A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): “Lactosome” Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy
Abstract
:1. Introduction
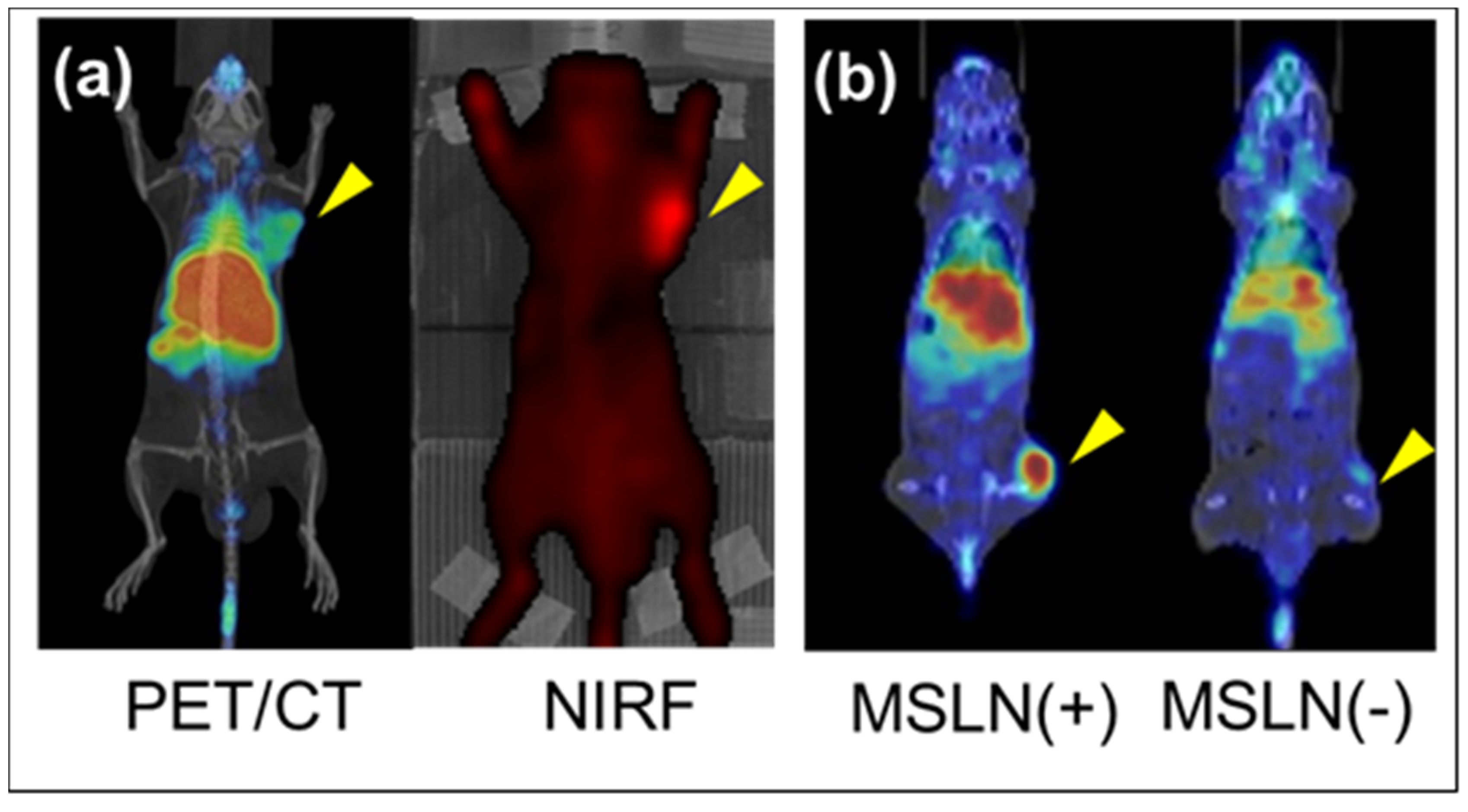
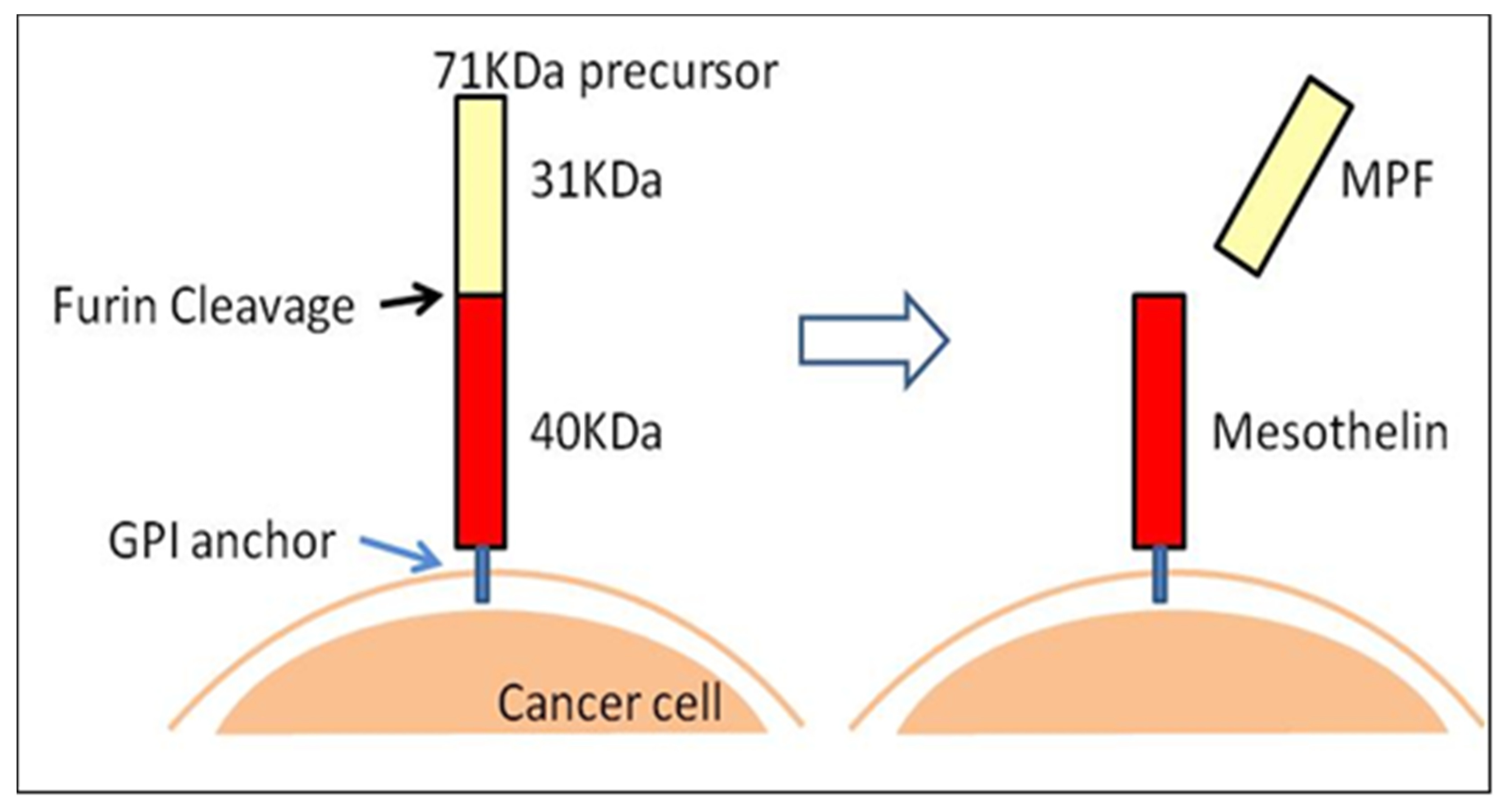
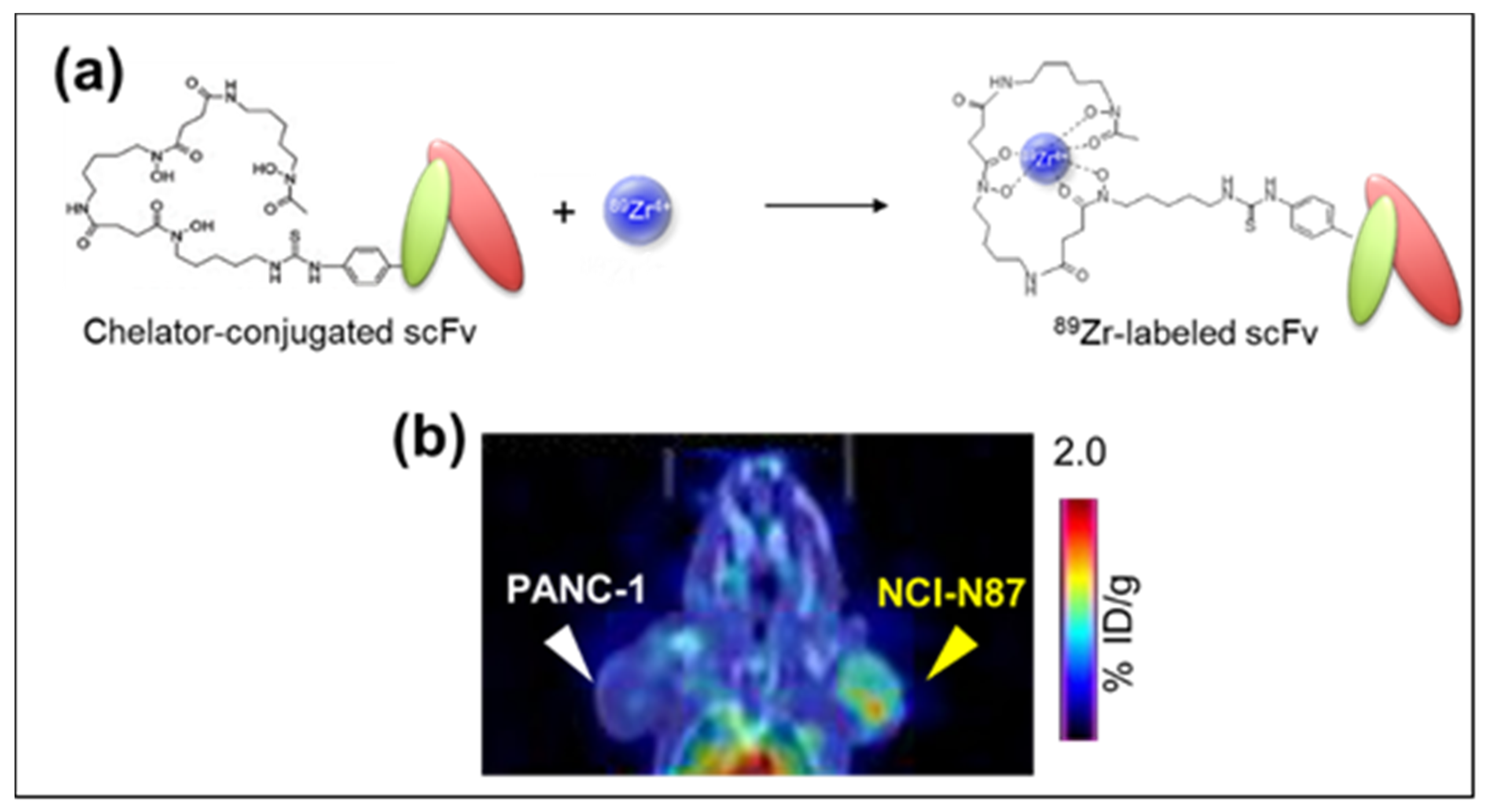
2. 89Zr-labeled PET Imaging and Anti-Mesothelin (MSLN) Single Chain Variable Fragment (scFv)
