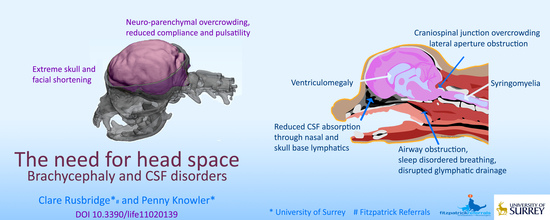
The Need for Head Space: Brachycephaly and Cerebrospinal Fluid Disorders
Abstract
:1. Introduction
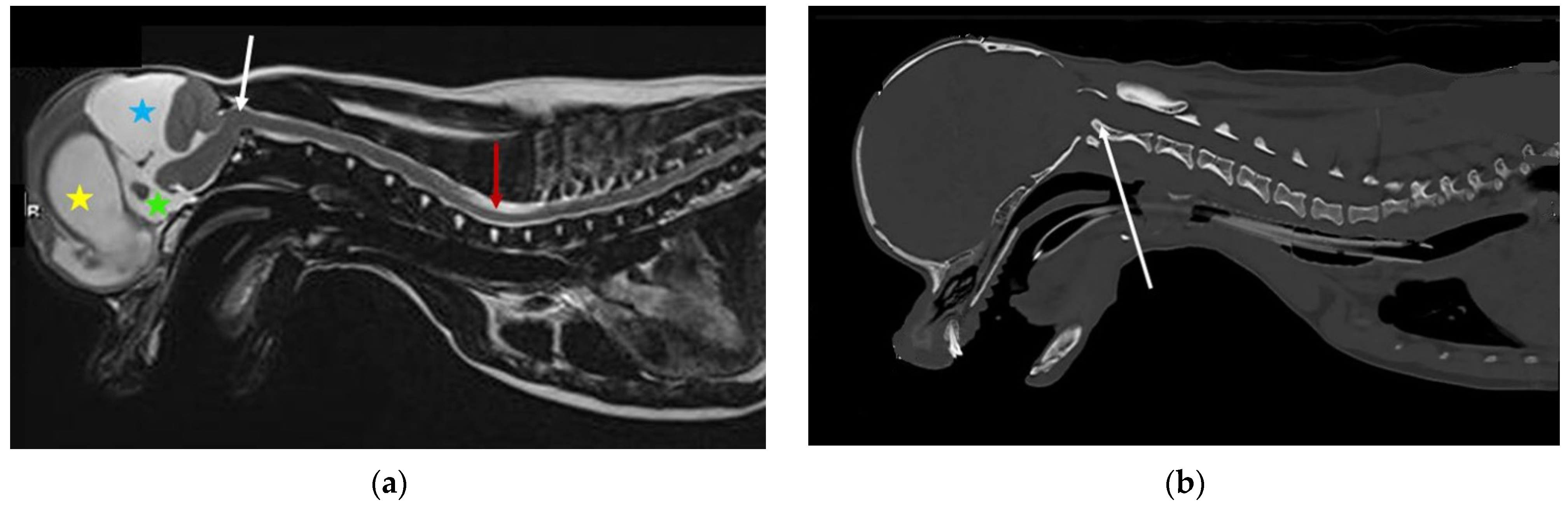
2. Effect of Brachycephaly on Brain Conformation, Compliance, and Pulsatility
3. Cerebrospinal Fluid Absorption and Movement
4. Effect of Brachycephaly on the Absorption of CSF through Lymphatics
4.1. Reduction of the Cribriform Plate and Nasal Mucosa
4.2. Reduction of Skull Base Foramen Volume
5. Cranial Venous Stenosis
6. Effect of Brachycephaly on Respiration and Sleep
6.1. Upper Respiratory Tract Conformation
6.2. Negative Intrathoracic Pressure
7. Effect of Brachycephaly on Craniospinal Junction Conformation
8. Effect of Brachycephaly on Conformation of the Lateral Aperture of the Fourth Ventricle
9. CSF Disorders in Brachycephalic Animals
9.1. Ventriculomegaly and Hydrocephalus
9.1.1. Characteristics
9.1.2. Proposed Contributory Pathophysiology in Brachycephaly
9.2. Quadrigeminal Cistern Expansion
9.2.1. Characteristics
9.2.2. Proposed Contributory Pathophysiology in Brachycephaly
9.3. Chiari-Like Malformation Associated Pain
9.3.1. Characteristics
9.3.2. Proposed Contributory Pathophysiology in Brachycephaly
9.4. Syringomyelia
9.4.1. Characteristics
9.4.2. Proposed Contributory Pathophysiology in Brachycephaly
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Packer, R.M.A.; O’Neill, D.G.; Fletcher, F.; Farnworth, M.J. Great expectations, inconvenient truths, and the paradoxes of the dog-owner relationship for owners of brachycephalic dogs. PLoS ONE 2019, 14, e0219918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skipper, A. The ‘Dog Doctors’ of Edwardian London: Elite Canine Veterinary Care in the Early Twentieth Century. Soc. Hist. Med. 2019. [Google Scholar] [CrossRef] [Green Version]
- Stockyard, C.R. The Genetic and Endocrinic Basis for Differences in Form and Behaviour. In Anatomical Memoirs; Wistar Institute of Anatomy and Biology: Philadelphia, PA, USA, 1941; Volume 19, pp. 40–357. [Google Scholar]
- Geiger, M.; Haussman, S. Cranial Suture Closure in Domestic Dog Breeds and Its Relationships to Skull Morphology. Anat. Rec. 2016, 299, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Volk, H.; Klingler, M.; Failing, K.; Kramer, M.; Ondreka, N. Closure of the cranial base synchondroses in mesaticephalic and brachycephalic dogs in comparison to Cavalier King Charles Spaniels. Vet. Radiol. Ultrasound 2013, 54, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.J.; Kampschulte, M.; Enderlein, S.; Gorgas, D.; Lang, J.; Ludewig, E.; Fischer, A.; Meyer-Lindenberg, A.; Schaubmar, A.R.; Failing, K.; et al. The Relationship between Brachycephalic Head Features in Modern Persian Cats and Dysmorphologies of the Skull and Internal Hydrocephalus. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2017, 31, 1487–1501. [Google Scholar] [CrossRef]
- Lindsay, W.L. Insanity in the Lower Animals. Br. Foreign Med. Chir. Rev. 1871, 48, 178–213. [Google Scholar] [PubMed]
- Taylor, R. Catalogue of the Hunterian Collection in the Museum of the Royal College of Surgeons in London. In Part II The Pathological Preparations in a Dried State; Royal College of Surgeons: London, UK, 1830; p. 39. [Google Scholar]
