1. Introduction
C-type lectin receptors play an important role in recognition of the two major fungal cell wall polysaccharide ligands exposed at the host–pathogen interface. DC-SIGN recognizes abundantly exposed N-mannan in the outer cell wall, whereas Dectin-1 recognizes nanoscale exposures of β-(1,3)-glucan. Recognition of β-glucan by Dectin-1 contributes to phagocytosis, oxidative burst, regulation of transcription, production of inflammatory cytokines and chemokines, and initiation of adaptive immunity [
1]. DC-SIGN is known to mediate intercellular adhesion, as well as antigen uptake and signaling in dendritic cells (DCs) [
2]. We are examining the relationship between Dectin-1 and DC-SIGN to understand, in a simplified model, how an effective host–pathogen contact is built. We focused on the earliest events in fungal contact site biogenesis.
Initial pathogen capture and formation of a stable contact site are the earliest events that must occur for signaling through antifungal receptors to initiate. Our previous work with zymosan particles demonstrated that human monocyte-derived dendritic cells (DC) form durable contacts between the DC plasma membrane and extracellular fungal particles, which may be important for the antigen gathering functions of these cells [
3]. Rapid formation of adhesive contact site structures is especially important for
C. albicans capture under conditions involving fluid shear stress, for example by reticuloendothelial macrophages capturing yeast in the bloodstream. Fungal recognition under fluid shear also pertains to phagocytes interacting with
Candida in the oropharyngeal cavity, a major site of mucocutaneous candidiasis, where the host–pathogen interaction is subject to salivary flow.
Various authors have described the accumulation of pattern recognition receptors, such as Dectin-1 and DC-SIGN, at fungal contact sites [
4,
5,
6]. Immune cells must mobilize receptors to these contact sites for activation, crosstalk and amplification of signaling that directs downstream immune responses. In fact, these contact sites achieve an ordered segregation of molecular components with a peripheral zone enriched in the large transmembrane phosphatase CD45 and a central zone where DC-SIGN and Dectin-1 concentrates. Such “phagocytic synapses” can also involve the development of barriers to molecular diffusion that support specialized signaling processes occurring therein [
7,
8]. These findings suggest that PRRs are recruited to fungal contacts in some fashion to support their enrichment at these sites. Active and passive transport processes might conceivably account for observed receptor recruitment, but the molecular mechanisms of innate immunoreceptor recruitment in contact sites with
C. albicans have not been defined.
Previous studies from our group and others have shown the enrichment of DC-SIGN and CD-206 at fungal contact sites [
4,
5,
6,
9]. These studies are typically conducted at longer time scales of hours, which is relevant to processes such as cytokine response and cytotoxic effector responses. However, there is much less information on the dynamics of pattern recognition receptors at fungal contact sites on the time scale of minutes—a time scale that is relevant to the earliest signaling events necessary for innate immune fungal recognition. In the intensely studied immunologic synapse, it is known that immunoreceptors in the T cell/Antigen-Presenting Cell (APC) immune synapse are actively transported into the synapse within minutes via their coupling to a centripetal RHOA/myosin II-dependent actomyosin flow (AMF) [
10]. Likewise, we previously demonstrated that that Dectin-1 stimulation by glucan activates mechanical contractility signaling via a RHOA/ROCK/myosin II signaling module within minutes post-stimulation [
11]. Thus, the central hypothesis tested in this study is that Dectin-1 activates a transport mechanism, through RHOA/ROCK/myosin II-dependent signaling processes, which facilitates the recruitment of DC-SIGN to the contact site. This would be expected to improve fungal particle retention by providing higher-avidity adhesive interactions with the fungal cell wall.
We used a micropipette-micromanipulation approach to provide very high spatiotemporal control over host–pathogen contact site formation. We report that Dectin-1, in collaboration with DC-SIGN, does promote improved capture of C. albicans yeast. This occurs through improved recruitment of DC-SIGN to the contact site in a manner that is dependent upon Dectin-1 signaling via RHOA, ROCK and myosin II. These findings provide a high-resolution view of early events in receptor recruitment processes that tailor the earliest stages of the innate immune antifungal response.
2. Materials and Methods
2.1. Cell Culture
HEK-293 cells (ATCC, Manassas, VA, USA; #CRL-1573) were cultured in DMEM containing 10% FBS, 1% penicillin-streptomycin, 2 mM L-glutamine and 1 mM sodium pyruvate at 37 °C, in a 5% CO2 environment in an incubator. The identity and mycoplasma-free status of the cell line were independently confirmed by submission to ATCC Human Cell Line authentication (STR) and mycoplasma detection (PCR) services.
2.2. Transfection
mApple-Dectin1A-C-10 was a gift from Michael Davidson (Addgene, Watertown, MA, USA; plasmid # 54883). pEGFP-DC-SIGN was a generous gift from Ken Jacobson [
12]. pUNO1-hDectin-1a was purchased from Invivogen (San Diego, CA, USA; #puno1-hdectin1a). mCardinal-Lifeact-7 was a gift from Michael Davidson (Addgene, Watertown, MA, USA; plasmid #54663). Transfection with plasmid was performed following standard protocols for FuGENE 6 (Promega, Madison, WI, USA; #E2691). Cells were selected for stable expression using Geneticin (G418 sulfate) (Thermo Fisher Scientific, Waltham, MA, USA; #10131035; for pEGFP-DC-SIGN) at 400 μg/mL or Blasticidin (Invivogen, San Diego, CA, USA; #ant-bl-05; for pUNO1-hDectin-1a) at 20 μg/mL for 2 weeks.