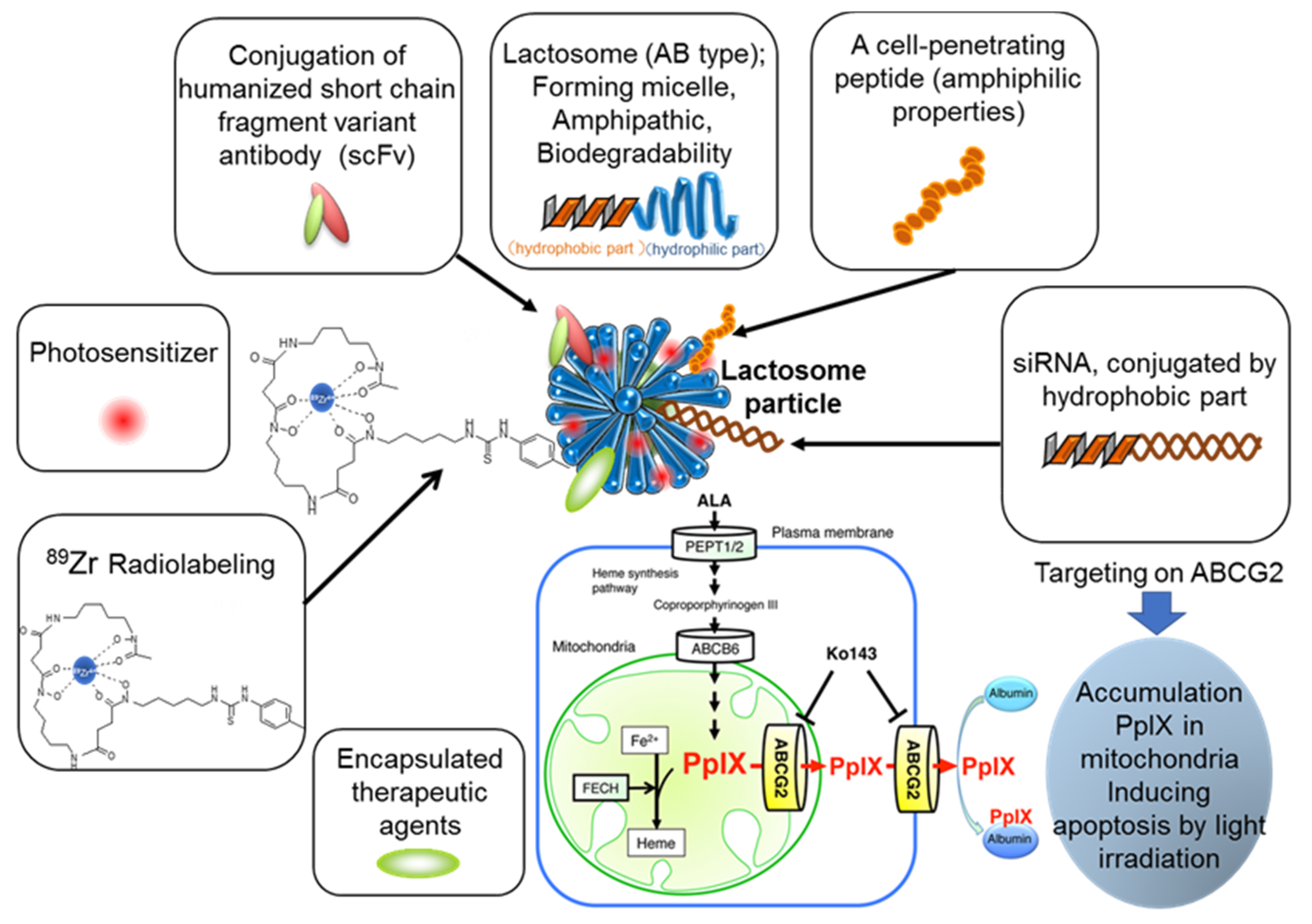
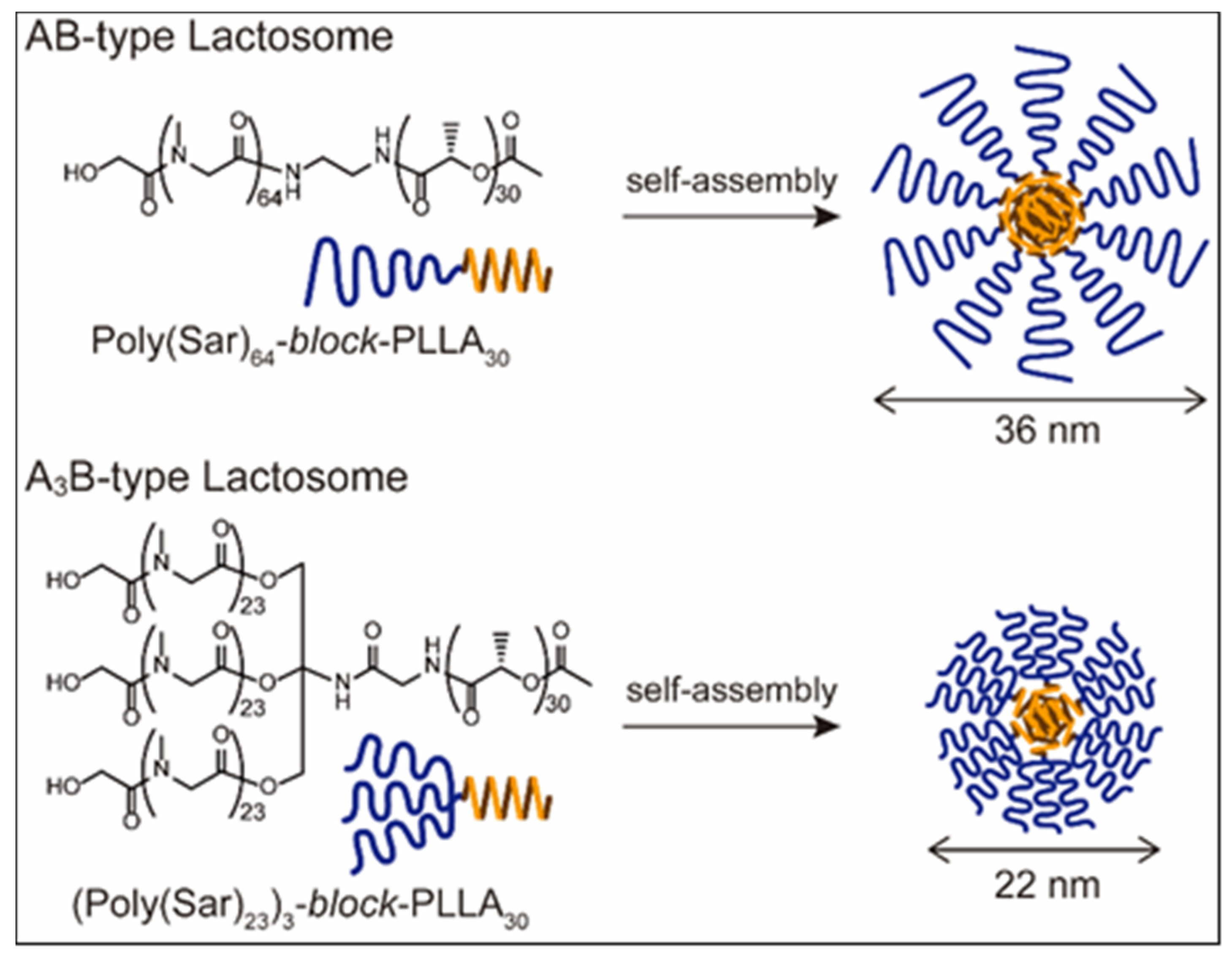
3. Polymeric Micelle-Type DDS Carrier as the “Core” of Theranostics Technology
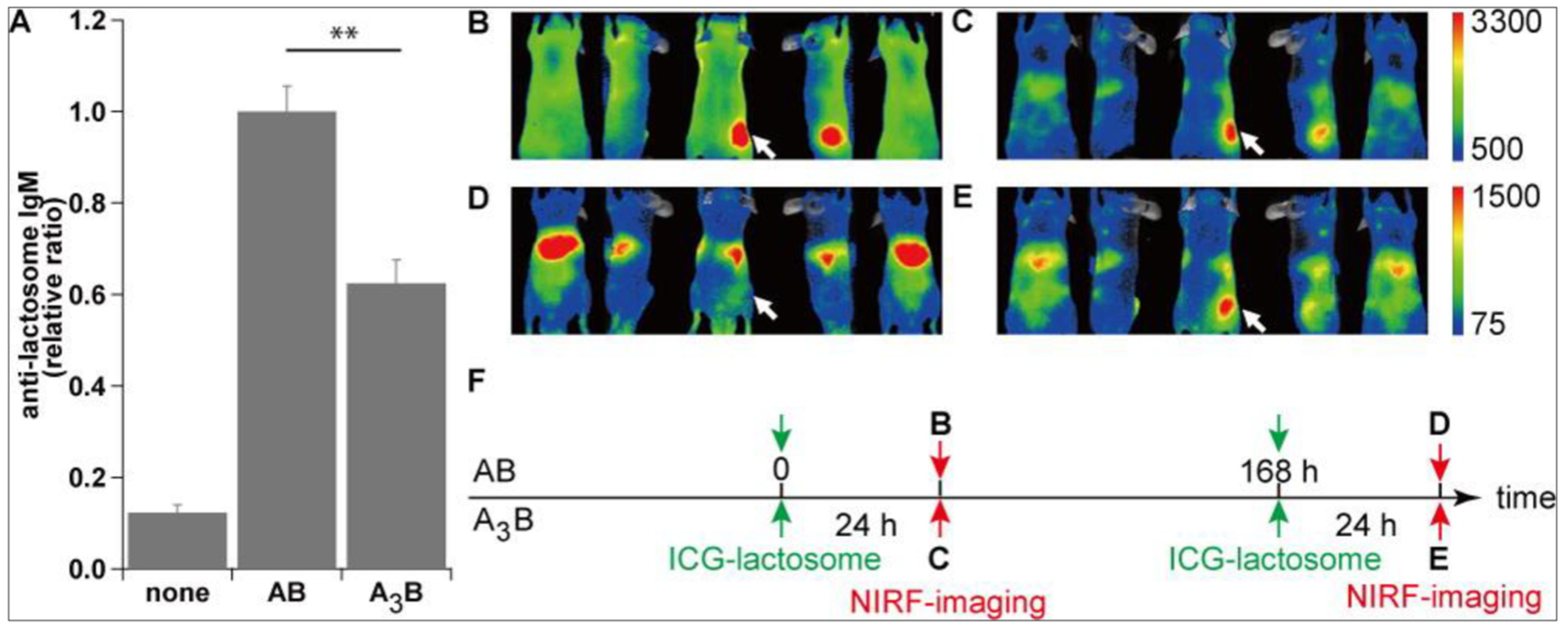
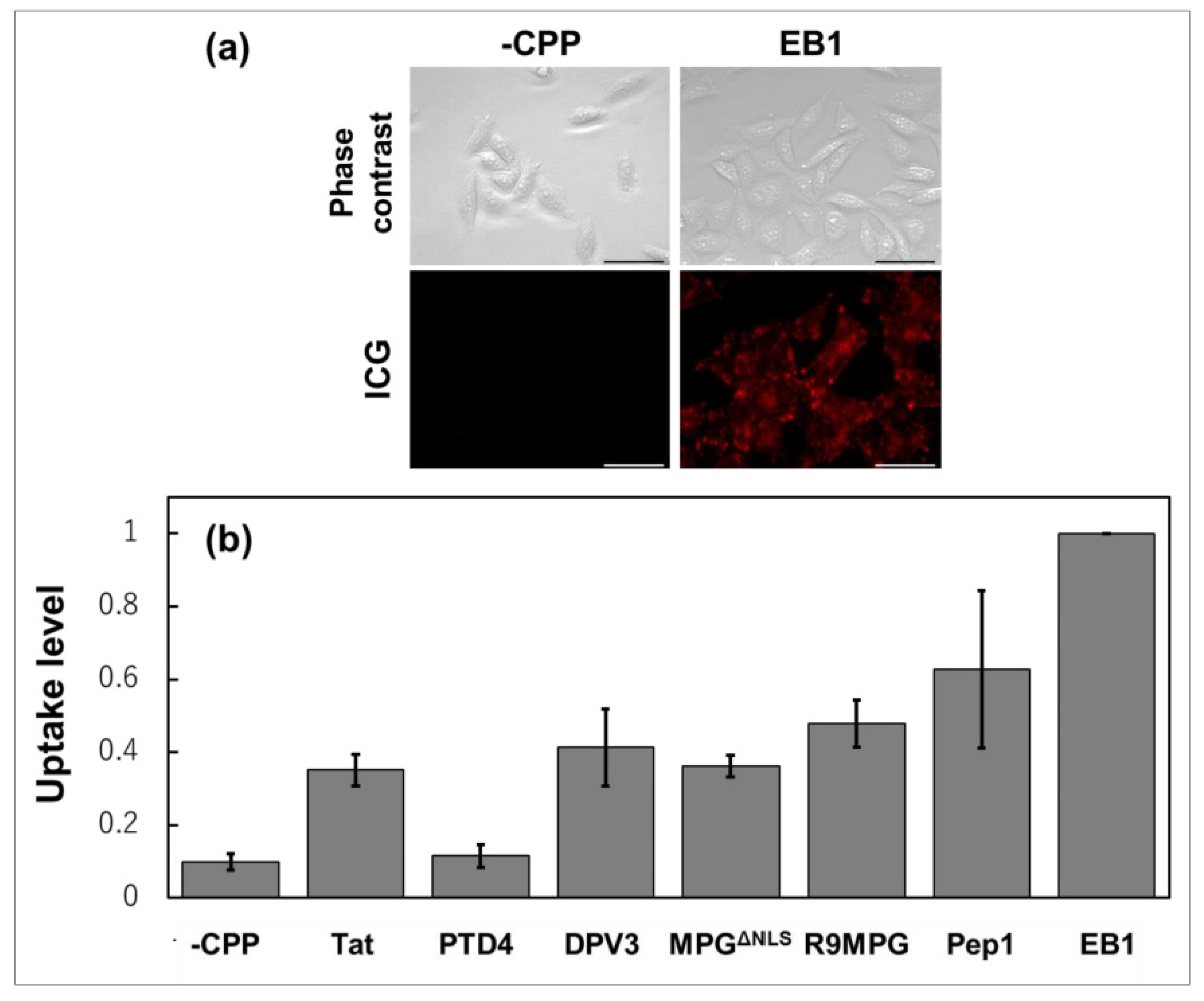
4. Lactosome Mediated RNAi.
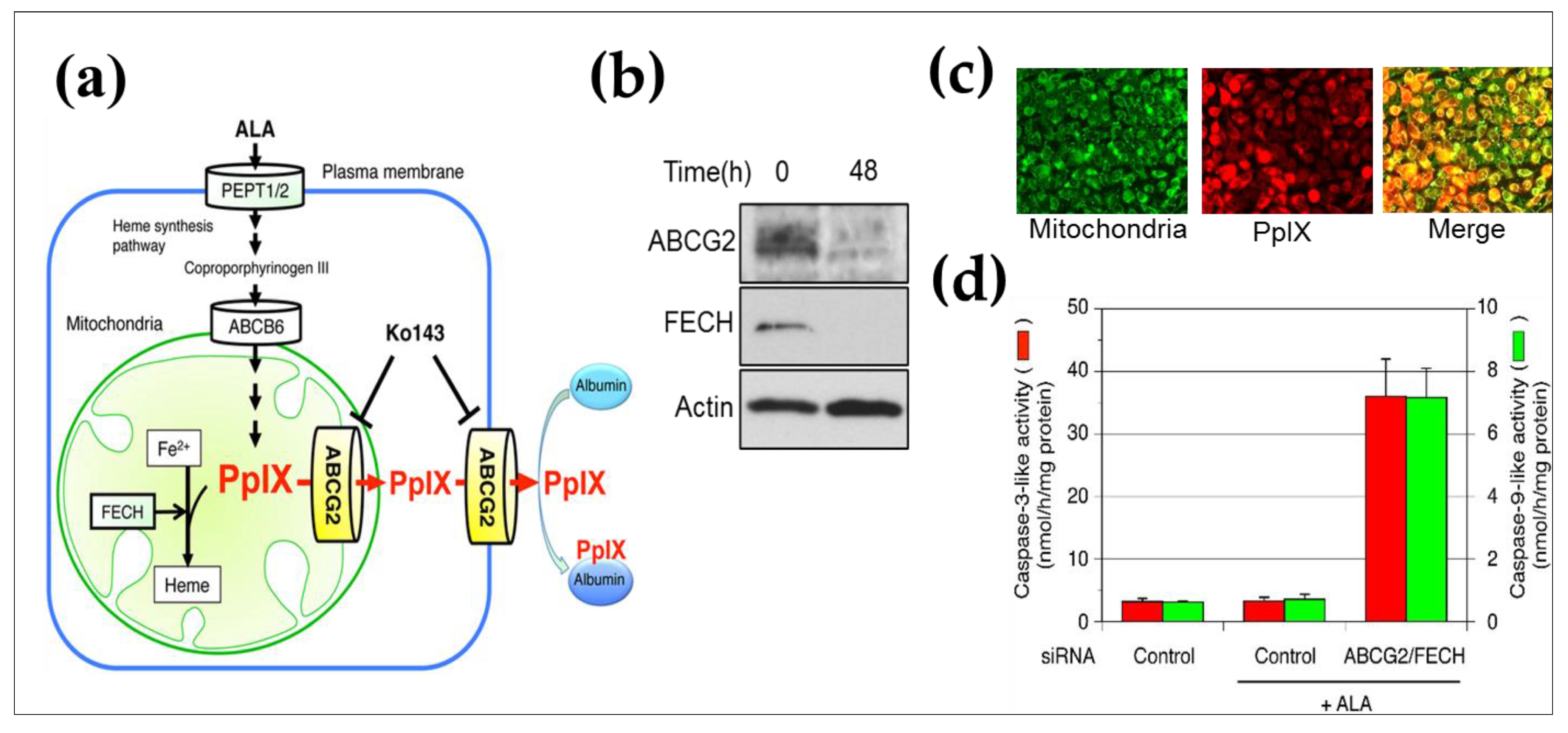
5. ABCG2 Knockdown by RNAi
6. Future Perspective and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Warner, S. Diagnostics plus therapy = theranostics. Scientist 2004, 38–39. [Google Scholar]
- Seidlin, S.M.; Marinelli, L.D.; Oshry, E. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J. Am. Med. Assoc. 1946, 132, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Deb, N.; Goris, M.; Trisler, K.; Fowler, S.; Saal, J.; Ning, S.; Becker, M.; Marquez, C.; Knox, S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 1289–1297. [Google Scholar]
- Maresca, K.P.; Hillier, S.M.; Femia, F.J.; Keith, D.; Barone, C.; Joyal, J.L.; Zimmerman, C.N.; Kozikowski, A.P.; Barrett, J.A.; Eckelman, W.C.; et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J. Med. Chem. 2009, 52, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.; Merkin, R.; Maresca, K.; Zimmerman, C.; Barrett, J.; Tesson, M.; Eckelman, W.; Mairs, R.; Joyal, J.; Babich, J. [131I]MIP-1375, a small molecule prostate-specific membrane antigen (PSMA) inhibitor for targeted therapy of prostate cancer (PCa). J. Nucl. Med. 2011, 52, 361. [Google Scholar]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet. Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar]
- Saumya, S.; Saloni, J.; Deepak, K.; Shankar, S.; Mukesh, S. A Review on Theranostics: An Approach to Targeted Diagnosis and Therapy. Asian J. Pharm. Res. Dev. 2019, 7, 63–69. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. Ca Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Yuan, A.; Wu, J.; Tang, X.; Zhao, L.; Xu, F.; Hu, Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J. Pharm Sci. 2013, 102, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Jadeja, P.; Tambe, P.; Vu, K.; Yuan, B.; Nguyen, K.T. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 2013, 3, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely Triggered Nano-Theranostics For Cancer Applications. Nanotheranostics. 2017, 1, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Mitsuyama, S. New challenges in the field of breast cancer therapy-do we need surgery for the patients with breast cancer? Breast Cancer. 2007, 14, 37–38. [Google Scholar] [CrossRef]
- Meurisse, M.; Defechereux, T.; Meurisse, N.; Bataille, Y.; Hamoir, E. New challenges in the treatment of early breast cancer or surgery for early breast cancer... can less be more? Acta Chir. Belg. 2002, 102, 97–109. [Google Scholar] [CrossRef]
- Giacomantonio, C.A.; Temple, W.J. Quality of cancer surgery: Challenges and controversies. Surg. Oncol. Clin. North Am. 2000, 9, 51–60. [Google Scholar] [CrossRef]
- Farolfi, A.; Lima, G.M.; Oyen, W.; Fanti, S. Molecular Imaging and Theranostics-A Multidisciplinary Approach. Semin. Nucl. Med. 2019, 49, 247–254. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sasaki, T.; Takenaka, F.; Yakushiji, H.; Fujii, Y.; Kishi, Y.; Kita, S.; Shen, L.; Kumon, H.; Matsuura, E. A novel PET imaging using (6)(4)Cu-labeled monoclonal antibody against mesothelin commonly expressed on cancer cells. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharm. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Dimitrov, D.S.; Marks, J.D. Therapeutic antibodies: Current state and future trends--is a paradigm change coming soon? Methods Mol. Biol. 2009, 525, 1–27. [Google Scholar]
- Gura, T. Therapeutic antibodies: Magic bullets hit the target. Nature 2002, 417, 584–586. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. scFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 980250. [Google Scholar] [CrossRef]
- Chowdhury, P.S.; Wu, H. Tailor-made antibody therapeutics. Methods 2005, 36, 11–24. [Google Scholar] [CrossRef]
- Hussein, W.M.; Cheong, Y.S.; Liu, C.; Liu, G.; Begum, A.A.; Attallah, M.A.; Moyle, P.M.; Torchilin, V.P.; Smith, R.; Toth, I. Peptide-based targeted polymeric nanoparticles for siRNA delivery. Nanotechnology 2019, 30, 415604. [Google Scholar] [CrossRef] [PubMed]
- Frère, Y.; Danicher, L.; Muller, S. Peptide Nanostructured Conjugates for Therapeutics: The Example of P140 Peptide for the Treatment of Systemic Lupus Erythematosus. Pept. Mater. Nanostructures Appl. 2013, 14, 385–415. [Google Scholar]
- Makino, A.; Yamahara, R.; Ozeki, E.; Kimura, S. Preparation of novel polymer assemblies, "lactosome", composed of poly(L-lactic acid) and poly(sarcosine). Chem. Lett. 2007, 36, 1220–1221. [Google Scholar] [CrossRef]
- Hara, E.; Ueda, M.; Kim, C.J.; Makino, A.; Hara, I.; Ozeki, E.; Kimura, S. Suppressive immune response of poly-(sarcosine) chains in peptide-nanosheets in contrast to polymeric micelles. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2014, 20, 570–577. [Google Scholar] [CrossRef]
