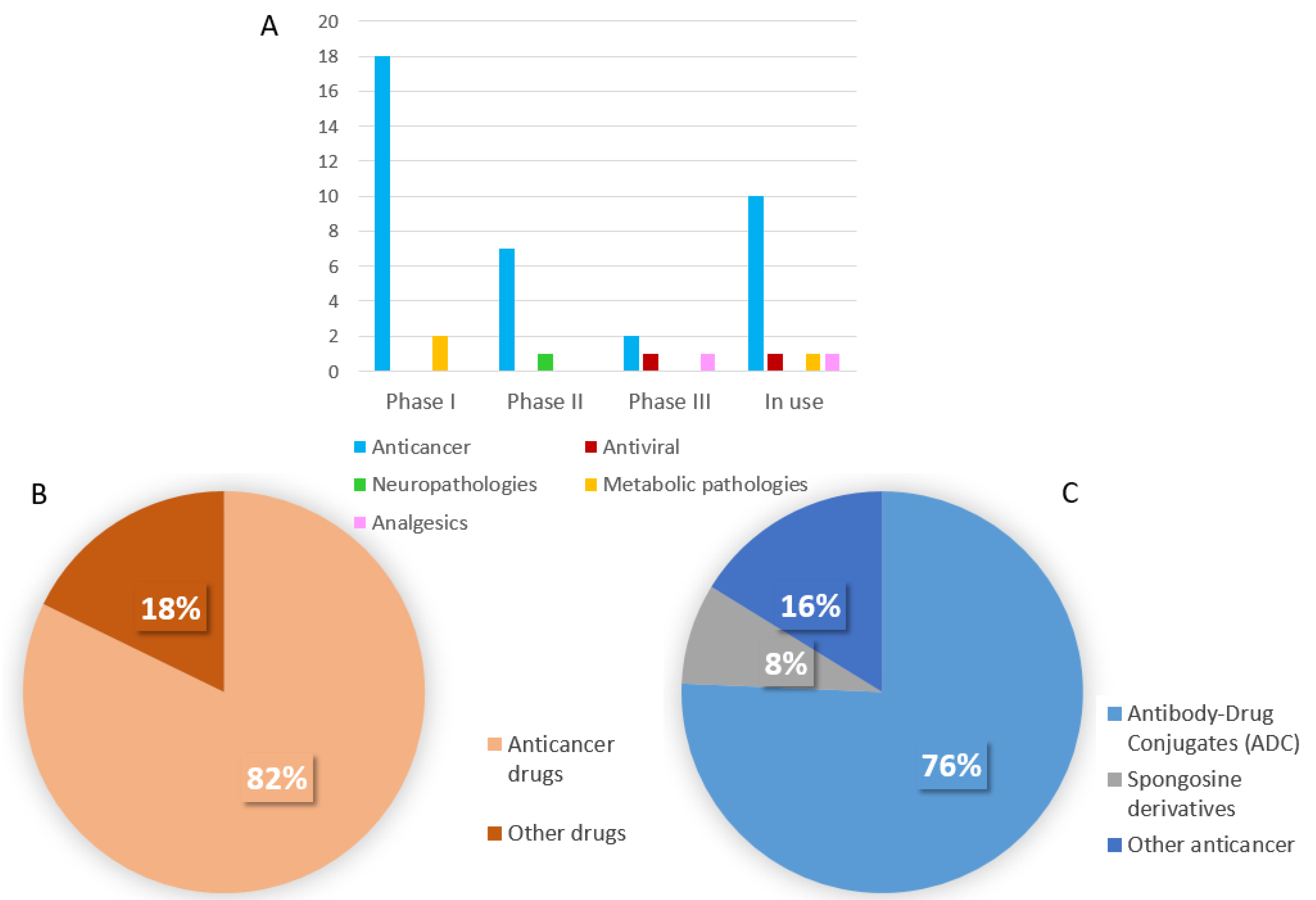
3. New Marine Drugs under Clinical Trial
3.1. Phase III
3.2. Phases II
3.3. Phase I
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AA: arachidonic acid | FDA: food and drug administration |
| ADA: adenosine deaminase | HSV: Herpex symplex virus |
| ADC: antibody-drug conjugate | IgG: immunoglobulin |
| AL: light chain amyloidosis | LA: linoleic acid |
| ALA: α-linolenic acid | LBL: lymphoblastic lymphoma |
| sALCL: systemic anaplastic large T-cell lymphoma | Mab: monoclonal antibody |
| ALL: acute lymphoblastic leukemia | MDR: multi drug resistance |
| AML: acute myeloid leukemia | MDM2: murine double minute 2 |
| Ara-A: arabinofuranosyl-adenine, adenine-arabinoside, vidarabine | MM: multiple myeloma |
| Ara-C: cytosine arabinoside or arabinosyl cytosine or cytarabine | MMAE: monomethylauristatin E |
| Ara-G: guanosine-arabinoside | MMAF: monomethylauristatin F |
| Ara-T: arabinofuranosylthymine, spongothymidine | NSCLC: non-small cell lung cancer phosphorylase |
| Ara-CTP: cytosine arabinoside triphosphate | PNP: purine nucleoside |
| Ara-GTP: guanosine arabinoside triphosphate | PDC: probody drug conjugate |
| ASCT: autologous stem cell transplantation | ORR: overall response rate |
| BCMA: B-cell maturation antigen | PUFA: Polyunsaturated fatty acid |
| CGRP: calcitonin gene-related peptide | sALCL: systemic anaplastic large cell lymphoma |
| CHMP: committee for medicinal products for human use (EMA) | SCLC: small cell lung cancer |
| CLL: chronic lymphatic leukaemia | SMDC: small molecule drug conjugate |
| CNS: central nervous system | T-ALL: T-cell acute lymphoblastic leukaemia |
| DHA: docosahexaenoic acid | T-LBL: T-cell lymphoblastic lymphoma |
| EFA: essential fatty acid | TC-NER: Transcription-nucleotide excision repair |
| EMT: epithelial-mesenchymal transition | TME: tumor microenvironment |
| EPA: eicosapentaenoic acid | TNF: tumor necrosis factor |
| EMA: european medicine agency | VDSC: voltage-dependent sodium channel |
| EU: european Union | VLDL: very low density lipoprotein |
| FA: fatti acid |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, K.H. Drugs from the oceans: Marine natural products as leads for drug discovery. Chimia 2017, 71, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, H.; Li, Q.; Wu, Y. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [Green Version]