2.3. Micropipette
The 1.5 mm outer diameter and 1.12 mm inner diameter borosilicate glass capillaries were purchased from World Precision Instruments (Sarasota, FL, USA; # TW150-4). We optimized fabrication procedures to obtain micropipettes with 2 μm diameter tips. We used a microelectrode puller (World Precision Instruments, Sarasota, FL, USA; #micrPUL-1000) for pulling micropipettes. Our final protocol for pulling micropipette of 2 μm is shown in
Table 1.
2.4. Fungal Culture
C. albicans clinical isolate TRL035 was obtained as previously described [
13]. The isolate was stored as single-use glycerol stock aliquots −80 °C. This stock was transferred to 5 mL of sterile yeast extract-peptone-dextrose (YPD) medium (Becton Dickinson, Franklin Lakes, NJ, USA; #242820) at a concentration of 1 × 10
5 cell/mL of YPD and grown for 16 h at 30 °C, with a shaking speed of 300 rpm. The glycerol stock contained 4 × 10
7 yeast/mL and was previously calibrated to provide 3 × 10
8/mL yeast cells at the late log phase under the stated growth conditions.
2.5. Silicone Chambers
We used silicone isolators (Grace Bio-labs, Bend, OR, USA; # 665116 and # 665203) on cover glass in configurations as shown below (
Figure 1). The whole chamber was sterilized by passing it through a Bunsen burner blue flame 5 times.
2.6. Contact Site Studies
HEK-293 cells were transfected with mApple-Dectin1A-C-10 and stable lines were generated, as described above. mApple-Dectin1A-C-10 stable line cells were transiently transfected with pEGFP-DC-SIGN 2 days before each experiment. On the next day, cells and yeast were seeded into separate compartments of sterilized silicone chambers in a configuration as shown below (
Figure 2).
Cells were stained with CellMask Deep Red Plasma Membrane stain (CMDR) (Thermo Fisher Scientific, Waltham, MA, USA; #C10046). Original stock CMDR was freshly diluted 1:100 in the culture medium to form a working stock solution. A volume of 10 μL of working stock CMDR was added per 1 mL of the culture medium in the culture vessel. Depending on the condition, along with CMDR, various inhibitors were added during this stage. Blebbistatin (Sigma-Aldrich, St. Louis, MO, USA; #203390) at 12.5 μM, or Y-27632 (Sigma-Aldrich, St. Louis, MO, USA; #Y0503) at 5 μM, was added for 1 h. For RHO inhibitor conditions, cells were treated with C3 transferase (Cytoskeleton Inc., Denver, CO, USA; #CT04) 1.5 μg/mL for 2 h. Both CMDR and inhibitor were added to the culture dish at the same time. TRL035
C. albicans was stained with Fluorescent Brightener 28 (Calcofluor White; Sigma-Aldrich, St. Louis, MO, USA; #F3543). A volume of 25 μL of 1 mg/mL Calcofluor White was used to stain 1 mL of yeast in PBS pH 7.4 for 15 min. Then yeast was washed three times with PBS and vortexed for 15 min.
C. albicans was added to smaller chambers as shown in
Figure 2. Glucose oxidase (Sigma-Aldrich, St. Louis, MO, USA; # G2133) and catalase (Sigma-Aldrich St. Louis, MO, USA; # C100) were added to the chamber at a concentration of 0.5 and 40 μg/mL, respectively, during data acquisition to reduce photobleaching.
Micropipettes were filled with PBS using MicroFil (World Precision Instruments, Sarasota, FL, USA; #MF28G-5) and syringe. The PBS-filled micropipette was then attached to the microelectrode holder (World Precision Instruments, Sarasota, FL, USA; # MPH415). The inlet of the microelectrode holder was attached to the 1 mL syringe using Luer lock via plastic tubing.
For micromanipulation, we used a Sensapex (Oulu, Finland) uMp micromanipulator. We attached the microelectrode holder to the micromanipulator using an electrode handle (World Precision Instruments, Sarasota, FL, USA; #2505). The silicon chamber was then placed on the FV1000 laser-scanning confocal microscope (Olympus, Center Valley, PA, USA) with the temperature controlled at 37 °C, at 5% CO2. We used a 60× super-corrected, 1.40 NA, Plan-Apochromat oil immersion objective for imaging cells. We replaced one microscope eye piece with a Centering Telescope eyepiece (Olympus, Center Valley, PA, USA; #U-CT30-2), which allowed a separate focal plane in each eyepiece for ease in positioning the micropipette.
