Effects of Schlemm’s Canal Expansion: Biomechanics and MIGS Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Experimental Protocol
2.3. Imaging System and Scanning Procedures
2.4. Data Analysis
3. Results
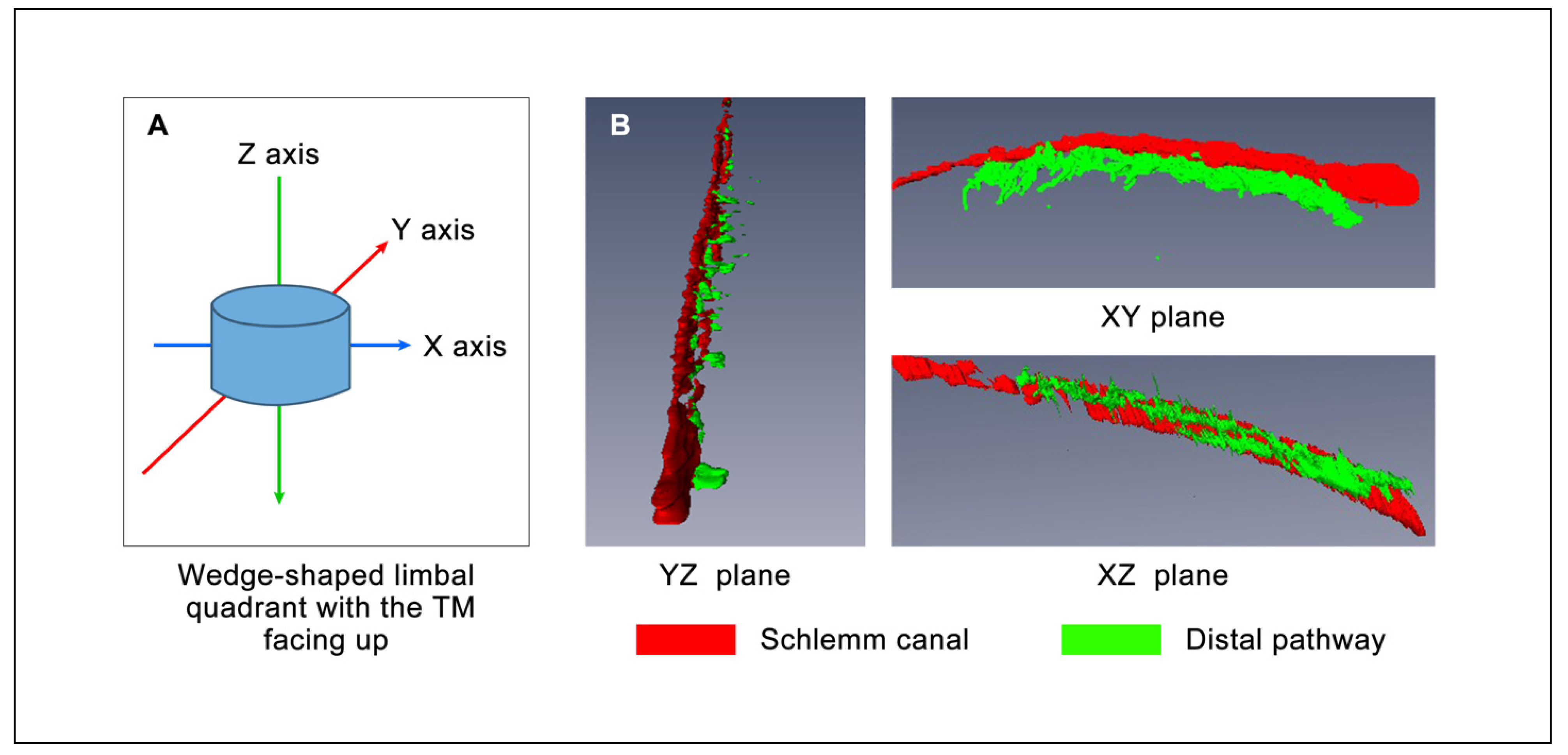
3.1. D Volumetric Imaging
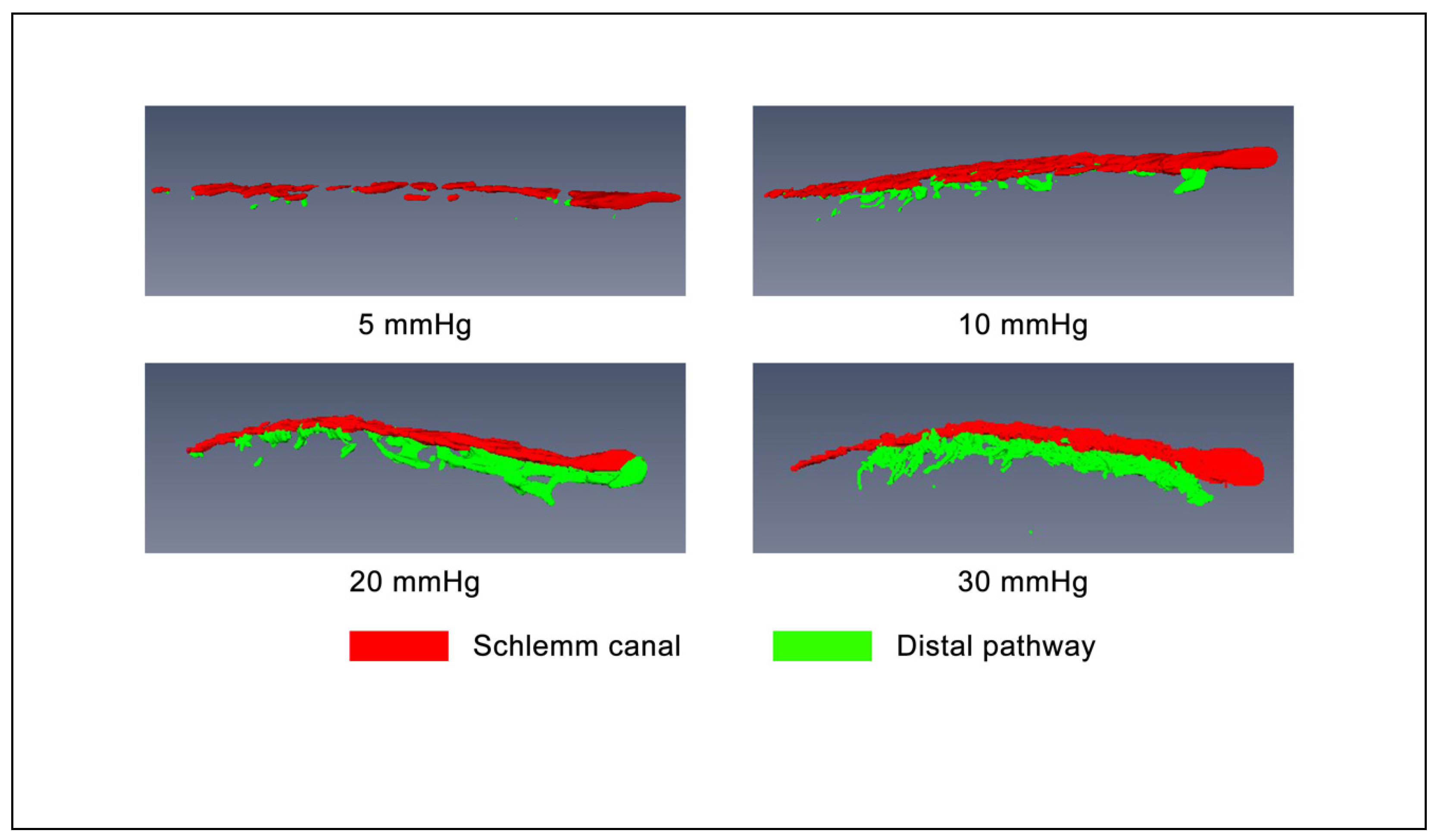
3.2. Morphology with 2D Cross-Sectional Imaging
3.3. Volumetric Stress-Strain Curves (Elastance Curves)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schirmer, K.E. Reflux of blood in the canal of Schlemm, quantitated. Can. J. Ophthalmol. 1969, 4, 40–44. [Google Scholar]
- Suson, E.B.; Schultz, R.O. Blood in Schlemm’s canal in glaucoma suspects. A study of the relationship between blood-filling pattern and outflow facility in ocular hypertension. Arch. Ophthalmol. 1969, 81, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, K.E. Gonioscopic Assessment of Blood in Schlemm’s Canal: Correlation with glaucoma tests. Arch. Ophthalmol. 1971, 85, 263–267. [Google Scholar] [CrossRef]
- Johnstone, M.; Martin, E.; Jamil, A. Pulsatile flow into the aqueous veins: Manifestations in normal and glaucomatous eyes. Exp. Eye Res. 2011, 92, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, M.; Xin, C.; Tan, J.; Martin, E.; Wen, J.; Wang, R.K. Aqueous outflow regulation—21st-century concepts. Prog. Retin. Eye Res. 2020, 100917. [Google Scholar] [CrossRef]
- Khatib, T.Z.; Meyer, P.A.; Lusthaus, J.; Manyakin, I.; Mushtaq, Y.; Martin, K.R. Hemoglobin Video Imaging Provides Novel In Vivo High-Resolution Imaging and Quantification of Human Aqueous Outflow in Patients with Glaucoma. Ophthalmol. Glaucoma 2019, 2, 327–335. [Google Scholar] [CrossRef]
- Lusthaus, J.A.; Khatib, T.Z.; Meyer, P.A.R.; McCluskey, P.; Martin, K.R. Aqueous outflow imaging techniques and what they tell us about intraocular pressure regulation. Eye 2021, 35, 216–235. [Google Scholar] [CrossRef]