- Leiman, D.A.; Riff, B.P.; Morgan, S.; Metz, D.C.; Falk, G.W.; French, B.; Umscheid, C.A.; Lewis, J.D. Alginate therapy is effective treatment for GERD symptoms: A systematic review and meta-analysis. Dis. Esophagus. 2017, 30, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bo, G. Giuseppe Brotzu and the discovery of cephalosporins. Clin. Microbiol. Infect. 2000, 6, 6–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Jimenez, P.C.; Wilke, D.V.; Costa-Lotufo, L.V. Marine drugs for cancer: Surfacing biotechnological innovations from the oceans. Clinics 2018, 73. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U. Marine-derived pharmaceuticals-challenges and opportunities. Biomol. Ther. 2018, 24, 561–571. [Google Scholar] [CrossRef] [Green Version]
- DepoCyte: Withdrawn application|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/depocyte#key-facts-section (accessed on 7 October 2021).
- Schabel, F.M., Jr. The Antiviral Activity of 9-β-D-Arabinofuranosyladenine (ARA-A). Chemotherapia 1968, 13, 321–338. [Google Scholar] [CrossRef]
- Buchanan, R.A.; Hess, F. Vidarabine (Vira-A®): Pharmacology and clinical experience. Pharmacol. Ther. 1980, 8, 143–171. [Google Scholar] [CrossRef]
- Dicioccio, R.A.; Srivastava, B.I.S. Kinetics of Inhibition of Deoxynucleotide-Polymerizing Enzyme Activities from Normal and Leukemic Human Cells by 9-beta-D-Arabinofuranosyladenine 5’-Triphosphate and 1-beta-D-Arabinofuranosylcytosine 5’-Triphosphate. Eur. J. Biochem. 1977, 79, 411–418. [Google Scholar] [CrossRef]
- Rose, K.M.; Jacob, S.T. Selective inhibition of RNA polyadenylation by Ara-ATP in vitro: A possible mechanism for antiviral action of Ara-A. Biochem. Biophys. Res. Commun. 1978, 81, 1418–1424. [Google Scholar] [CrossRef]
- Sloan, B.J.; Kielty, J.K.; Miller, F.A. Effect of a Novel Adenosine Deaminase Inhibitor (Co-Vidarabine, Co-V) upon the Antiviral Activity in Vitro and in vivo of Vidarabine (Vira-Atm) for DNA Virus Replication. Ann. N. Y. Acad. Sci. 1977, 284, 60–80. [Google Scholar] [CrossRef]
- Rodriguez, G. Fludarabine phosphate. A new anticancer drug with significant activity in patients with chronic lymphocytic leukemia and in patients with lymphoma. Investig. New Drugs 1994, 12, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.R.; McTavish, D.; Faulds, D. Fludarabine. Drugs 1993, 45, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways. J. Proteom. 2019, 190, 12–20. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, M.; Cruz, L.J.; Hunkapiller, M.W.; Gray, W.R.; Olivera, B.M. Isolation and structure of a peptide toxin from the marine snail Conus magus. Arch. Biochem. Biophys. 1982, 218, 329–334. [Google Scholar] [CrossRef]
- Wie, C.S.; Derian, A. Ziconotide, StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459151/ (accessed on 7 February 2021).