- Di Ieva, A.; Bruner, E.; Davidson, J.; Pisano, P.; Haider, T.; Stone, S.S.; Cusimano, M.D.; Tschabitscher, M.; Grizzi, F. Cranial sutures: A multidisciplinary review. Child. Nerv. Syst. Chns Off. J. Int. Soc. Pediatric Neurosurg. 2013, 29, 893–905. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Bosmia, A.N.; Cohen-Gadol, A.A. The human calvaria: A review of embryology, anatomy, pathology, and molecular development. Child. Nerv. Syst. 2012, 28, 23–31. [Google Scholar] [CrossRef]
- Virchow, R. Uber den cretinismus, namentilich in franken, und uber pathologische schadelformen. Verh Phys. Med. Ges. Wurzbg. 1851, 2, 230–256. [Google Scholar]
- Roberts, T.; McGreevy, P.; Valenzuela, M. Human induced rotation and reorganization of the brain of domestic dogs. PLoS ONE 2010, 5, e11946. [Google Scholar] [CrossRef] [Green Version]
- Knowler, S.P.; Cross, C.; Griffiths, S.; McFadyen, A.K.; Jovanovik, J.; Tauro, A.; Kibar, Z.; Driver, C.J.; Ragione, R.M.L.; Rusbridge, C. Use of morphometric mapping to characterise symptomatic chiari-like malformation, secondary syringomyelia and associated brachycephaly in the cavalier king charles spaniel. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomycz, L.D.; Hale, A.T.; George, T.M. Emerging Insights and New Perspectives on the Nature of Hydrocephalus. Pediatric Neurosurg. 2017, 52, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Bering, E.A., Jr.; Yagi, M.; Tsugane, R.; Hara, M.; Amano, Y.; Asai, T. Bulk flow in the cerebrospinal fluid system of the dog. Acta Neurol. Scand. 1975, 51, 1–11. [Google Scholar] [CrossRef]
- Orešković, D.; Klarica, M. The formation of cerebrospinal fluid: Nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 2010, 64, 241–262. [Google Scholar] [CrossRef] [Green Version]
- Oi, S.; Di Rocco, C. Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Child. Nerv. Syst. 2006, 22, 662–669. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaca-Carreira, J.; Temel, Y.; Hescham, S.A.; Jahanshahi, A. Transependymal Cerebrospinal Fluid Flow: Opportunity for Drug Delivery? Mol. Neurobiol. 2018, 55, 2780–2788. [Google Scholar] [CrossRef] [Green Version]
- Bulat, M.; Klarica, M. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res. Rev. 2011, 65, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [Green Version]
- Albertini, R.; Bianchi, R. Aquaporins and glia. Curr. Neuropharmacol. 2010, 8, 84–91. [Google Scholar] [CrossRef]
- Thomas, J.H. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J. R. Soc. Interface R. Soc. 2019, 16, 20190572. [Google Scholar] [CrossRef] [Green Version]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [Green Version]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Decker, Y.; Müller, A.; Ineichen, B.V.; Proulx, S.T. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J. Exp. Med. 2019, 216, 2492–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalbe, G. Die Arachnoidalraum, ein Lymphraum und sein Zusammenhang mit dem Perichoriordalraum. Zbl Med. Wiss Zent. Fur. Die Med. Wiss. 1869, 7, 465–467. [Google Scholar]
- Zakharov, A.; Papaiconomou, C.; Johnston, M. Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat. Res. Biol. 2004, 2, 139–146. [Google Scholar] [CrossRef]
- Leeds, S.E.; Kong, A.K.; Wise, B.L. Alternative pathways for drainage of cerebrospinal fluid in the canine brain. Lymphology 1989, 22, 144–146. [Google Scholar] [PubMed]