- Hara, E.; Ueda, M.; Makino, A.; Hara, I.; Ozeki, E.; Kimura, S. Factors influencing in vivo disposition of polymeric micelles on multiple administrations. Acs Med. Chem. Lett. 2014, 5, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Hara, E.; Makino, A.; Kurihara, K.; Yamamoto, F.; Ozeki, E.; Kimura, S. Pharmacokinetic change of nanoparticulate formulation "Lactosome" on multiple administrations. Int. Immunopharmacol. 2012, 14, 261–266. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, A.; Matsuura, E.; Ozeki, E.; Matsui, H.; Watanabe, K.; Ohtsuki, T. Enhanced cellular uptake of lactosomes using cell-penetrating peptides. Sci. Technol. Adv. Mater. 2016, 17, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release. Off. J. Control. Release Soc. 2014, 174, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, U. Cell-penetrating peptides: Design, synthesis, and applications. Acs Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Endoh, T.; Ohtsuki, T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv. Drug Deliv. Rev. 2009, 61, 704–709. [Google Scholar] [CrossRef]
- Canete, M.; Villanueva, A.; Dominguez, V.; Polo, S.; Juarranz, A.; Stockert, J.C. Meso-tetraphenylporphyrin: Photosensitizing properties and cytotoxic effects on cultured tumor cells. Int. J. Oncol. 1998, 13, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Selbo, P.K.; Hogset, A.; Prasmickaite, L.; Berg, K. Photochemical internalisation: A novel drug delivery system. Tumour Biol. 2002, 23, 103–112. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, G.; Zhang, X.; Xu, F.; Xiong, X.; Zhou, S. A Step-by-Step Multiple Stimuli-Responsive Nanoplatform for Enhancing Combined Chemo-Photodynamic Therapy. Adv. Mater. 2017, 29, 1605357. [Google Scholar] [CrossRef]
- Shieh, M.J.; Peng, C.L.; Lou, P.J.; Chiu, C.H.; Tsai, T.Y.; Hsu, C.Y.; Yeh, C.Y.; Lai, P.S. Non-toxic phototriggered gene transfection by PAMAM-porphyrin conjugates. J. Control. Release. Off. J. Control. Release Soc. 2008, 129, 200–206. [Google Scholar] [CrossRef]
- de Bruin, K.G.; Fella, C.; Ogris, M.; Wagner, E.; Ruthardt, N.; Brauchle, C. Dynamics of photoinduced endosomal release of polyplexes. J. Control. Release. Off. J. Control. Release Soc. 2008, 130, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in nuclear medicine practice. Onco Targets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapp, K.N.; Lea, W.B.; Johnson, M.S.; Tann, M.; Fletcher, J.W.; Hutchins, G.D. The impact of image reconstruction bias on PET/CT 90Y dosimetry after radioembolization. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55, 1452–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, K.; Ueda, M.; Hara, I.; Hara, E.; Sano, K.; Makino, A.; Ozeki, E.; Yamamoto, F.; Saji, H.; Togashi, K.; et al. Inflammation-induced synergetic enhancement of nanoparticle treatments with DOXIL® and 90Y-Lactosome for orthotopic mammary tumor. J. Nanopart. Res. 2016, 18, 137. [Google Scholar] [CrossRef]
- Borjesson, P.K.; Jauw, Y.W.; Boellaard, R.; de Bree, R.; Comans, E.F.; Roos, J.C.; Castelijns, J.A.; Vosjan, M.J.; Kummer, J.A.; Leemans, C.R.; et al. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 2133–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, F.T.; Scott, A.M. Immuno-PET for tumor targeting. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2003, 44, 1282–1283. [Google Scholar]
- Verel, I.; Visser, G.W.; van Dongen, G.A. The promise of immuno-PET in radioimmunotherapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2005, 46 (Suppl. 1), 164S–171S. [Google Scholar]
- Verel, I.; Visser, G.W.; Boellaard, R.; Stigter-van Walsum, M.; Snow, G.B.; van Dongen, G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2003, 44, 1271–1281. [Google Scholar]
- Dijkers, E.C.; de Vries, E.G.; Kosterink, J.G.; Brouwers, A.H.; Lub-de Hooge, M.N. Immunoscintigraphy as potential tool in the clinical evaluation of HER2/neu targeted therapy. Curr. Pharm. Des. 2008, 14, 3348–3362. [Google Scholar] [CrossRef]
- Hara, E.; Makino, A.; Kurihara, K.; Ueda, M.; Hara, I.; Kawabe, T.; Yamamoto, F.; Ozeki, E.; Togashi, K.; Kimura, S. Radionuclide therapy using nanoparticle of 131I-Lactosome in combination with percutaneous ethanol injection therapy. J. Nanopart. Res. 2013, 15, 2131. [Google Scholar] [CrossRef]
- Jang, J.W.; Park, Y.M.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chang, U.I.; Nam, S.W.; Kim, B.S. Therapeutic efficacy of multimodal combination therapy using transcatheter arterial infusion of epirubicin and cisplatin, systemic infusion of 5-fluorouracil, and additional percutaneous ethanol injection for unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004, 54, 415–420. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, Y.C.; Cao, Y.; Cai, X.Y.; Tan, Y.T.; Shi, Y.X.; Jiang, W.Q. Radiofrequency ablation combined with systemic chemotherapy in nasopharyngeal carcinoma liver metastases improves response to treatment and survival outcomes. J. Surg. Oncol. 2012, 106, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. Acs Nano 2013, 7, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. The effect of photoimmunotherapy followed by liposomal daunorubicin in a mixed tumor model: A demonstration of the super-enhanced permeability and retention effect after photoimmunotherapy. Mol. Cancer Ther. 2014, 13, 426–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, K.; Ueda, M.; Hara, I.; Ozeki, E.; Togashi, K.; Kimura, S. Polymeric Micelle of A(3)B-Type Lactosome as a Vehicle for Targeting Meningeal Dissemination. Nanomaterials 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, K.; Kumar, S.; Ross, K.A.; Gautam, S.; Poelaert, B.; Nasser, M.W.; Aithal, A.; Bhatia, R.; Wannemuehler, M.J.; Narasimhan, B.; et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018, 417, 35–46. [Google Scholar] [CrossRef]