- Goldmann, H. Uber Abflussdruck und Glasstab-phanomen. Pathogenese des einfachen Glaukoms. Ophthalmologica 1948, 116, 193. [Google Scholar]
- Ascher, K.W. The Aqueous Veins: Biomicroscopic Study of the Aqueous Humor Elimination; Charles C. Thomas: Springfield, IL, USA, 1961. [Google Scholar]
- Kagemann, L.; Wang, B.; Wollstein, G.; Ishikawa, H.; Mentley, B.; Sigal, I.A.; Bilonick, R.A.; Schuman, J.S. Trabecular Meshwork Response to Pressure Elevation in the Living Human Eye. J. Vis. Exp. 2015, e52611. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Reif, R.; Zhi, Z.; Shen, T.; Wang, R.K.; Martin, E.; Johnstone, M. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo nonhuman primate eyes. J. Biomed. Opt. 2012, 17, 076026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; An, L.; Lan, G.; Johnstone, M.; Malchow, D.; Wang, R.K. Extended imaging depth to 12 mm for 1050-nm spectral domain optical coherence tomography for imaging the whole anterior segment of the human eye at 120-kHz A-scan rate. J. Biomed. Opt. 2013, 18, 016012. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Song, S.; Johnstone, M.A.; Wang, R.K.; Wen, J.C. Trabecular meshwork motion in normal compared with glaucoma eyes. Investig. Ophthalmol. Vis Sci. 2020, 61, 21. [Google Scholar] [CrossRef]
- Hariri, S.; Johnstone, M.; Jiang, Y.; Padilla, S.; Zhou, Z.; Reif, R.; Wang, R.K. Platform to investigate aqueous outflow system structure and pressure-dependent motion using high-resolution spectral domain optical coherence tomography. J. Biomed. Opt. 2014, 19, 106013. [Google Scholar] [CrossRef]
- Xin, C.; Johnstone, M.; Wang, N.; Wang, R.K. OCT Study of Mechanical Properties Associated with Trabecular Meshwork and Collector Channel Motion in Human Eyes. PLoS ONE 2016, 11, e0162048. [Google Scholar] [CrossRef] [Green Version]