- Sokolowski, W.; Czubaj, N.; Skibniewski, M.; Barszcz, K.; Kupczynska, M.; Kinda, W.; Kielbowicz, Z. Rostral cranial fossa as a site for cerebrospinal fluid drainage—volumetric studies in dog breeds of different size and morphotype. BMC Vet. Res. 2018, 14, 162. [Google Scholar] [CrossRef]
- Wagner, F.; Ruf, I. “Forever young”—Postnatal growth inhibition of the turbinal skeleton in brachycephalic dog breeds (Canis lupus familiaris). Anat. Rec. 2021. [Google Scholar] [CrossRef]
- Mollanji, R.; Bozanovic-Sosic, R.; Zakharov, A.; Makarian, L.; Johnston, M.G. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. American journal of physiology. Regul. Integr. Comp. Physiol. 2002, 282, R1593–R1599. [Google Scholar] [CrossRef] [Green Version]
- Mollanji, R.; Bozanovic-Sosic, R.; Silver, I.; Li, B.; Kim, C.; Midha, R.; Johnston, M. Intracranial pressure accommodation is impaired by blocking pathways leading to extracranial lymphatics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1573–R1581. [Google Scholar] [CrossRef] [Green Version]
- Knowler, S.P.; Dumas, E.; Spiteri, M.; McFadyen, A.K.; Stringer, F.; Wells, K.; Rusbridge, C. Facial changes related to brachycephaly in Cavalier King Charles Spaniels with Chiari-like malformation associated pain and secondary syringomyelia. J. Vet. Intern. Med. 2020, 34, 237–246. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.-H.; Kim, S.H.; Ham, J.-S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.J.; Ondreka, N.; Rummel, C.; Volk, H.; Sauerbrey, M.; Kramer, M. Volume reduction of the jugular foramina in Cavalier King Charles Spaniels with syringomyelia. BMC Vet. Res. 2012, 8, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusbridge, C.; Knowler, S.P.; Pieterse, L.; McFadyen, A.K. Chiari-like malformation in the Griffon Bruxellois. J. Small Anim. Pract. 2009, 50, 386–393. [Google Scholar] [CrossRef]
- Brühl, K.; Stoeter, P.; Wietek, B.; Schwarz, M.; Humpl, T.; Schumacher, R.; Spranger, J. Cerebral spinal fluid flow, venous drainage and spinal cord compression in achondroplastic children: Impact of magnetic resonance findings for decompressive surgery at the cranio-cervical junction. Eur. J. Pediatr. 2001, 160, 10–20. [Google Scholar] [CrossRef]
- Fenn, J.; Schmidt, M.J.; Simpson, H.; Driver, C.J.; Volk, H.A. Venous sinus volume in the caudal cranial fossa in Cavalier King Charles spaniels with syringomyelia. Vet. J. 2013, 197, 896–897. [Google Scholar] [CrossRef]
- McGonnell, I.M.; Akbareian, S.E. Like a hole in the head: Development, evolutionary implications and diseases of the cranial foramina. Semin. Cell Dev. Biol. 2019, 91, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischfield, M.A.; Robson, C.D.; Gilette, N.M.; Chim, S.M.; Sofela, F.A.; DeLisle, M.M.; Gelber, A.; Barry, B.J.; MacKinnon, S.; Dagi, L.R.; et al. Cerebral Vein Malformations Result from Loss of Twist1 Expression and BMP Signaling from Skull Progenitor Cells and Dura. Dev. Cell 2017, 42, 445–461.e445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.C.; Troconis, E.L.; Kalmar, L.; Price, D.J.; Wright, H.E.; Adams, V.J.; Sargan, D.R.; Ladlow, J.F. Conformational risk factors of brachycephalic obstructive airway syndrome (BOAS) in pugs, French bulldogs, and bulldogs. PLoS ONE 2017, 12, e0181928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, R.M.; Tivers, M.S. Strategies for the management and prevention of conformation-related respiratory disorders in