- Hassan, R.; Bera, T.; Pastan, I. Mesothelin: A new target for immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 3937–3942. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.; Ho, M. Mesothelin targeted cancer immunotherapy. Eur. J. Cancer 2008, 44, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argani, P.; Iacobuzio-Donahue, C.; Ryu, B.; Rosty, C.; Goggins, M.; Wilentz, R.E.; Murugesan, S.R.; Leach, S.D.; Jaffee, E.; Yeo, C.J.; et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 3862–3868. [Google Scholar]
- Baba, K.; Ishigami, S.; Arigami, T.; Uenosono, Y.; Okumura, H.; Matsumoto, M.; Kurahara, H.; Uchikado, Y.; Kita, Y.; Kijima, Y.; et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J. Surg. Oncol. 2012, 105, 195–199. [Google Scholar] [CrossRef]
- Hassan, R.; Kreitman, R.J.; Pastan, I.; Willingham, M.C. Localization of mesothelin in epithelial ovarian cancer. Appl. Immunohistochem. Mol. Morphol. Aimm 2005, 13, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2003, 16, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Tchou, J.; Wang, L.C.; Selven, B.; Zhang, H.; Conejo-Garcia, J.; Borghaei, H.; Kalos, M.; Vondeheide, R.H.; Albelda, S.M.; June, C.H.; et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res. Treat. 2012, 133, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Dainty, L.A.; Risinger, J.I.; Morrison, C.; Chandramouli, G.V.; Bidus, M.A.; Zahn, C.; Rose, G.S.; Fowler, J.; Berchuck, A.; Maxwell, G.L. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol. Oncol. 2007, 105, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbach, D.; Onda, M.; Voigt, A.; Dawczynski, K.; Wittig, S.; Hassan, R.; Gruhn, B.; Pastan, I. Mesothelin, a possible target for immunotherapy, is expressed in primary AML cells. Eur. J. Haematol. 2007, 79, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Feng, M.; Kim, H.; Phung, Y.; Kleiner, D.E.; Gores, G.J.; Qian, M.; Wang, X.W.; Ho, M. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J. Cancer. 2010, 1, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Qian, M.; Ho, M. The role of mesothelin in tumor progression and targeted therapy. Anti Cancer Agents Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.; Schweizer, C.; Lu, K.F.; Schuler, B.; Remaley, A.T.; Weil, S.C.; Pastan, I. Inhibition of mesothelin-CA-125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: Implications for cancer therapy. Lung Cancer 2010, 68, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, A.; Hirono, S.; Tani, M.; Kawai, M.; Okada, K.; Miyazawa, M.; Kitahata, Y.; Nakamura, Y.; Noda, T.; Yokoyama, S.; et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012, 103, 739–746. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, C.A.; Chen, P.J.; Chiang, Y.C.; Chen, Y.L.; Mao, T.L.; Lin, H.W.; Lin Chiang, W.H.; Cheng, W.F. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem. J. 2012, 442, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servais, E.L.; Colovos, C.; Rodriguez, L.; Bograd, A.J.; Nitadori, J.; Sima, C.; Rusch, V.W.; Sadelain, M.; Adusumilli, P.S. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2478–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin confers pancreatic cancer cell resistance to TNF-alpha-induced apoptosis through Akt/PI3K/NF-kappaB activation and IL-6/Mcl-1 overexpression. Mol. Cancer. 2011, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis 2011, 32, 1013–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.C.; Chen, C.A.; Hsieh, C.Y.; Lee, C.N.; Su, Y.N.; Hu, Y.H.; Cheng, W.F. Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem. J. 2009, 424, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.F.; Huang, C.Y.; Chang, M.C.; Hu, Y.H.; Chiang, Y.C.; Chen, Y.L.; Hsieh, C.Y.; Chen, C.A. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br. J. Cancer 2009, 100, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Pastan, I.; Willingham, M.C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer 1992, 50, 373–381. [Google Scholar] [CrossRef]
- Hassan, R.; Wu, C.; Brechbiel, M.W.; Margulies, I.; Kreitman, R.J.; Pastan, I. 111Indium-labeled monoclonal antibody K1: Biodistribution study in nude mice bearing a human carcinoma xenograft expressing mesothelin. Int. J. Cancer 1999, 80, 559–563. [Google Scholar] [CrossRef]