We identified a suitable single C. albicans yeast for capture and then adjusted the microscope’s focal plane to ~20 μm above that yeast. We focused the Centering Telescope eyepiece on the tip of micropipette. The telescope eyepiece was then focused at a plane closer to the yeast, then we lowered the tip of micropipette via micromanipulator into the lower focal plane toward the yeast, while being monitored through the telescopic eyepiece. This process was repeated until we reached the level of fungus. This was performed to avoid breaking of micropipette tip while lowering it. We always lowered the telescopic eyepiece focus first and then adjusted the micropipette level. Once at the same level as C. albicans, we applied negative pressure using the syringe to capture a single yeast on the micropipette tip. Then, we manipulated C. albicans to the chamber with HEK-293 cells by moving from the yeast chamber, over the isolator barrier, and translating into the cell chamber, keeping the tip submerged at all times. Finally, C. albicans was brought near the plasma membrane of a HEK-293 cell expressing the appropriate receptors, as verified by their fluorescent protein tags.
Confocal fluorescence microscopic observation of contacts sites was conducted with the following parameters. Calcofluor White (a marker for all yeast) was excited with a 50 mW, 405 nm diode laser operated at 1% power, and CMDR was excited with a 20 mW, 635 nm diode laser operated at 0.5% power. EGFP–DC-SIGN was excited with a 20 mW, 473 nm diode laser operated at 1% power. mApple-Dectin-1 was excited with a 20 mW 559 nm laser at 1% power. These lines were reflected to the specimen by a 405/473/559/ 635 multi-edge main dichroic element and routed through a confocal pinhole (110 mm diameter) to secondary dichroic followed by bandpass emission filters in front of two independent PMT detectors and two independent high-sensitivity GaAsP PMT detectors (HSD). Specifically, the emission light passed by the main dichroic was directed to PMT1 (Calcofluor White channel) via reflection from the SDM473 dichroic and passage through a BA430-455 nm bandpass filter. For the CMDR channel, light from SDM473 was directed to a 640 nm shortpass dichroic and BA575-675 nm bandpass filter. Light from 640 nm shortpass dichroic was directed to SDM560 filter cube to HSD1 (the EGFP–DC-SIGN channel) via passage through a BA490-540 nm bandpass filter. For fluorescence microscopic observation of mApple-Dectin-1, light was directed via a SDM560 filter cube to HSD2 via passage through a BA575–675 nm bandpass filter. A supercorrected 60× oil lens (NA 1.42) with 3× zoom was used to capture images. Further, a subregion of interest for image scanning was selected such that the region was small enough to be scanned at a rate of 0.400 s per frame. Overall, the pixel size for all images was 7.24 pixels per micron. Imaging was started and then, with the micromanipulator, contact was made between C. albicans and HEK-293 cells. The contact site was imaged for 10 min total duration after contact initiation.
2.7. Polystyrene Bead Control for Contact Site Studies
For making Dextran-coated beads, we used 5 μm streptavidin-coated polystyrene beads (Spherotech, Lake Forest, IL, USA; #VP-60-5). We used 1,1′-carbonyldiimidazole (Sigma-Aldrich, St. Louis, MO, USA; #115533) in a DMSO-based system as described by Tam et al. [
6] to conjugate Dextran (Sigma-Aldrich, St. Louis, MO, USA; # 31388) with beads. The rest of the procedure for making contact and imaging was exactly the same as used for TRL035
C. albicans.
2.8. FRAP Studies
For FRAP studies, the exact same steps as mentioned above for the contact site studies were followed. Then the TRL035 contact site was allowed to mature for 10 min after contact. Then a rectangular FRAP window was selected so that it included the whole of the contact site. Imaging was started and 5 frames were collected pre-bleach. Then, we photobleached the contact area with 473 nm and 559 nm lasers, 100% power for 500 ms. Imaging was continued for 10 min to quantify recovery. FRAP analysis was performed using easyFRAP [
14].
2.9. Analysis of Contacts Site Data
For quantifying the contact site, we used the Fiji distribution of ImageJ. We demarcated overlapping pixels between the dilated Calcofluor White channel and the CMDR channel. These overlapping pixels denote the contact site. All further calculation of the Mean Fluorescence Intensity (MFI) for DC-SIGN, Dectin-1 channel and their normalizations were performed from these contact site pixels only. The detailed steps followed were as follows. Calcofluor White (405 channel) was thresholded to make a fungal mask and converted to binary. The binary fungal mask was then dilated 2 times. The fungal mask was divided by 255 to make all pixel values 1. This is essential for calculating overlapping areas in next steps. The CMDR (635 channel) was thresholded and converted to binary to create a CMDR mask. The CMDR mask was divided by 255. The fungal mask was multiplied by CMDR mask to mark overlapping pixels as those demarcating the contact site mask, within which each pixel had a numerical value of 1. The remaining non-mask pixels had a numerical value of 0. Overlapping pixels were multiplied with Dectin-1, DC-SIGN and CMDR raw pixel intensities, creating masked Dectin-1, DC-SIGN and CMDR datasets. The RawDensity, which is the sum of intensities of all pixels in a dataset, was calculated for each of the contact site-masked DC-SIGN, Dectin-1 and CMDR datasets. The same calculation was performed for the contact site mask image, which provides the area (pixels) of the contact site mask. The Mean Fluorescence Intensity (MFI) per pixel for DC-SIGN, and Dectin-1, and CMDR was calculated by dividing the RawDensity for each of these datasets by the contact site area in pixels. To normalize for variable amount of membrane in a contact site, we divided the MFI of DC-SIGN and Dectin-1 by the corresponding contact site CMDR MFI. Finally, to control for possible differential expression/staining of individual HEK-293 cells, we expressed the above normalized receptor MFI signals as a percentage of their value at time 0, on a per cell basis.