brachycephalic dogs. Vet. Med. 2015, 6, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, J.A.; Holt, D.E.; Reetz, J.A.; Clarke, D.L. Signalment, clinical presentation, concurrent diseases, and diagnostic findings in 28 dogs with dynamic pharyngeal collapse (2008–2013). J. Vet. Intern. Med. 2015, 29, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Facin, A.C.; Uscategui, R.A.R.; Maronezi, M.C.; Pavan, L.; Menezes, M.P.; Montanhim, G.L.; Camacho, A.A.; Feliciano, M.A.R.; Moraes, P.C. Liver and spleen elastography of dogs affected by brachycephalic obstructive airway syndrome and its correlation with clinical biomarkers. Sci. Rep. 2020, 10, 16156. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Tivers, M.S.; Burn, C.C. Impact of Facial Conformation on Canine Health: Brachycephalic Obstructive Airway Syndrome. PLoS ONE 2015, 10, e0137496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, S.; Miyazaki, M.; Yamashita, Y.; Ouyang, C.; Yui, M.; Nakahashi, M.; Shimizu, S.; Aoki, I.; Morohoshi, Y.; McComb, J.G. Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinberg, D.A.; Mark, A.S. Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology 1987, 163, 793–799. [Google Scholar] [CrossRef]
- Bert, R.J.; Settipalle, N.; Tiwana, E.; Muddasani, D.; Nath, R.; Wellman, B.; Mihlon, F.; Negahdar, M.; Amini, A.; Boakye, M. The relationships among spinal CSF flows, spinal cord geometry, and vascular correlations: Evidence of intrathecal sources and sinks. American journal of physiology. Regul. Integr. Comp. Physiol. 2019, 317, R470–R484. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Sugita, Y.; Iijima, S.; Teshima, Y.; Shimizu, T.; Nishimura, N.; Tsutsumi, T.; Hayashi, H.; Kaneda, H.; Hishikawa, Y. Marked episodic elevation of cerebrospinal fluid pressure during nocturnal sleep in patients with sleep apnea hypersomnia syndrome. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 214–219. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Fung, S.H.; Zhang, Y.J.; Verma, A.K. Sleep-Disordered Breathing and Idiopathic Normal-Pressure Hydrocephalus: Recent Pathophysiological Advances. Curr. Neurol. Neurosci. Rep. 2019, 19, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauglund, N.L.; Pavan, C.; Nedergaard, M. Cleaning the sleeping brain—The potential restorative function of the glymphatic system. Curr. Opin. Physiol. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Reidenberg, J.S.; Laitman, J.T. Effect of basicranial flexion on larynx and hyoid position in rats: An experimental study of skull and soft tissue interactions. Anat. Rec. 1991, 230, 557–569. [Google Scholar] [CrossRef]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, D.A.N. Growth of the Cranial Base. In Fundamentals of Craniofacial Growth; Dixon, A.D., Hoyte, D.A.N., Ronning, O., Eds.; CRC Pres LLC: Boca Raton, FL, USA, 1997; pp. 258–333. [Google Scholar]