- Ho, M.; Feng, M.; Fisher, R.J.; Rader, C.; Pastan, I. A novel high-affinity human monoclonal antibody to mesothelin. Int. J. Cancer 2011, 128, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.; Sogawa, C.; Tsuji, A.B.; Sudo, H.; Sugyo, A.; Uehara, T.; Hino, O.; Yoshii, Y.; Fujibayashi, Y.; Fukumura, T.; et al. Development of positron emission tomography imaging by 64Cu-labeled Fab for detecting ERC/mesothelin in a mesothelioma mouse model. Nucl. Med. Commun. 2010, 31, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Connett, J.M.; Schwarz, S.W.; Rocque, P.A.; Guo, L.W.; Philpott, G.W.; Zinn, K.R.; Meares, C.F.; Welch, M.J. Copper-64-labeled antibodies for PET imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1992, 33, 1685–1691. [Google Scholar]
- Ping Li, W.; Meyer, L.A.; Capretto, D.A.; Sherman, C.D.; Anderson, C.J. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother. Radiopharm. 2008, 23, 158–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakushiji, H.; Kobayashi, K.; Takenaka, F.; Kishi, Y.; Shinohara, M.; Akehi, M.; Sasaki, T.; Ohno, E.; Matsuura, E. Novel single-chain variant of antibody against mesothelin established by phage library. Cancer Sci. 2019, 110, 2722–2733. [Google Scholar] [CrossRef] [Green Version]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics—Application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Sonali, M.K.V.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Datta Maroti Pawde, N.; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70–86. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Richter, A.W.; Akerblom, E. Polyethylene glycol reactive antibodies in man: Titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int. Arch. Allergy Appl. Immunol. 1984, 74, 36–39. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef]
- Ganson, N.J.; Kelly, S.J.; Scarlett, E.; Sundy, J.S.; Hershfield, M.S. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 2006, 8, R12. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Hwang, S.H.; Park, J.S.; Park, H.S.; Shin, Y.S. Anaphylaxis to Polyethylene Glycol (Colyte(R)) in a Patient with Diverticulitis. J. Korean Med. Sci. 2016, 31, 1662–1663. [Google Scholar] [CrossRef] [Green Version]
- Chanan-Khan, A.; Szebeni, J.; Savay, S.; Liebes, L.; Rafique, N.M.; Alving, C.R.; Muggia, F.M. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): Possible role in hypersensitivity reactions. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2003, 14, 1430–1437. [Google Scholar] [CrossRef]
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Makino, A.; Kizaka-Kondoh, S.; Yamahara, R.; Hara, I.; Kanzaki, T.; Ozeki, E.; Hiraoka, M.; Kimura, S. Near-infrared fluorescence tumor imaging using nanocarrier composed of poly(L-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide. Biomaterials 2009, 30, 5156–5160. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Hara, E.; Hara, I.; Yamahara, R.; Kurihara, K.; Ozeki, E.; Yamamoto, F.; Kimura, S. Control of in vivo blood clearance time of polymeric micelle by stereochemistry of amphiphilic polydepsipeptides. J. Control. Release. Off. J. Control. Release Soc. 2012, 161, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Birke, A.; Fischer, K.; Schmidt, M.; Barz, M. Solution Properties of Polysarcosine: From Absolute and Relative Molar Mass Determinations to Complement Activation. Macromolecules 2018, 51, 2653–2661. [Google Scholar] [CrossRef]
- Makino, A.; Kimura, S. Solid tumor-targeting theranostic polymer nanoparticle in nuclear medicinal fields. Sci. World J. 2014, 2014, 424513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kimura, Y. Macromolecular Organization of Poly(L-lactide)-block-Polyoxyethylene into Bio-Inspired Nano-Architectures. Macromol. Biosci. 2002, 2, 11–23. [Google Scholar] [CrossRef]
- Yamamoto, F.; Yamahara, R.; Makino, A.; Kurihara, K.; Tsukada, H.; Hara, E.; Hara, I.; Kizaka-Kondoh, S.; Ohkubo, Y.; Ozeki, E.; et al. Radiosynthesis and initial evaluation of (18)F labeled nanocarrier composed of poly(L-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide. Nucl. Med. Biol. 2013, 40, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Funayama, T.; Sakane, M.; Abe, T.; Hara, I.; Ozeki, E.; Ochiai, N. Intraoperative Near-infrared Fluorescence Imaging with Novel Indocyanine Green-Loaded Nanocarrier for Spinal Metastasis: A Preliminary Animal Study. Open Biomed. Eng. J. 2012, 6, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Kiwada, H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int. J. Pharm. 2008, 354, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release. Off. J. Control. Release Soc. 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Asai, T.; Hatanaka, K.; Akai, S.; Ishii, T.; Kenjo, E.; Ishida, T.; Kiwada, H.; Tsukada, H.; Oku, N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int. J. Pharm. 2010, 392, 218–223. [Google Scholar] [CrossRef]
- Obukhanych, T.V.; Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Mosier, D.E.; Subbarao, B. Thymus-independent antigens: Complexity of B-lymphocyte activation revealed. Immunol. Today 1982, 3, 217–222. [Google Scholar] [CrossRef]