2.10. Yeast Capture Assay
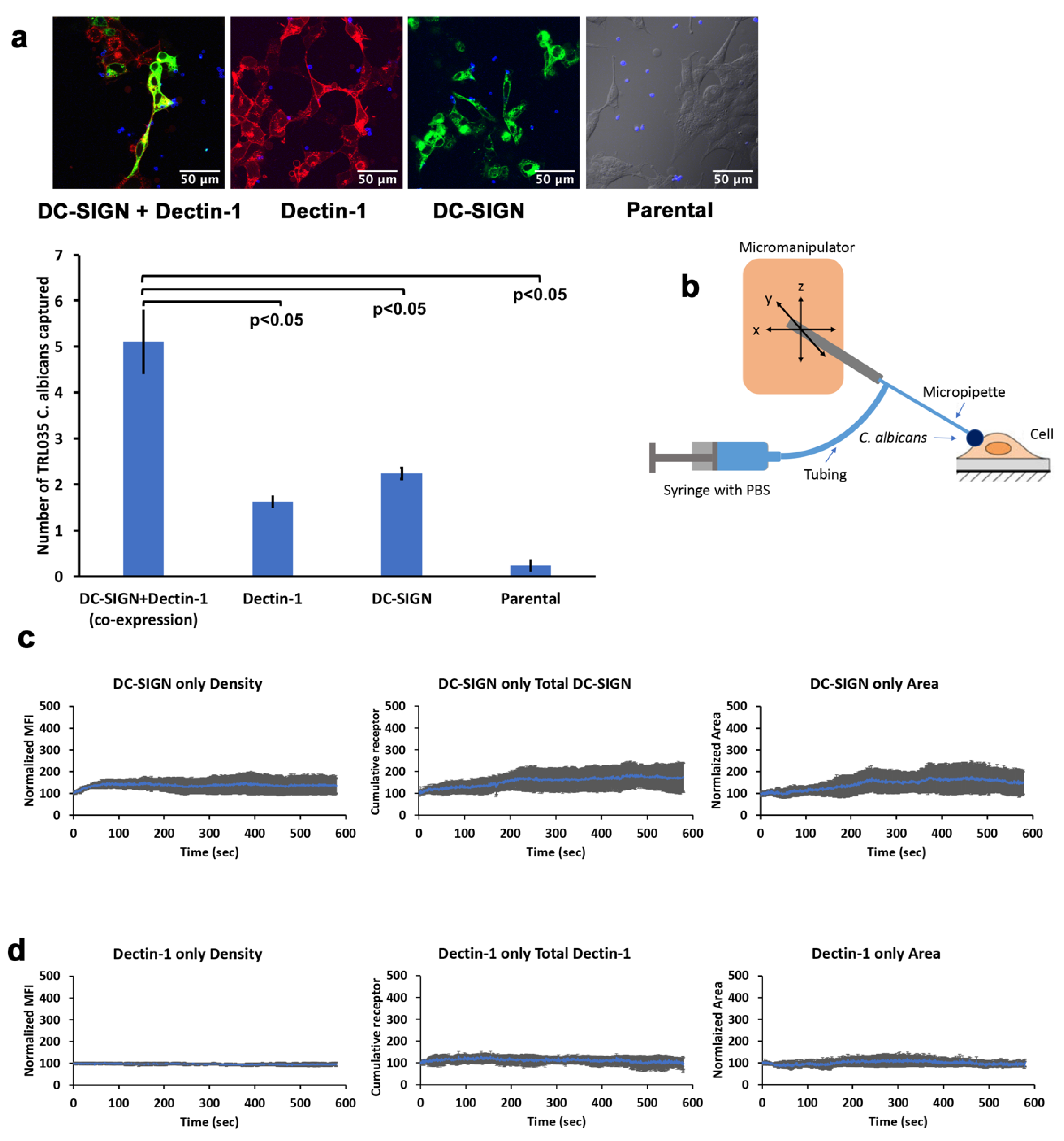
For the yeast capture assay, we used HEK-293 cells and stably transfected mApple-Dectin1A-C-10 cells were used or transiently transfected with pEGFP-DC-SIGN. For the DC-SIGN-only condition, parental cells were transiently transfected with pEGFP-DC-SIGN. Overall, the following 4 conditions were used for experiments, (1) EGFP-DC-SIGN + mApple-Dectin-1, (2) EGFP-DC-SIGN, (3) mApple-Dectin-1, and (4) untransfected. Cells were seeded at 2.5 × 104/dish overnight in 35 mm glass bottom dishes (MatTek, Ashland, MA, USA; #P35G-1.5-14-C). Then TRL035 C. albicans stained with Calcofluor White were added to the dishes at 10 × 104/dish. Then the dishes were kept on rocker shaker (Southwest Science, Roebling, NJ, USA;# BR200) for 30 min. Cells were then washed 3 times with PBS and fixed with 4% PFA, and the number of fungi attached to each cell of interest were counted under microscope with an imaging condition similar to that described in the contact site studies. Binding studies with cells expressing both DC-SIGN and Dectin-1 enumerated yeast binding only to cells confirmed to co-express both receptors.