- Dubrul, E.L.; Laskin, D.M. Preadaptive potentialities of the mammalian skull: An experiment in growth and form. Am. J. Anat. 1961, 109, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Knowler, S.P.; Galea, G.L.; Rusbridge, C. Morphogenesis of Canine Chiari Malformation and Secondary Syringomyelia: Disorders of Cerebrospinal Fluid Circulation. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Donnally, I.C.; Munakomi, S.; Varacallo, M. Basilar Invagination. In StatPearls, StatPearls Publishing Copyright © 2020; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Shoja, M.M.; Ramdhan, R.; Jensen, C.J.; Chern, J.J.; Oakes, W.J.; Tubbs, R.S. Embryology of the craniocervical junction and posterior cranial fossa, part I: Development of the upper vertebrae and skull. Clin. Anat. 2018, 31, 466–487. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M.F. The Role of the Craniocervical Junction in Craniospinal Hydrodynamics and Neurodegenerative Conditions. Neurol. Res. Int. 2015, 2015, 794829. [Google Scholar] [CrossRef] [Green Version]
- Williams, B. Pathogenesis of syringomyelia. Acta Neurochir. 1993, 123, 159–165. [Google Scholar] [CrossRef]
- Rai, S.K.; Rai, P. Volume change theory for syringomyelia: A new perspective. Asian J. Neurosurg. 2015, 10, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLahunta, A.; Glass, E.; Kent, M. Cerebrospinal Fluid and Hydrocephalous. In Veterinary Neuroanatomy and Clinical Neurology, 4th ed.; Elsevier: St Louis, MO, USA, 2014; pp. 78–101. [Google Scholar]
- Rekate, H.L. The definition and classification of hydrocephalus: A personal recommendation to stimulate debate. Cereb. Fluid Res. 2008, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolini, G.; Ricciardi, M.; Caldin, M. Multidetector computed tomographic and low-field magnetic resonance imaging anatomy of the quadrigeminal cistern and characterization of supracollicular fluid accumulations in dogs. Vet. Radiol. Ultrasound 2016, 57, 259–268. [Google Scholar] [CrossRef]
- Matiasek, L.A.; Platt, S.R.; Shaw, S.; Dennis, R. Clinical and magnetic resonance imaging characteristics of quadrigeminal cysts in dogs. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2007, 21, 1021–1026. [Google Scholar] [CrossRef]
- Fenstermacher, J.D.; Ghersi-Egea, J.F.; Finnegan, W.; Chen, J.L. The rapid flow of cerebrospinal fluid from ventricles to cisterns via subarachnoid velae in the normal rat. Acta neurochirurgica. Supplement 1997, 70, 285–287. [Google Scholar] [CrossRef]
- Yoon, J.S.; Nam, T.K.; Kwon, J.T.; Park, S.W.; Park, Y.S. CSF flow pathways through the ventricle-cistern interfaces in kaolin-induced hydrocephalus rats-laboratory investigation. Child. Nerv. Syst. Chns Off. J. Int. Soc. Pediatric Neurosurg. 2015, 31, 2277–2281. [Google Scholar] [CrossRef]
- Rusbridge, C. New considerations about Chiari-like malformation, syringomyelia and their management. Practice 2020, 42, 252–267. [Google Scholar] [CrossRef]
- Rusbridge, C.; McFadyen, A.K.; Knower, S.P. Behavioral and clinical signs of Chiari-like malformation-associated pain and syringomyelia in Cavalier King Charles spaniels. J. Vet. Intern. Med. 2019, 33, 2138–2150. [Google Scholar] [CrossRef]
- Knowler, S.P.; Kiviranta, A.-M.; McFadyen, A.K.; Jokinen, T.S.; La Ragione, R.M.; Rusbridge, C. Craniometric analysis of the hindbrain and craniocervical junction of chihuahua, affenpinscher and cavalier king charles spaniel dogs with and without syringomyelia secondary to chiari-like malformation. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Cross, H.R.; Cappello, R.; Rusbridge, C. Comparison of cerebral cranium volumes between cavalier King Charles spaniels with Chiari-like malformation, small breed dogs and Labradors. J. Small Anim. Pract. 2009, 50, 399–405. [Google Scholar] [CrossRef]