- Kim, C.J.; Hara, E.; Shimizu, A.; Sugai, M.; Kimura, S. Activation of B1a cells in peritoneal cavity by T cell-independent antigen expressed on polymeric micelle. J. Pharm. Sci. 2015, 104, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Mima, Y.; Hashimoto, Y.; Ukawa, M.; Ando, H.; Kiwada, H.; Ishida, T. Anti-PEG IgM and complement system are required for the association of second doses of PEGylated liposomes with splenic marginal zone B cells. Immunobiology 2015, 220, 1151–1160. [Google Scholar] [CrossRef]
- Shimizu, T.; Ishida, T.; Kiwada, H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology 2013, 218, 725–732. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ichihara, M.; Shimizu, T.; Ishida, T.; Kiwada, H. Ex-vivo/in-vitro anti-polyethylene glycol (PEG) immunoglobulin M production from murine splenic B cells stimulated by PEGylated liposome. Biol. Pharm. Bull. 2013, 36. [Google Scholar] [CrossRef] [Green Version]
- Mond, J.J.; Vos, Q.; Lees, A.; Snapper, C.M. T cell independent antigens. Curr. Opin Immunol. 1995, 7, 349–354. [Google Scholar] [CrossRef]
- Mond, J.J.; Lees, A.; Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ishida, T.; Kiwada, H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J. Control. Release. Off. J. Control. Release Soc. 2007, 119, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Urata, Y.; Anraku, M.; Watanabe, H.; Kadowaki, D.; Sakai, H.; Horinouchi, H.; Kobayashi, K.; Tsuchida, E.; Maruyama, T.; et al. Hemoglobin vesicles, polyethylene glycol (PEG)ylated liposomes developed as a red blood cell substitute, do not induce the accelerated blood clearance phenomenon in mice. Drug Metab Dispos. Biol. Fate Chem. 2009, 37, 2197–2203. [Google Scholar] [CrossRef]
- Hara, E.; Makino, A.; Kurihara, K.; Sugai, M.; Shimizu, A.; Hara, I.; Ozeki, E.; Kimura, S. Evasion from accelerated blood clearance of nanocarrier named as "Lactosome" induced by excessive administration of Lactosome. Biochim. Biophys. Acta. 2013, 1830, 4046–4052. [Google Scholar] [CrossRef]
- Kurihara, K.; Ueda, M.; Hara, I.; Ozeki, E.; Togashi, K.; Kimura, S. Control of in vivo disposition and immunogenicity of polymeric micelles by adjusting poly(sarcosine) chain lengths on surface. J. Nanopart. Res. 2017, 19, 242. [Google Scholar] [CrossRef]
- Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Rational design of peptide nanotubes for varying diameters and lengths. J. Pept Sci. Off. Publ. Eur. Pept. Soc. 2011, 17, 94–99. [Google Scholar] [CrossRef]
- Nomura, A.; Okayasu, K.; Ohno, K.; Fukuda, T.; Tsujii, Y. Lubrication Mechanism of Concentrated Polymer Brushes in Solvents: Effect of Solvent Quality and Thereby Swelling State. Macromolecules 2011, 44, 5013–5019. [Google Scholar] [CrossRef]
- Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Structure and Properties of High-Density Polymer Brushes Prepared by Surface-InitiatedLiving Radical Polymerization. In Surface-Initiated Polymerization I; Jordan, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 197, pp. 1–45. [Google Scholar]
- Makino, A.; Hara, E.; Hara, I.; Ozeki, E.; Kimura, S. Size control of core-shell-type polymeric micelle with a nanometer precision. Langmuir Acs J. Surf. Colloids 2014, 30, 669–674. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L. Delivery of siRNA therapeutics: Barriers and carriers. Aaps J. 2010, 12, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.H.; Zeng, R.F.; Fang, S.; Dai, Q.S.; Li, H.P.; Long, J.T. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int. J. Pharm. 2014, 474, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Davis, M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015, 14, 843–856. [Google Scholar] [CrossRef]
- Huang, C.; Li, M.; Chen, C.; Yao, Q. Small interfering RNA therapy in cancer: Mechanism, potential targets, and clinical applications. Exp. Opin. Targets. 2008, 12, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.A.; Nam, Y.S. Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics 2014, 4, 1211–1232. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Pattni, B.S.; Abouzeid, A.H.; Torchilin, V.P. Nanopreparations to overcome multidrug resistance in cancer. Adv. Drug Deliv. Rev. 2013, 65, 1748–1762. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Xiao, Z.; Votruba, A.R.; Vilos, C.; Farokhzad, O.C. Differentially charged hollow core/shell lipid-polymer-lipid hybrid nanoparticles for small interfering RNA delivery. Angew. Chem. 2011, 50, 7027–7031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Wu, J.; Liu, Y.; Saw, P.E.; Tao, W.; Yu, M.; Zope, H.; Si, M.; Victorious, A.; Rasmussen, J.; et al. Multifunctional Envelope-Type siRNA Delivery Nanoparticle Platform for Prostate Cancer Therapy. Acs Nano 2017, 11, 2618–2627. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Oupicky, D. Conjugate Polyplexes with Anti-Invasive Properties and Improved siRNA Delivery In Vivo. Bioconjug Chem. 2018, 29, 296–305. [Google Scholar] [CrossRef]
- Meade, B.R.; Dowdy, S.F. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv. Drug Deliv. Rev. 2007, 59, 134–140. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Z.; Zhang, Y.; Lv, H.; Zhou, J.; Li, C.; Hou, L.; Zhang, Q. Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials 2012, 33, 9246–9258. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Tanaka, G.; Futaki, S. Cell-penetrating peptides (CPPs) as a vector for the delivery of siRNAs into cells. Mol. Biosyst. 2013, 9, 855–861. [Google Scholar] [CrossRef]