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance — A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367. [Google Scholar] [CrossRef]
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular dsease. Mol. Cell. Biochem. 2021, 476, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Bhat, S.A.; Ara, A. Omega-3 fatty acids and the treatment of depression: A review of scientific evidence. Integr. Med. Res. 2015, 4, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, R.; Censi, S.; Cimaglia, P. The journey of omega-3 fatty acids in cardiovascular medicine. Eur. Heart J. Suppl. 2020, 22, J49–J53. [Google Scholar] [CrossRef] [PubMed]
- Omega-3 Acid Ethyl Esters-Containing Medicinal Products for Oral in Use in Secondary Prevention after mYocardial Infarction. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/omega-3-acid-ethyl-esters-containing-medicinal-products-oral-use-secondary-prevention-after (accessed on 7 October 2021).
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef] [Green Version]
- Buie, L.W.; Epstein, S.S.; Lindley, C.M. Nelarabine: A novel purine antimetabolite antineoplastic agent. Clin. Ther. 2007, 29, 1887–1899. [Google Scholar] [CrossRef]
- Kadia, T.M.; Gandhi, V. Nelarabine in the treatment of pediatric and adult patients with T-cell acute lymphoblastic leukemia and lymphoma. Expert Rev. Hematol. 2017, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Atriance|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/atriance (accessed on 7 October 2021).
- EMA, Yondelis|European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/product-information/yondelis-epar-product-information_it.pdf (accessed on 7 October 2021).
- Drug Trials Snapshots: YONDELIS. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-yondelis (accessed on 7 October 2021).
- Li, W.W.; Takahashi, N.; Jhanwar, S.; Cordon-Cardo, C.; Elisseyeff, Y.; Jimeno, J.; Faircloth, G.; Bertino, J.R. Sensitivity of Soft Tissue Sarcoma Cell Lines to ChemotherapeuticAgents: Identification of Ecteinascidin-743 as a Potent Cytotoxic Agent. Clin. Canc. Res. 2001, 7, 2908–2911. [Google Scholar]
- Larsen, A.K.; Galmarini, C.M.; D’Incalci, M. Unique features of trabectedin mechanism of action. Cancer Chemother. Pharmacol. 2016, 77, 663–671. [Google Scholar] [CrossRef]
- Swami, U.; Shah, U.; Goel, S. Eribulin in cancer treatment. Mar. Drugs 2015, 13, 5016–5058. [Google Scholar] [CrossRef] [Green Version]
- Halaven|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/halaven (accessed on 7 October 2021).
- Fda, Halaven Highlights Of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201532lbl.pdf (accessed on 7 October 2021).
