Radiomics Analysis for 177Lu-DOTAGA-(l-y)fk(Sub-KuE) Targeted Radioligand Therapy Dosimetry in Metastatic Prostate Cancer—A Model Based on Clinical Example
Abstract
1. Introduction
2. Results
2.1. Clinical Response
2.2. Biochemical and Molecular Response
2.3. Dosimetric Results
3. Discussion
The Limitations of the Study
4. Materials and Methods
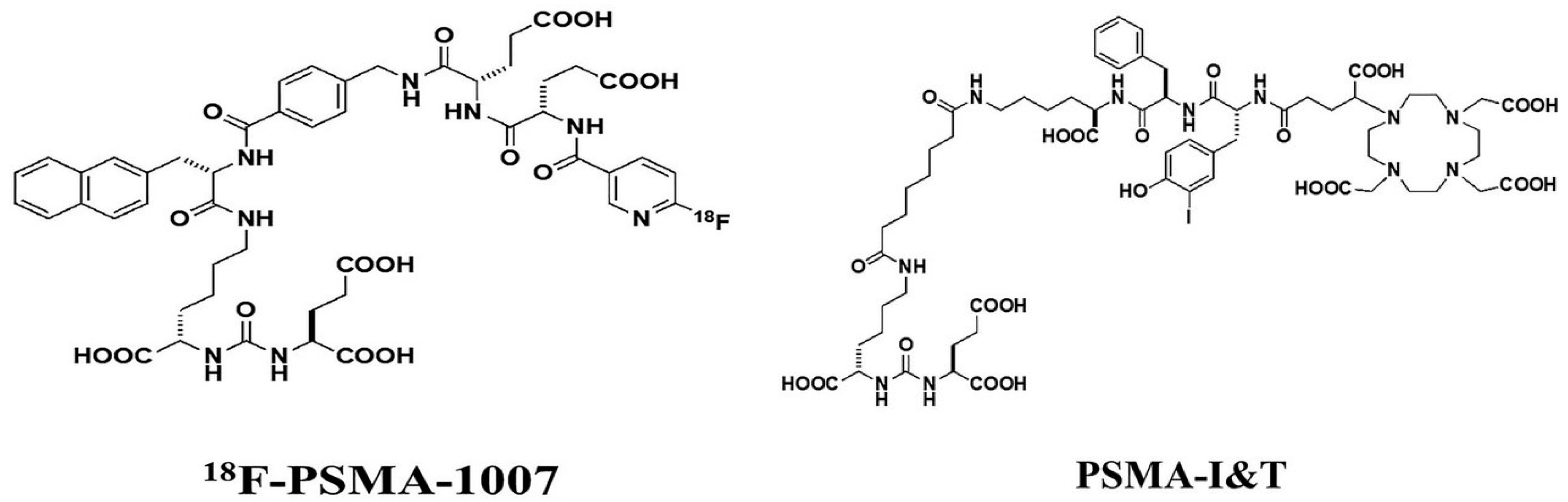
4.1. The Patient and 18F-PSMA-1007 PET/CT
4.2. Manufacturing, Administration and Side Effects of Lu-PSMA
4.3. Equipment and Study Protocols
4.4. Image Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| CT | computed tomography |
| DRT | Dosimetry Research Tool |
| ECOG | Eastern Cooperative Oncology Group Performance Status |
| ETCH | East Tallinn Central Hospital |
| LDH | lactate dehydrogenase |
| mCRPC | metastatic castration resistant prostate cancer |
| MIP | maximum intensity projection |
| MTV | metabolic tumor volume |
| N/A | not applicable |
| PET/CT | positron emission tomography/computed tomography |
| PR | partial response |
| PSA | prostate specific antigen |
| PSMA | prostate specific membrane antigen |
| PSMA I&T | DOTAGA-(l-y)fk(Sub-KuE) |
| RLT | radioligand therapy |
| SPECT | single photon emission computed tomography |
| SPECT/CT | single photon emission computed tomography/computed tomography |
| SUV | standardized uptake value |
| SUVmax | maximum standardized uptake value |
| TAC | time-activity curve |
| VOI | volume of interest |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Tan, N.; Bavadian, N.; Calais, J.; Oyoyo, U.; Kim, J.; Turkbey, I.B.; Mena, E.; Davenport, M.S. Imaging of Prostate Specific Membrane Antigen Targeted Radiotracers for the Detection of Prostate Cancer Biochemical Recurrence after Definitive Therapy: A Systematic Review and Meta-Analysis. J. Urol. 2019, 202, 231–240. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the Vision of Radioligand Therapy for Prostate Cancer Becoming a Reality? An Overview of the Phase III VISION Trial and Its Importance for the Future of Theranostics. J. Nucl. Med. 2019, 60, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Rawal, S.; Goel, H.C.; Rao, S.A. Evaluation of response in patients of metastatic castration resistant prostate cancer undergoing systemic radiotherapy with lutetium177-prostate-specific membrane antigen: A comparison between response evaluation criteria in solid tumors, positron-emission tomography response criteria in solid tumors, European organization for research and treatment of cancer, and MDA criteria assessed by gallium 68-prostate-specific membrane antigen positron-emission tomography-computed tomography. Urol. Ann. 2019, 11, 155–162. [Google Scholar] [CrossRef]