2.11. TIRF Microscopy
HEK-293 cells were stably transfected with pUNO1-hDectin-1a and mCardinal-LifeAct-7. These cells were transiently transfected with EGFP-DC-SIGN 1 day before experiment. Cells were dissociated from dish surfaces using brief exposure to 0.25% trypsin-EDTA followed by addition of the protein-rich medium to rapidly quench trypsin activity and by washing into a fresh culture medium. Then these cells were put on 35 mm dish coated with β-glucan. Cells were allowed to settle down on these surfaces in an incubator for 30 min. Then the cell membrane was observed under an Olympus (Center Valley, PA, USA) IX83 TIRF/Single Molecule Localization Microscope. The 488 and 561 nm lasers were used to excite EGFP-DC-SIGN and mCardinal-LifeAct, respectively. The cell membrane was observed for 5 min. To address the potential concern that trypsinization would damage transmembrane receptors sufficiently to render cells non-responsive to fungal ligands, we loaded cells with Fluo-4 calcium dye, trypsinized and settled them on glucan-coated glass as above, and observed calcium flux upon Dectin-1 contact with glucan-coated glass. We observed calcium flux on glucan-coated glass but not on glucan-free glass surfaces, demonstrating that the cells remained functional following trypsinization (data not shown).
2.12. Coating Dishes with β-Glucan
The central glass region of the glass bottom 35 mm dishes (MatTek, Ashland, MA, USA; #P35G-1.5-14-C) was coated with β-glucan to permit TIRF microscopic observations of early contact site membrane dynamics using a coupling chemistry previously reported by Tam et al. [
6]. The following procedure was used to produce these surfaces. A volume of 200 μL of 0.01
w/
v poly-l-lysine aqueous solution (Sigma-Aldrich, St. Louis, MO, USA; P4707) was put on the central portion of the 35 mm dishes and adsorption was permitted to occur for 30 min. Excess solution was removed and the dishes were washed with DMSO 3 times. Poly-l-lysine amines were activated by immersion in 0.5 M 1,1′-carbonyldiimidazole (Sigma-Aldrich, St. Louis, MO, USA; #115533) in DMSO for 1 h. The dishes were washed with DMSO 3 times. A 10 mM medium-molecular-weight (145 kDa) β-(1,3)-glucan from ImmunoResearch Inc. (Eagan, MN, USA) in DMSO was added to the dishes. The reaction was allowed to incubate overnight, excess solution was removed, and the dishes were washed with water 3 times.
2.13. Phagocytosis Assay
TRL035
C. albicans was first stained with Fluorescent Brightener 28 (Calcofluor White) (Sigma-Aldrich #F3543). A volume of 25 μL of 1 mg/mL Calcofluor White was used to stain 1 mL of 16 h fungal culture in PBS (Thermo Fisher Scientific, Waltham, MA, USA; #10010023) pH 7.4 for 15 min. Then cells were washed 3 times with PBS and stained with 75 μM CypHer5E NHS ester (Cytiva, Marlborough, MA, USA; #15401) for 1 h at 25 °C [
13].
C. albicans was then added to the culture dishes with HEK-293 cells transiently transfected with pEGFP-DC-SIGN for 1 h and observed under a microscope for increased fluorescence in the CypHer5E channel as an indicator of fungi which had been phagocytosed.
2.14. Agent-Based Modeling
4. Discussion
At
C. albicans contacts with immune cells, which have been described as the “phagocytic synapse”, Goodridge et al. showed the accumulation of Dectin-1 within contact sites between myeloid cell types and model fungal particles and fungal pathogen cells wherein regulatory tyrosine phosphatases CD45 and CD148 were excluded from Dectin-1-rich zones of the contact [
7]. The evident recruitment of immunoreceptors at these cellular synaptic structures and alterations to their patterns of lateral mobility in the membrane suggest that it is important to understand the molecular mechanisms responsible for their construction. In the case of fungal host–pathogen contacts, many studies have examined receptor distribution at tens of minutes to hours due to the difficulty of achieving precise control over contact site formation that is necessary for examining the earliest stages of host–pathogen interaction. We have overcome that problem in this study by the use of micropipette-micromanipulator-based application of fungal particles to cells with high spatiotemporal precision.
We looked at the dynamics of DC-SIGN and Dectin-1 recruitment at the ≤10 min time scale. We propose that events occurring at these earliest stages of contact site formation are important to promote pathogen capture and to stabilize the phagocytic synapse. DC-SIGN showed significant contact site recruitment within the first 10 min, whereas Dectin-1 did not show recruitment within this period, relative to its initial density. DC-SIGN is optimized for high-avidity interactions with fungal pathogens due to its tetramerization via its stalk domain and its organization into multi-tetramer nanodomains [
17,
18,
23]. Improvements in the efficiency of contact site recruitment of DC-SIGN are likely to be important for determining the ability of the phagocytic synapse to retain a fungal pathogen, especially under circumstances where fluid shear forces could destabilize the contact site before internalization of the particle can take place.