- Xin, C.; Wang, R.K.; Song, S.; Shen, T.; Wen, J.; Martin, E.; Jiang, Y.; Padilla, S.; Johnstone, M. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp. Eye Res. 2017, 158, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hann, C.R.; Bentley, M.D.; Vercnocke, A.; Ritman, E.L.; Fautsch, M.P. Imaging the aqueous humor outflow pathway in human eyes by three-dimensional micro-computed tomography (3D micro-CT). Exp. Eye Res. 2011, 92, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Bentley, M.D.; Hann, C.R.; Fautsch, M.P. Anatomical Variation of Human Collector Channel Orifices. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1153–1159. [Google Scholar] [CrossRef]
- Johnstone, M. Intraocular pressure control through linked trabecular meshwork and collector channel motion. In Glaucoma Research and Clinical Advances: 2016 to 2018; Samples, J.R., Knepper, P.A., Eds.; Kugler Publications: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Smit, B.A.; Johnstone, M.A. Effects of viscoelastic injection into Schlemm’s canal in primate and human eyes: Potential relevance to viscocanalostomy. Ophthalmology 2002, 109, 786–792. [Google Scholar] [CrossRef]
- Johnstone, M.A.; Grant, W.M. Microsurgery of Schlemm’s Canal and the Human Aqueous Outflow System. Am. J. Ophthalmol. 1973, 76, 906–917. [Google Scholar] [CrossRef]
- Vranka, J.A.; Staverosky, J.A.; Raghunathan, V.; Acott, T.S. Elevated pressure influences relative distribution of segmental regions of the trabecular meshwork. Exp. Eye Res. 2020, 190, 107888. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.D.; Xu, J.; Gong, L.; Gong, H. Variations in active outflow along the trabecular outflow pathway. Exp. Eye Res. 2016, 146, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Butt, A.; Chien, J.L.; Ghassibi, M.P.; Furlanetto, R.L.; Netto, C.F.; Liu, Y.; Kirkland, W.; Liebmann, J.M.; Ritch, R.; et al. Characteristics and variations of in vivo Schlemm’s canal and collector channel microstructures in enhanced-depth imaging optical coherence tomography. Br. J. Ophthalmol. 2017, 1016, 808–813. [Google Scholar] [CrossRef]