- Seitz, A.K.; Rauscher, I.; Haller, B.; Krönke, M.; Luther, S.; Heck, M.M.; Horn, T.; Gschwend, J.E.; Schwaiger, M.; Eiber, M.; et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 602–612. [Google Scholar] [CrossRef]
- Vija, A.H.; Cachovan, M. Automated Internal Dosimetry Research Tool Using Quantitative SPECT for the Lu177 Theranostic Application. J. Nucl. Med. 2017, 58 (Suppl. 1). [Google Scholar]
- Kairemo, K.; Joensuu, T. Lu-177-PSMA treatment for metastatic prostate cancer: Case examples of major responses. Clin Transl Imaging 2018, 6, 223–237. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Okamoto, S.; Thieme, A.; Allmann, J.; D’Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.J.; Tamaki, N.; et al. Radiation dosimetry for 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer: Absorbed dose in normal organs and tumor lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Essler, M.; Biersack, H.J. PSMA-Based Theranostics: A Step-by-Step Practical Approach to Diagnosis and Therapy for mCRPC Patients. Semin. Nucl. Med. 2020, 50, 98–109. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Friederike, V.Ã.; Gosewisch, A.; Kaiser, L.; Gildehaus, F.; Todica, A.; Bartenstein, P.; Boening, G.; Ilhan, H. Pretherapeutic SUV as a predictive parameter for therapy response in PSMA radioligand therapy—Correlation of pre- and posttherapeutic SUV in 68Ga-PSMA-11 PET with absorbed dose and PSA-response. J. Nucl. Med. 2020, 61 (Suppl. 1). [Google Scholar]
- Ljungberg, M.; Celler, A.; Konijnenberg, M.W.; Eckerman, K.F.; Dewaraja, Y.K.; Sjögreen-Gleisner, K. MIRD Pamphlet No. 26: Joint EANM/MIRD Guidelines for Quantitative 177Lu SPECT Applied for Dosimetry of Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Hänscheid, H.; Lapa, C.; Buck, A.K.; Lassmann, M.; Werner, R.A. Dose Mapping After Endoradiotherapy with 177Lu-DOTATATE/DOTATOC by a Single Measurement After 4 Days. J. Nucl. Med. 2018, 59, 75–81. [Google Scholar] [CrossRef] [PubMed]



| No of RLT Cycle | Method | Right Kidney | Left Kidney | Liver | Spleen | Parotid Glands | Submandibular Glands | Lacrimal Glands |
|---|---|---|---|---|---|---|---|---|
| Gy/GBq | ||||||||
| 1st | Voxel | 0.31 | 0.30 | 0.03 | 0.01 | 0.11 | 0.24 | 0.80 |
| MIRD | 0.36 | 0.35 | 0.03 | 0.03 | ||||
| 2nd | Voxel | 0.39 | 0.35 | 0.03 | 0.03 | 0.12 | 0.20 | 0.51 |
| MIRD | 0.42 | 0.40 | 0.04 | 0.04 | ||||
| 3rd | Voxel | 0.37 | 0.34 | 0.03 | 0.03 | 0.11 | 0.04 | 0.37 |
| MIRD | 0.40 | 0.39 | 0.04 | 0.03 | ||||