- Shetty, N.; Gupta, S. Eribulin drug review. South Asian J. Cancer 2014, 3, 57–59. [Google Scholar] [CrossRef]
- McBride, A.S.; Butler, K. Eribulin mesylate: A novel halichondrin B analogue for the treatment of metastatic breast cancer. Am. J. Health-Syst. Pharm 2012, 69, 745–755. [Google Scholar] [CrossRef]
- Engene, N.; Tronholm, A.; Salvador-Reyes, L.A.; Luesch, H.; Paul, V.J. Caldora penicillata gen. nov., comb. nov. (Cyanobacteria), a pantropical marine species with biomedical relevance. J. Phycol. 2015, 51, 670–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, R.; Petit, G.R.; Hamel, E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem. Pharmacol. 1990, 39, 1941–1949. [Google Scholar] [CrossRef]
- Gravanis, I.; Tzogani, K.; Hennik, P.V.; Graeff, P.D.; Schmitt, P.; Mueller-Berghaus, J.; Salmonson, T.; Gisselbrecht, C.; Laane, E.; Bergmann, L.; et al. The European Medicines Agency Review of Brentuximab Vedotin (Adcetris) for the Treatment of Adult Patients With Relapsed or Refractory CD30+ Hodgkin Lymphoma or Systemic Anaplastic Large Cell Lymphoma: Summary of the Scientific Assessment of the Committee. Oncologist 2016, 21, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Van De Donk, N.W.C.J.; Dhimolea, E. Brentuximab vedotin. mAbs 2012, 4, 458–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adcetris|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/adcetris (accessed on 7 October 2021).
- Elez, M.E.; Tabernero, J.; Geary, D.; Macarulla, T.; Kang, S.P.; Kahatt, C.; Soto-Matos Pita, A.; Fernandez Teruel, C.; Siguero, M.; Cullell-Young, M.; et al. First-in-human phase i study of lurbinectedin (PM01183) in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 2205–2214. [Google Scholar] [CrossRef] [Green Version]
- Farago, A.F.; Drapkin, B.J.; Lopez-Vilarino de Ramos, J.A.; Galmarini, C.M.; Núñez, R.; Kahatt, C.; Paz-Ares, L. ATLANTIS: A Phase III study of lurbinectedin/doxorubicin versus topotecan or cyclophosphamide/doxorubicin/vincristine in patients with small-cell lung cancer who have failed one prior platinum-containing line. Futur. Oncol. 2019, 15, 231–239. [Google Scholar] [CrossRef]
- Belgiovine, C.; Bello, E.; Liguori, M.; Craparotta, I.; Mannarino, L.; Paracchini, L.; Beltrame, L.; Marchini, S.; Galmarini, C.M.; Mantovani, A. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br. J. Cancer 2017, 117, 628–638. [Google Scholar] [CrossRef]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef]
- Shinn, L.T.; Vo, K.A.; Reeves, D.J. Lurbinectedin: A New Treatment Option for Relapsed/Refractory Small-Cell Lung Cancer. Ann. Pharmacother 2021, 55, 1172–1179. [Google Scholar] [CrossRef]
- Public-Summary-Opinion-Orphan-Designation-Lurbinectedin-Treatment-Ovarian-Cancer. Available online: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/12/1053-public-summary-opinion-orphan-designation-lurbinectedin-treatment-ovarian-cancer_en.pdf (accessed on 7 October 2021).
- Public Summary of Opinion on Orphan Designation Lurbinectedin for the Treatment of Small Cell Lung Cancer. Available online: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/19/2143-public-summary-opinion-orphan-designation-lurbinectedin-treatment-small-cell-lung-cancer_en.pdf (accessed on 7 October 2021).
- Rajput, P.S.; Khan, S.R.; Singh, P.; Chawla, P.A. Treatment of Small Cell Lung Cancer with Lurbinectedin: A Review. Anticancer Agents Med. Chem. 2021. [Google Scholar] [CrossRef]
- Mehta, A.; Forero-Torres, A. Development and Integration of Antibody–Drug Conjugate in Non-Hodgkin Lymphoma. Curr. Oncol. Rep. 2015, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Polivy|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/polivy#:~:text=Polivy%20is%20a%20cancer%20medicine,other%20medicines%2C%20bendamustine%20and%20rituximab (accessed on 7 October 2021).
- FDA. Polivy. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-chemoimmunotherapy-regimen-patients-relapsed-or-refractory-diffuse-large-b-cell (accessed on 7 October 2021).
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- A Study Comparing the Efficacy and Safety of Polatuzumab Vedotin with Rituximab-Cyclophosphamide, Doxorubicin, and Prednisone (R-CHP) versus Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants with Diffuse Large B-Cell Lymphoma (POLARIX). Available online: https://clinicaltrials.gov/ct2/show/NCT03274492 (accessed on 7 October 2021).
- Alt, M.; Stecca, C.; Tobin, S.; Jiang, D.M.; Sridhar, S.S. Enfortumab Vedotin in urothelial cancer. Ther. Adv. Urol. 2020, 12. [Google Scholar] [CrossRef]
- FDA Approves New Type of Therapy to Treat Advanced Urothelial Cancer|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-type-therapy-treat-advanced-urothelial-cancer (accessed on 7 October 2021).