- Cheng, C.J.; Saltzman, W.M. Enhanced siRNA delivery into cells by exploiting the synergy between targeting ligands and cell-penetrating peptides. Biomaterials 2011, 32, 6194–6203. [Google Scholar] [CrossRef] [Green Version]
- Nakase, I.; Akita, H.; Kogure, K.; Graslund, A.; Langel, U.; Harashima, H.; Futaki, S. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc. Chem. Res. 2012, 45, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Miki, S.; Kobayashi, S.; Haraguchi, T.; Nakata, E.; Hirakawa, K.; Sumita, K.; Watanabe, K.; Okazaki, S. The molecular mechanism of photochemical internalization of cell penetrating peptide-cargo-photosensitizer conjugates. Sci. Rep. 2015, 5, 18577. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Bae, K.H.; Kim, J.S.; Nam, Y.S.; Park, T.G. Intracellular delivery of paclitaxel using oil-free, shell cross-linked HSA--multi-armed PEG nanocapsules. Biomaterials 2011, 32, 8635–8644. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.; Rosenthal-Aizman, K.; Saar, K.; Eiriksdottir, E.; Jiang, Y.; Sassian, M.; Ostlund, P.; Hallbrink, M.; Langel, U. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem. Pharmacol. 2006, 71, 416–425. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Lee, H.J. Mechanistic studies of intracellular delivery of proteins by cell-penetrating peptides in cyanobacteria. Bmc Microbiol. 2013, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Yang, H.; Stanley, J.T.; Holloway, P.H.; Moudgil, B.M.; Walter, G.; Mericle, R.A. Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots. Chem. Commun. 2005, 25, 3144–3146. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.; Carlesso, N.; Tung, C.H.; Tang, X.W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Vives, E.; Schmidt, J.; Pelegrin, A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Biophys. Acta 2008, 1786, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. Aaps J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Horsey, A.J.; Cox, M.H.; Sarwat, S.; Kerr, I.D. The multidrug transporter ABCG2: Still more questions than answers. Biochem. Soc. Trans. 2016, 44, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Susanto, J.; Lin, Y.H.; Chen, Y.N.; Shen, C.R.; Yan, Y.T.; Tsai, S.T.; Chen, C.H.; Shen, C.N. Porphyrin homeostasis maintained by ABCG2 regulates self-renewal of embryonic stem cells. PLoS ONE 2008, 3, e4023. [Google Scholar] [CrossRef] [Green Version]
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; Van Der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plosch, T.; Kuipers, F.; Elferink, R.P.; Rosing, H.; et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, M.S.H.; Nishiyama, Y.; Ohtsuki, T.; Watanabe, K.; Kobuchi, H.; Kobayashi, K.; Matsuura, E. Lactosome-Conjugated siRNA Nanoparticles for Photo-Enhanced Gene Silencing in Cancer Cells. J. Pharm. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Berg, K.; Dietze, A.; Kaalhus, O.; Hogset, A. Site-specific drug delivery by photochemical internalization enhances the antitumor effect of bleomycin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 8476–8485. [Google Scholar] [CrossRef] [Green Version]
- Prasmickaite, L.; Cekaite, L.; Hellum, M.; Hovig, E.; Hogset, A.; Berg, K. Transcriptome changes in a colon adenocarcinoma cell line in response to photochemical treatment as used in photochemical internalisation (PCI). Febs Lett. 2006, 580, 5739–5746. [Google Scholar] [CrossRef] [Green Version]
- Weyergang, A.; Selbo, P.K.; Berg, K. Photochemically stimulated drug delivery increases the cytotoxicity and specificity of EGF-saporin. J. Control. Release. Off. J. Control. Release Soc. 2006, 111, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.S.; Lou, P.J.; Peng, C.L.; Pai, C.L.; Yen, W.N.; Huang, M.Y.; Young, T.H.; Shieh, M.J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release. Off. J. Control. Release Soc. 2007, 122, 39–46. [Google Scholar] [CrossRef]
- Allikmets, R.; Schriml, L.M.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar]
- Haider, A.J.; Cox, M.H.; Jones, N.; Goode, A.J.; Bridge, K.S.; Wong, K.; Briggs, D.; Kerr, I.D. Identification of residues in ABCG2 affecting protein trafficking and drug transport, using co-evolutionary analysis of ABCG sequences. Biosci Rep. 2015, 35, e00241. [Google Scholar] [CrossRef] [Green Version]
- Aronica, E.; Gorter, J.A.; Redeker, S.; van Vliet, E.A.; Ramkema, M.; Scheffer, G.L.; Scheper, R.J.; van der Valk, P.; Leenstra, S.; Baayen, J.C.; et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 2005, 46, 849–857. [Google Scholar] [CrossRef]
- Gutmann, H.; Hruz, P.; Zimmermann, C.; Beglinger, C.; Drewe, J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 2005, 70, 695–699. [Google Scholar] [CrossRef]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.E.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Regula, J.; MacRobert, A.J.; Gorchein, A.; Buonaccorsi, G.A.; Thorpe, S.M.; Spencer, G.M.; Hatfield, A.R.; Bown, S.G. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX--a pilot study. Gut 1995, 36, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J.; Cooper, M.T.; Mang, T.S. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg. Med. 1990, 10, 485–488. [Google Scholar] [CrossRef]