| 4th | Voxel | 0.43 | 0.36 | 0.04 | 0.05 | 0.12 | 0.21 | 0.30 |
| MIRD | 0.47 | 0.42 | 0.05 | 0.05 | ||||
| Cycle of RLT | LLN | DLN1 | DLN2 | B1 | B2 | B3 |
|---|---|---|---|---|---|---|
| Gy/GBq | ||||||
| 1st | 6.89 | 25.57 | 6.8 | 5.55 | 6.25 | 3.75 |
| 2nd | 6.03 | 9.43 | 3.04 | 0.64 | 1.85 | 1.96 |
| 3rd | 2.84 | 8.3 | 2.59 | 0.48 | 0.97 | 1.86 |
| 4th | 2.72 | 7.24 | 2.91 | N/A | N/A | N/A |
| Cycle of RLT | LLN | DLN1 | DLN2 | B1 | B2 | B3 | |
|---|---|---|---|---|---|---|---|
| 1st; Volume (mL) | 0.67 | 3.9 | 0.6 | 1.5 | 0.4 | 0.3 | |
| SUVmax at | 4 h | 2.69 | 33.56 | 24.73 | 9.5 | 5.72 | 2.9 |
| 24 h | 2.64 | 14.36 | 11.2 | 4.21 | 2.33 | 0.96 | |
| 48 h | 2.06 | 9.8 | 8.9 | 2.61 | 1.56 | 0.66 | |
| 2nd; Volume (mL) | 0.37 | 1.22 | 0.4 | 1.5 | 0.4 | 0.3 | |
| SUVmax at | 4 h | 1.14 | 5.73 | 3.56 | 1.83 | 1.23 | 1.32 |
| 24 h | 0.73 | 2.28 | 1.56 | 0.92 | 0.42 | 0.27 | |
| 48 h | 0.68 | 1.43 | 1.28 | 0.64 | 0.29 | 0.15 | |
| 3rd; Volume (mL) | 0.1 | 0.82 | 0.26 | 1.5 | 0.4 | 0.3 | |
| SUVmax at | 4 h | N/A | 3.12 | N/A | 1.02 | N/A | N/A |
| 24 h | N/A | 1.19 | 0.94 | 0.52 | N/A | N/A | |
| 48 h | N/A | 0.76 | N/A | 0.35 | N/A | N/A | |
| 4th; Volume (mL) | 0.1 | 0.5 | 0.13 | 1.5 | 0.4 | 0.3 | |
| SUVmax at | 4 h | N/A | 1.96 | N/A | 0.66 | N/A | N/A |
| 24 h | N/A | 0.79 | 0.5 | 0.32 | N/A | N/A | |
| 48 h | N/A | 0.52 | N/A | 0.22 | N/A | N/A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelk, E.; Ruuge, P.; Rohtla, K.; Poksi, A.; Kairemo, K. Radiomics Analysis for 177Lu-DOTAGA-(l-y)fk(Sub-KuE) Targeted Radioligand Therapy Dosimetry in Metastatic Prostate Cancer—A Model Based on Clinical Example. Life 2021, 11, 170. https://doi.org/10.3390/life11020170
Kelk E, Ruuge P, Rohtla K, Poksi A, Kairemo K. Radiomics Analysis for 177Lu-DOTAGA-(l-y)fk(Sub-KuE) Targeted Radioligand Therapy Dosimetry in Metastatic Prostate Cancer—A Model Based on Clinical Example. Life. 2021; 11(2):170. https://doi.org/10.3390/life11020170
Chicago/Turabian StyleKelk, Eve, Priit Ruuge, Kristi Rohtla, Anne Poksi, and Kalevi Kairemo. 2021. "Radiomics Analysis for 177Lu-DOTAGA-(l-y)fk(Sub-KuE) Targeted Radioligand Therapy Dosimetry in Metastatic Prostate Cancer—A Model Based on Clinical Example" Life 11, no. 2: 170. https://doi.org/10.3390/life11020170
APA StyleKelk, E., Ruuge, P., Rohtla, K., Poksi, A., & Kairemo, K. (2021). Radiomics Analysis for 177Lu-DOTAGA-(l-y)fk(Sub-KuE) Targeted Radioligand Therapy Dosimetry in Metastatic Prostate Cancer—A Model Based on Clinical Example. Life, 11(2), 170. https://doi.org/10.3390/life11020170