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- A Study to Evaluate Enfortumab Vedotin versus (vs) Chemotherapy in Subjects with Previously Treated Locally Advanced or Metastatic Urothelial Cancer (EV-301). Available online: https://clinicaltrials.gov/ct2/show/NCT03474107 (accessed on 7 October 2021).
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13. [Google Scholar] [CrossRef]
- Drug Approval Package: BLENREP. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761158Orig1s000TOC.cfm (accessed on 7 October 2021).
- Blenrep|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/blenrep (accessed on 7 October 2021).
- Becnel, M.R.; Lee, H.C. The role of belantamab mafodotin for patients with relapsed and/or refractory multiple myeloma. Ther. Adv. Hematol. 2020, 11. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can some marine-derived fungal metabolites become actual anticancer agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Herzig, P.; Pishali Bejestani, E.; Buchi, M.; Ritschard, R.; Lloyd, G.K.; Mohanlal, R.; Tonra, J.R.; Huang, L.; Heinzelmann, V.; et al. Plinabulin, a Distinct Microtubule-Targeting Chemotherapy, Promotes M1-Like Macrophage Polarization and Anti-tumor Immunity. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; El-Khoueiry, A.; Hafez, N.; Lakhani, N.; Mamdani, H.; Rodon, J.; Sanborn, R.E.; Garcia-Corbacho, J.; Boni, V.; Stroh, M.; et al. Phase I, First-in-Human Study of the Probody Therapeutic CX-2029 in adults with advanced solid tumor malignancies. Clin. Cancer Res. 2021, 2021 27, 4521–4530. [Google Scholar] [CrossRef]
- Martínez-Díez, M.; Guillén-Navarro, M.J.; Pera, B.; Bouchet, B.P.; Martínez-Leal, J.F.; Barasoain, I.; Cuevas, C.; Andreu, J.M.; García-Fernández, L.F.; Díaz, J.F.; et al. PM060184, a new tubulin binding agent with potent antitumor activity including P-glycoprotein over-expressing tumors. Biochem. Pharmacol. 2014, 88, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Hongpaisan, J.; Sun, M.K.; Alkon, D.L. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J. Neurosci. 2011, 31, 630–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Sato, J.; Tamura, K.; Shimomura, A.; Ikezawa, H.; Nomoto, M.; Furuuchi, K.; et al. First-in-HumanPhase 1 Study of MORAb-202, an Antibody-Drug Conjugate comprising Farletuzumab li nked to eribulin Mesylate, in patients with folate receptor-α-positive advanced solid tumors. Clin. Cancer Res. 2021, 27, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y.; et al. Site-selective modification strategies in antibody–drug conjugates. Chem. Soc. Rev. 2021, 50, 1305–1353. [Google Scholar] [CrossRef]
- Zawieja, P.; Kornprobst, J.-M.; Métais, P. 3-(2,4-Dimethoxybenzylidene)-anabaseine: A promising candidate drug for Alzheimer’s disease? Geriatr. Gerontol. Int. 2012, 12, 365–371. [Google Scholar] [CrossRef]


| Generic Name | Brand Name/s | Date of Marketing Authorisation | Natural Source | Chemical Class | Clinical Use |
|---|---|---|---|---|---|
| Cytarabine | Cytosar-U Aracytin C.- Hospira | 1969 (FDA) | Sponge | Nucleoside | Leukemia |
| Vidarabine | Vira-A | 1976 (FDA) | Sponge | Nucleoside | Antiviral |
| Fludarabine | Fludara | 1992 (FDA) 1994 (EMA) | Sponge | Nucleoside | Leukemia |
| Ziconotide | Prialt | 2004 (FDA) 2005 (EMA) | Mollusk | Peptide | Chronic pain |
| Omega-3 acid ethyl esters | Lovaza (US) Eskim (EU) and others | 2004 (FDA) 2005 (EMA) | Fish | PUFA | Hypertriglyceridemia |