- Shaw, T.A.; McGonnell, I.M.; Driver, C.J.; Rusbridge, C.; Volk, H.A. Increase in cerebellar volume in Cavalier King Charles Spaniels with Chiari-like malformation and its role in the development of syringomyelia. PLoS ONE 2012, 7, e33660. [Google Scholar] [CrossRef]
- Vadivelu, S.; Bolognese, P.; Milhorat, T.H.; Mogilner, A.Y. Occipital neuromodulation for refractory headache in the Chiari malformation population. Prog. Neurol. Surg. 2011, 24, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.; Loftus, J.R.; Oliu, C.J.; Bagci, A.M.; Lee, S.H.; Ertl-Wagner, B.; Sekula, R.; Lichtor, T.; Green, B.A. Imaging-Based Features of Headaches in Chiari Malformation Type I. Neurosurgery 2015, 77, 96–103; discussion 103. [Google Scholar] [CrossRef] [Green Version]
- Brodbelt, A. The Biochemistry of Syringomyelia. In Syringomyelia: A Disorder of CSF Circulation; Flint, G., Rusbridge, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–278. [Google Scholar]
- Nalborczyk, Z.R.; McFadyen, A.K.; Jovanovik, J.; Tauro, A.; Driver, C.J.; Fitzpatrick, N.; Knower, S.P.; Rusbridge, C. MRI characteristics for “phantom” scratching in canine syringomyelia. BMC Vet. Res. 2017, 13, 340. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.J.; Loughin, C.A.; Dewey, C.W.; Marino, L.J.; Sackman, J.J.; Lesser, M.L.; Akerman, M.B. Morphometric features of the craniocervical junction region in dogs with suspected Chiari-like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am. J. Vet. Res. 2012, 73, 105–111. [Google Scholar] [CrossRef]
- Cerda-Gonzalez, S.; Olby, N.J.; Griffith, E.H. Medullary position at the craniocervical junction in mature cavalier king charles spaniels: Relationship with neurologic signs and syringomyelia. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 2015, 29, 882–886. [Google Scholar] [CrossRef]
- Kiviranta, A.-M.; Rusbridge, C.; Laitinen-Vapaavuori, O.; Hielm-Björkman, A.; Lappalainen, A.K.; Knowler, S.P.; Jokinen, T.S. Syringomyelia and Craniocervical Junction Abnormalities in Chihuahuas. J. Vet. Intern. Med. 2017, 31, 1771–1781. [Google Scholar] [CrossRef] [Green Version]
- Cirovic, S.; Lloyd, R.; Jovanovik, J.; Volk, H.A.; Rusbridge, C. Computer simulation of syringomyelia in dogs. BMC Vet. Res. 2018, 14, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, C.R.; Robertson, I.; Olby, N.J. Morphometric analysis of spinal cord termination in Cavalier King Charles Spaniels. J. Vet. Intern. Med. 2019, 33, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, M. The Filling Mechanism. In Syringomyelia: A Disorder of CSF Circulation; Flint, G., Rusbridge, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 87–101. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusbridge, C.; Knowler, P. The Need for Head Space: Brachycephaly and Cerebrospinal Fluid Disorders. Life 2021, 11, 139. https://doi.org/10.3390/life11020139
Rusbridge C, Knowler P. The Need for Head Space: Brachycephaly and Cerebrospinal Fluid Disorders. Life. 2021; 11(2):139. https://doi.org/10.3390/life11020139
Chicago/Turabian StyleRusbridge, Clare, and Penny Knowler. 2021. "The Need for Head Space: Brachycephaly and Cerebrospinal Fluid Disorders" Life 11, no. 2: 139. https://doi.org/10.3390/life11020139
APA StyleRusbridge, C., & Knowler, P. (2021). The Need for Head Space: Brachycephaly and Cerebrospinal Fluid Disorders. Life, 11(2), 139. https://doi.org/10.3390/life11020139