- Bedwell, J.; MacRobert, A.J.; Phillips, D.; Bown, S.G. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br. J. Cancer. 1992, 65, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef]
- Kuo, M.T. Redox regulation of multidrug resistance in cancer chemotherapy: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2009, 11, 99–133. [Google Scholar] [CrossRef] [Green Version]
- Jonker, J.W.; Musters, S.; Vlaming, M.L.; Plosch, T.; Gooijert, K.E.; Hillebrand, M.J.; Rosing, H.; Beijnen, J.H.; Verkade, H.J.; Schinkel, A.H. Breast cancer resistance protein (Bcrp1/Abcg2) is expressed in the harderian gland and mediates transport of conjugated protoporphyrin IX. Am. J. Physiol. Cell Physiol. 2007, 292, C2204–C2212. [Google Scholar] [CrossRef] [Green Version]
- Doss, M.; Sixel-Dietrich, F.; Verspohl, F. "Glucose effect" and rate limiting function of uroporphyrinogen synthase on porphyrin metabolism in hepatocyte culture: Relationship with human acute hepatic porphyrias. J. Clin. Chem. Clin. Biochem. Z. Fur. Klin. Chem. Und Klin. Biochem. 1985, 23, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Desuzinges-Mandon, E.; Arnaud, O.; Martinez, L.; Huche, F.; Di Pietro, A.; Falson, P. ABCG2 transports and transfers heme to albumin through its large extracellular loop. J. Biol. Chem. 2010, 285, 33123–33133. [Google Scholar] [CrossRef] [Green Version]
- Ogino, T.; Kobuchi, H.; Munetomo, K.; Fujita, H.; Yamamoto, M.; Utsumi, T.; Inoue, K.; Shuin, T.; Sasaki, J.; Inoue, M.; et al. Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Mol. Cell Biochem. 2011, 358, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Kriska, T.; Korytowski, W.; Girotti, A.W. Role of mitochondrial cardiolipin peroxidation in apoptotic photokilling of 5-aminolevulinate-treated tumor cells. Arch. Biochem. Biophys. 2005, 433, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, H.; Moriya, K.; Ogino, T.; Fujita, H.; Inoue, K.; Shuin, T.; Yasuda, T.; Utsumi, K.; Utsumi, T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS ONE 2012, 7, e50082. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Xie, T.; Schuetz, J.D. The role of transporters in cellular heme and porphyrin homeostasis. Pharm. Ther. 2007, 114, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Nagakawa, K.; Kobuchi, H.; Ogino, T.; Kondo, Y.; Inoue, K.; Shuin, T.; Utsumi, T.; Utsumi, K.; Sasaki, J.; et al. Phytoestrogen Suppresses Efflux of the Diagnostic Marker Protoporphyrin IX in Lung Carcinoma. Cancer Res. 2016, 76, 1837–1846. [Google Scholar] [CrossRef] [Green Version]
- Roh, Y.J.; Kim, J.H.; Kim, I.W.; Na, K.; Park, J.M.; Choi, M.G. Photodynamic Therapy Using Photosensitizer-Encapsulated Polymeric Nanoparticle to Overcome ATP-Binding Cassette Transporter Subfamily G2 Function in Pancreatic Cancer. Mol. Cancer Ther. 2017, 16, 1487–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, T.M.; Pratt, E.C.; Grimm, J. Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol. 2017, 12, 106–117. [Google Scholar] [CrossRef]
- Nomura, S.; Morimoto, Y.; Tsujimoto, H.; Arake, M.; Harada, M.; Saitoh, D.; Hara, I.; Ozeki, E.; Satoh, A.; Takayama, E.; et al. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using a near-infrared absorbent. Sci. Rep. 2020, 10, 9765. [Google Scholar] [CrossRef]
- Tsunoi, Y.; Araki, K.; Ozeki, E.; Hara, I.; Shiotani, A.; Terakawa, M.; Sato, S. Photoacoustic diagnosis of pharmacokinetics and vascular shutdown effects in photodynamic treatment with indocyanine green-lactosome for a subcutaneous tumor in mice. Photodiagnosis Photodyn. Ther. 2019, 26, 436–441. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, M.S.H.; Ohtsuki, T.; Takenaka, F.; Kobayashi, K.; Akehi, M.; Uji, H.; Kobuchi, H.; Sasaki, T.; Ozeki, E.; Matsuura, E. A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): “Lactosome” Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy. Life 2021, 11, 158. https://doi.org/10.3390/life11020158
Lim MSH, Ohtsuki T, Takenaka F, Kobayashi K, Akehi M, Uji H, Kobuchi H, Sasaki T, Ozeki E, Matsuura E. A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): “Lactosome” Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy. Life. 2021; 11(2):158. https://doi.org/10.3390/life11020158
Chicago/Turabian StyleLim, Melissa Siaw Han, Takashi Ohtsuki, Fumiaki Takenaka, Kazuko Kobayashi, Masaru Akehi, Hirotaka Uji, Hirotsugu Kobuchi, Takanori Sasaki, Eiichi Ozeki, and Eiji Matsuura. 2021. "A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): “Lactosome” Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy" Life 11, no. 2: 158. https://doi.org/10.3390/life11020158
APA StyleLim, M. S. H., Ohtsuki, T., Takenaka, F., Kobayashi, K., Akehi, M., Uji, H., Kobuchi, H., Sasaki, T., Ozeki, E., & Matsuura, E. (2021). A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): “Lactosome” Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy. Life, 11(2), 158. https://doi.org/10.3390/life11020158