| Nelarabine | Arranon (US) Atriance (EU) | 2005 (FDA) 2007 (EMA) | Sponge | Nucleoside | Leukemia |
| Trabectedin | Yondelis | 2007 (EMA) 2015 (FDA) | Tunicate | Alkaloid | Ovarian cancer, soft tissue sarcoma |
| Eribulin | Halaven | 2010 (FDA)2011 (EMA) | Sponge | Macrolide | Breast cancer |
| Brentuximab vedotin | Adcetris | 2011 (FDA) 2012 (EMA) | Mollusk/ cyanobacterium | ADC | Lymphomas |
| Lurbinectedin | Zepzelca | 2020 (FDA) | Tunicate | Alkaloid | Ovarian cancer |
| Polatuzumab vedotin | Polivy | 2019 (FDA) 2020 (EMA) | Mollusk/ cyanobacterium | ADC | Breast cancer |
| Enfortumab vedotin | Padcev | 2019 (FDA) 2021 (EMA) | Mollusk/ cyanobacterium | ADC | Urothelial cancer |
| Belantamab mafodotin | Blenrep | 2020 (FDA) 2020 (EMA) | Mollusk/ cyanobacterium | ADC | Multiple myeloma |
| Generic Name | Natural Source | Chemical Class | Clinical Use |
|---|---|---|---|
| PHASE III | |||
| Tetrodotoxin | Pufferfish | Alkaloid | Chronic pain |
| Plinabulin | Fungus | Diketopiperazine | Cancer |
| Marizomib (salinosporamide A) | Actinomycetes | γ-lactam-β-lactone | Cancer |
| Plitidepsin | Tunicate | Depsipeptide | COVID-19 |
| PHASE II | |||
| Bryostatin | Bryozoan | Macrolide lactone | Alzheimer |
| Plocabulin (PM060184) | Sponge | Polyketide | Cancer |
| Tisotumab vedotin | Mollusk/cyanobacterium | ADC | Cancer |
| Ladiratuzumab vedotin (SGN-LIV1A) | Mollusk/cyanobacterium | ADC | Cancer |
| Telisotuzumab vedotin | Mollusk/cyanobacterium | ADC | Cancer |
| CAB-ROR2 (BA-3021) | Mollusk/cyanobacterium | ADC | Cancer |
| CX-2029 | Mollusk/cyanobacterium | PDC | Cancer |
| W0101 | Mollusk/cyanobacterium | ADC | Cancer |
| PHASE I | |||
| GTS-21 (DMXBA) | Worm | Alkaloid | Obesity |
| Samrotamab vedotin | Mollusk/cyanobacterium | ADC | Cancer |
| Sirtratumab vedotin (ASG-15ME) | Mollusk/cyanobacterium | ADC | Cancer |
| SGN-CD48A | Mollusk/cyanobacterium | ADC | Cancer |
| ALT-P7 | Mollusk/cyanobacterium | ADC | Cancer |
| ARX788 | Mollusk/cyanobacterium | ADC | Cancer |
| Upifitamab rilsodotin (XMT-1536) | Mollusk/cyanobacterium | ADC | Cancer |
| AGS62P1 | Mollusk/cyanobacterium | ADC | Cancer |
| PF-06804103 | Mollusk/cyanobacterium | ADC | Cancer |
| Cofetuzumab pelidotin (ABBV-647) | Mollusk/cyanobacterium | ADC | Cancer |
| ZW-49 | Mollusk/cyanobacterium | ADC | Cancer |
| MRG003 | Mollusk/cyanobacterium | ADC | Cancer |
| STRO-002 | Sponge | ADC | Cancer |
| MORAb-202 | Sponge | ADC | Cancer |
| RC-88 | Mollusk/cyanobacterium | ADC | Cancer |
| SGN-B6A | Mollusk/cyanobacterium | ADC | Cancer |
| SGN-CD228A | Mollusk/cyanobacterium | ADC | Cancer |
| FOR-46 | Mollusk/cyanobacterium | ADC | Cancer |
| A-166 | Mollusk/cyanobacterium | ADC | Cancer |
| STI-6129 | Mollusk/cyanobacterium | ADC | Amyloidosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life 2021, 11, 1390. https://doi.org/10.3390/life11121390
Cappello E, Nieri P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life. 2021; 11(12):1390. https://doi.org/10.3390/life11121390
Chicago/Turabian StyleCappello, Emiliano, and Paola Nieri. 2021. "From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial" Life 11, no. 12: 1390. https://doi.org/10.3390/life11121390
APA StyleCappello, E., & Nieri, P. (2021). From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life, 11(12), 1390. https://doi.org/10.3390/life11121390