- Wang, K.; Johnstone, M.A.; Xin, C.; Song, S.; Padilla, S.; Vranka, J.A.; Acott, T.S.; Zhou, K.; Schwaner, S.A.; Wang, R.K.; et al. Estimating Human Trabecular Meshwork Stiffness by Numerical Modeling and Advanced OCT Imaging. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4809–4817. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.; Epstein, M. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Appl. Mech. Rev. 2002, 55, B103–B104. [Google Scholar] [CrossRef]
- Battista, S.A.; Lu, Z.; Hofmann, S.; Freddo, T.; Overby, D.R.; Gong, H. Reduction of the Available Area for Aqueous Humor Outflow and Increase in Meshwork Herniations into Collector Channels Following Acute IOP Elevation in Bovine Eyes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5346–5352. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, C.; Song, S.; Wang, N.; Wang, R.; Johnstone, M. Effects of Schlemm’s Canal Expansion: Biomechanics and MIGS Implications. Life 2021, 11, 176. https://doi.org/10.3390/life11020176
Xin C, Song S, Wang N, Wang R, Johnstone M. Effects of Schlemm’s Canal Expansion: Biomechanics and MIGS Implications. Life. 2021; 11(2):176. https://doi.org/10.3390/life11020176
Chicago/Turabian StyleXin, Chen, Shaozhen Song, Ningli Wang, Ruikang Wang, and Murray Johnstone. 2021. "Effects of Schlemm’s Canal Expansion: Biomechanics and MIGS Implications" Life 11, no. 2: 176. https://doi.org/10.3390/life11020176
APA StyleXin, C., Song, S., Wang, N., Wang, R., & Johnstone, M. (2021). Effects of Schlemm’s Canal Expansion: Biomechanics and MIGS Implications. Life, 11(2), 176. https://doi.org/10.3390/life11020176