In the absence of Dectin-1, there was a significant decrease in the recruitment of DC-SIGN. In recently published work, we showed that Dectin-1 stimulation by β-glucan gives rise to RHOA-mediated actomyosin activation for contractile mechanical force generation [
11]. Previous research on the immunological synapse has demonstrated the importance of RHO-GTPase-mediated actin cytoskeleton organization in adhesion and early immunological synapse formation [
24]. Further, the work of Tsourkas et al. with B cell synapses showed with stochastic simulations that the formation of the synapse occurs only if BCR mobility is enhanced by directed motor-driven transport [
25]. Further, Manzo et al. showed the role of lateral mobility of DC-SIGN nanoclusters in enhancing pathogen binding using Monte Carlo simulations [
18]. Because Dectin-1 activation appeared to enhance DC-SIGN recruitment, we tested the role of RHOA-mediated actomyosin activation downstream of Dectin-1, leading to active recruitment of DC-SIGN to
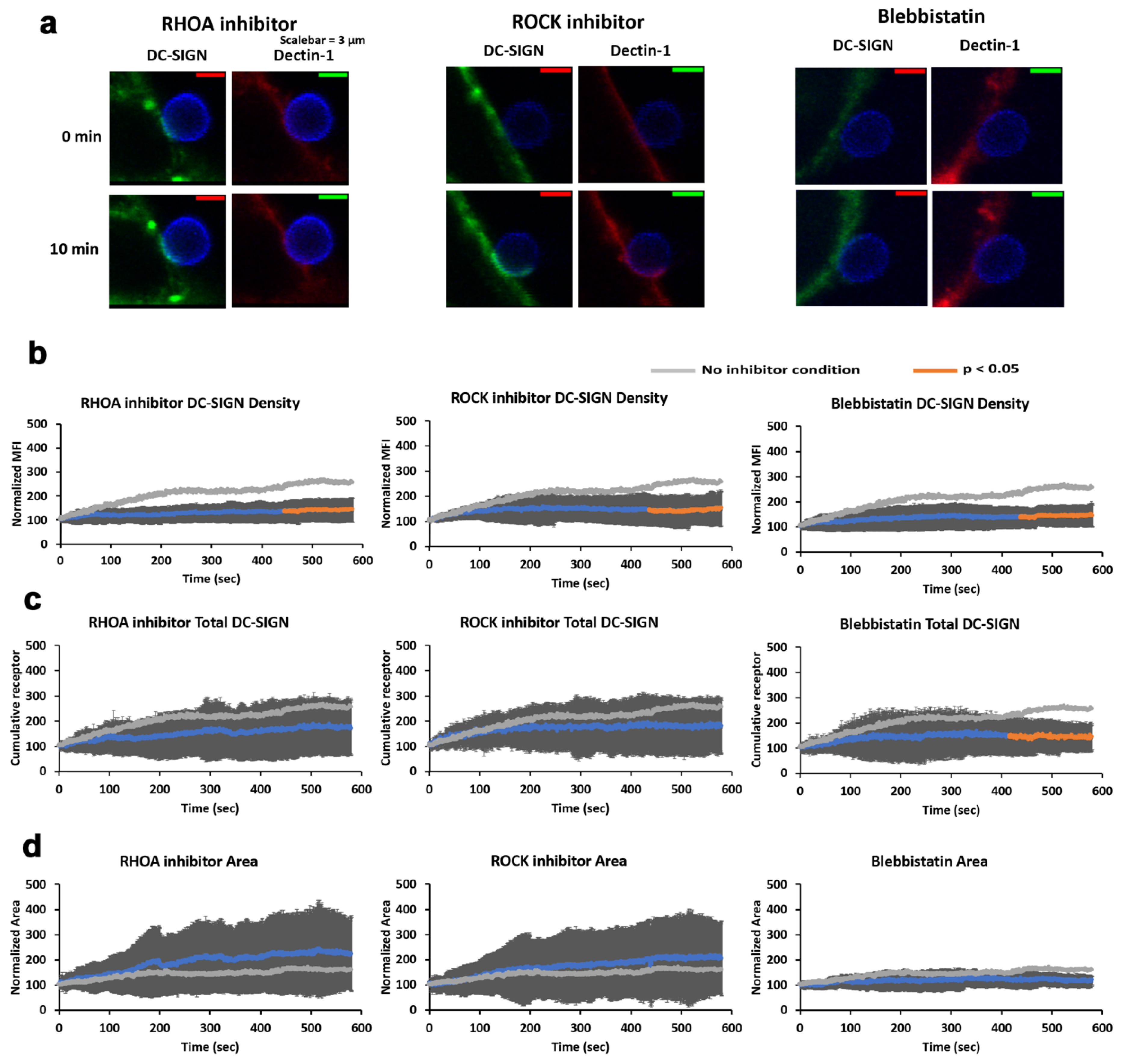
C. albicans contact sites. Using agent-based modeling, we found that a directed transport process for DC-SIGN recruitment was necessary in order for computational predictions of DC-SIGN recruitment kinetics to match experimentally observed rates of DC-SIGN recruitment to host–pathogen contact sites. To further support this finding experimentally, we found that inhibitors of RHOA, ROCK and myosin II decreased DC-SIGN recruitment to contact sites (
Figure 4). The density of contact site DC-SIGN achieved in the presence these inhibitors was similar to the DC-SIGN density predicted by computational modeling of contact site biogenesis under the assumption that DC-SIGN was only transported by passive diffusion followed by trapping in the contact via high-avidity interactions with cell wall N-mannans (
Figure 6). This result strongly suggests that RHOA, ROCK and myosin II are essential components of a Dectin-1-dependent active transport mechanism that enhances DC-SIGN recruitment to the sites of host–pathogen interaction within minutes of pathogen contact.
RHOA and ROCK inhibited contacts exhibited a larger total area than without inhibitor. We expect that the spreading of a cell over a pathogen surface to form a contact site is a net result of protrusive (e.g., RAC1-mediated branched actin in lamellar edges and membrane ruffles) and contractile processes (i.e., RHOA-mediated actomyosin contraction around the contact), both of which are unfolding over the time scale of our studies. The fact that RHOA pathway inhibitors allow greater cell spreading to form larger area contacts is compelling evidence that contractile mechanical forces are being generated during the first ten minutes of host–pathogen interaction with C. albicans.
We did not find significant enhancement of Dectin-1 density in contact sites relative to non-contact membrane within the first 10 min of
C. albicans contact. We think this is because sites of nanoscopic exposures of β-glucan on the
C. albicans wall surface are limited in spatial extent and sparsely distributed. As described by Ostrowski et al. for phagocytic synapse, upon initial clustering of cognate receptors, the ligands on the pathogen surface trigger the signaling pathways that could initiate signaling and phagocytosis [
8]. However, receptor density enrichment and continuous receptor-ligand interaction is required to complete internalization of phagocytic particle, otherwise phagocytosis will stall [
26]. Our results are consistent with a model of
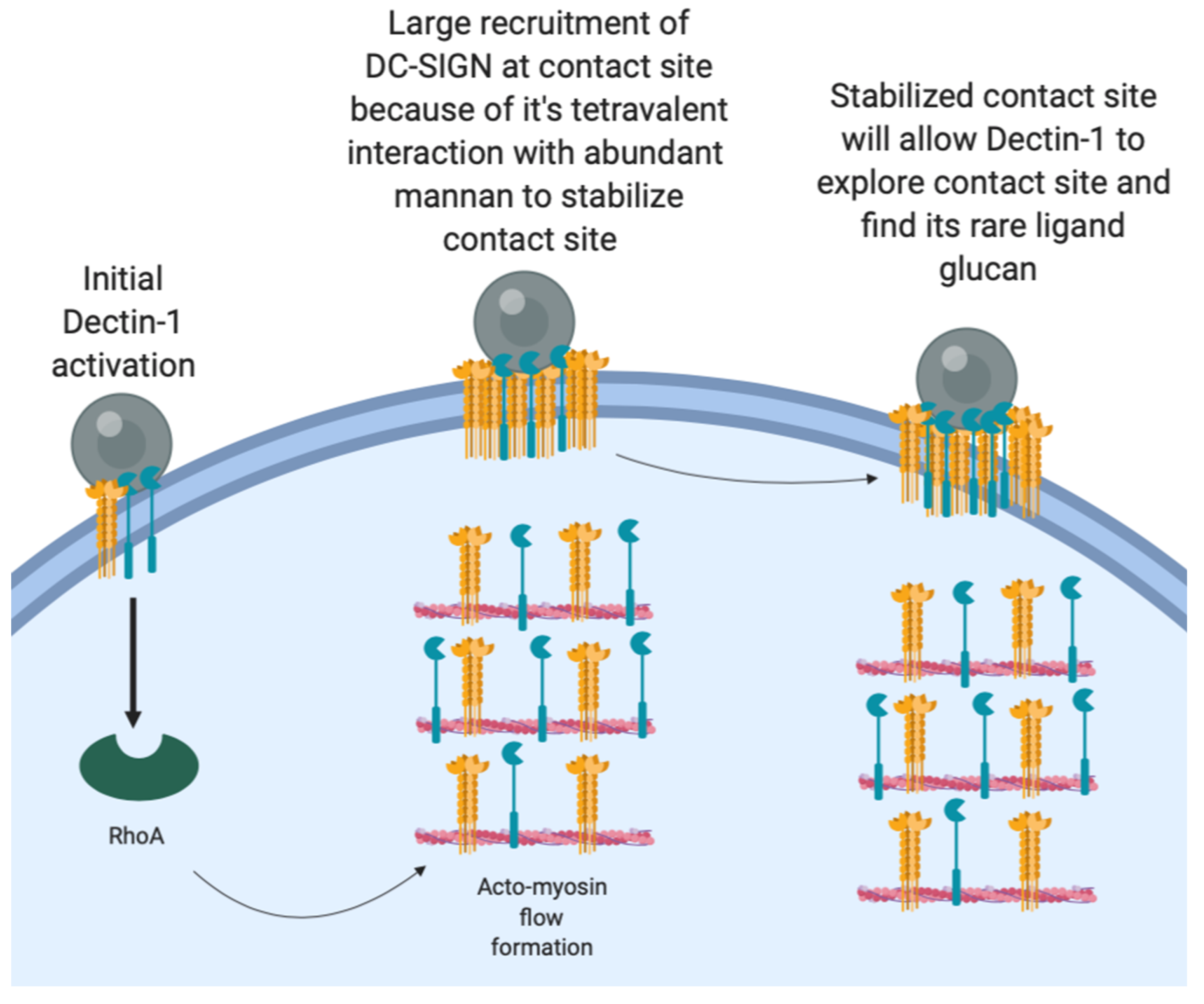
C. albicans recognition wherein initial Brownian diffusion of Dectin-1 leads to rapid Dectin-1 engagement and RHOA-mediated actomyosin flow formation (
Figure 6). Subsequently, DC-SIGN gets coupled to this actomyosin flow, which facilitates its efficient mobilization to the contact site. Soon after being drawn into the contact site, DC-SIGN nanodomains engage in high-avidity interactions with the fungal cell wall surface, which is important for strong retention of the fungal particle. The high fraction of pathogen-interacting DC-SIGN in the contact site is evident from our FRAP results where the majority of DC-SIGN is immobile over the time scale of minutes (
Figure 5). It is this prolonged pathogen retention that allows Dectin-1, the pro-phagocytic receptor, to engage in the more time-consuming process of searching for and integrating signaling from sparse sites of nanoscale glucan exposure. This is evident from a large mobile fraction of Dectin-1 in contacts sites at 10 min. At larger time scales, Dectin-1 may show significant enrichment within phagocytic contacts to amplify its signaling ultimately giving rise to phagocytosis. This is supported by Tam et al. showing accumulation of Dectin-1 within phagocytic synapse with glucan-coated particles at 30 min time scale [
6]. Further, Strijibis et al. showed importance BTK, VAV1 and F-actin accumulation within
C. albicans contact sites for efficient phagocytosis of fungus at an hour scale [
5]. Our results on the earliest stages of innate immune fungal recognition provide some increased insight into host–pathogen contact site evolution, which is evidently a complex, orchestrated process that involves many receptors being are recruited and activated across different time scales.
Yi et al. showed direct evidence for actin retrograde flow and actomyosin II arc contractions playing a role in driving TCR clusters at T-cell immunologic synapses [
10]. We hypothesize that DC-SIGN can be coupled to a similar actomyosin flow in the phagocytic synapse, leading to its early recruitment (
Figure 7).
In contrast to our model, Liu et al. showed a constitutive role of microtubule-based retrograde transport of DC-SIGN nanoclusters to bring pathogens to the perinuclear region for antigen processing [
21]. In this study, DC-SIGN nanoclusters were unladen with pathogen or attached to viral particles. It is possible that microtubule associated transport is a constitutive retrograde transport process involved in receptor recycling or antigen acquisition, but the conditions in Liu et al. did not involve AMF generation because Dectin-1–RHOA axis signaling was absent. Nevertheless, future studies could examine potential the contribution of microtubule-mediated transport of DC-SIGN to nascent fungal contacts. Cambi et al. discuss the possible role of DC-SIGN in direct phagocytosis of
C. albicans by immature dendritic cells [
4]. They show enrichment of DC-SIGN within phagosome. This finding is consistent with DC-SIGN’s prominent role in capturing fungal particles, though it also raises possible pro-phagocytic role of DC-SIGN. However, Rosa et al. showed that DC-SIGN plays a role in binding of zymosan particles but is uninvolved in coordinating phagocytic signaling of those particles. Consistent with our findings, Rosa et al. also highlighted the prominent co-localization of actin and Dectin-1 within zymosan contact site, as would be expected for Dectin-1-mediated actomyosin reorganization at the contact site [
19].
Geijtenbeek et al. and van Gisbergen et al. showed that DC-SIGN plays a role in intercellular adhesion of DCs with T cells [
27,
28]. Using an assay of rapid cellular capture of yeast under fluid shear conditions, we found that when Dectin-1 is co-expressed with DC-SIGN, cells could capture significantly more fungal particles than when any of these receptors are expressed individually. Thus, we concluded that the interplay between Dectin-1 and DC-SIGN is important for optimal fungal capture and retention in early fungal phagocytic contacts. ALS5 is a fungal amyloid mannoprotein adhesin which undergoes reorganization into nanodomains under shear. This reorganization of ALS5 under shear exposes binding sites for DC-SIGN, thus making fungal particle sticky for DC-SIGN binding. Thus, it is possible that early, large recruitment of DC-SIGN will improve avidity of interaction with
Candida through DC-SIGN-ALS5 interactions (or other mannoprotein adhesins) under flow conditions [
29], which could be pursued in future research.
We acknowledge the specificity limitations of Y27632 and C3-trasnferase, which are indeed generally inherent in this type of experimental approach. In our previously published work, we conducted a more extensive analysis tying the RhoA/ROCK/myosin II pathway to Dectin-1-mediated cellular contractile responses [
11]. Considering the role of this contractile pathway in formation of stress fibers which is linked to RHOA subtype [
30] and as mentioned in results 3.2, we do propose the working model that Dectin-1-mediated activation of RHOA/ROCK/myosin II contractile force generation plays role in formation of actomyosin arcs for DC-SIGN recruitment. Future work using a variety of experimental modalities would be helpful to fully test this model.
In conclusion, we showed that Dectin-1-mediated activation of RHOA–ROCK–myosin II axis plays important role in active recruitment of DC-SIGN to C. albicans contact sites. This is important for capture of fungal particles and the formation of stable host–pathogen contact sites.