Factors Affecting a Short-Term Response to Anti-VEGF Therapy in Diabetic Macular Edema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Intravitreal Anti-VEGF Injections
2.3. Imaging
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Baseline Ocular Characteristics
3.3. Response to Initial Anti-VEGF Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, G.S.; Cheung, N.; Simó, R.; Cheung, G.C.M.; Wong, T.Y. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017, 5, 143–155. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.-T.; D’Amico, D.J.; Folkman, J.; Yeo, T.-K.; Yeo, K.-T. Increased Vascular Endothelial Growth Factor Levels in the Vitreous of Eyes With Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef]
- Heier, J.S.; Korobelnik, J.-F.; Brown, D.M.; Schmidt-Erfurth, U.; Edoardo, M.; Midena, E.; Boyer, D.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology 2016, 123, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.P.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Mitchell, P.; Wong, T.Y. Management Paradigms for Diabetic Macular Edema. Am. J. Ophthalmol. 2014, 157, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McGuire, P.G.; Rangasamy, S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology 2015, 122, 1375–1394. [Google Scholar] [CrossRef]
- Dugel, P.U.; Layton, A.; Varma, R.B. Diabetic Macular Edema Diagnosis and Treatment in the Real World: An Analysis of Medicare Claims Data (2008 to 2010). Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Sophie, R.; Lu, N.; Campochiaro, P.A. Predictors of Functional and Anatomic Outcomes in Patients with Diabetic Macular Edema Treated with Ranibizumab. Ophthalmology 2015, 122, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.S.; Khurana, R.N.; Wieland, M.R.; Wang, P.-W.; Van Everen, S.A.; Tuomi, L. Influence of Glycosylated Hemoglobin on the Efficacy of Ranibizumab for Diabetic Macular Edema. Ophthalmology 2015, 122, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, S.; Tam, T.; Singh, R.P.; Kaiser, P.; Petkovsek, D.; Carneiro, G.; Zanella, M.T.; Ehlers, J.P. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J. Diabetes Complicat. 2014, 28, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B. Factors Associated With Changes in Visual Acuity and Central Subfield Thickness at 1 Year after Treatment for Diabetic Macular Edema with Ranibizumab. Arch. Ophthalmol. 2012, 130, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Koytak, A.; Altinisik, M.; Sari, E.S.; Artunay, O.; Akkan, J.C.U.; Tuncer, K. Effect of a single intravitreal bevacizumab injection on different optical coherence tomographic patterns of diabetic macular oedema. Eye 2013, 27, 716–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.H.; Yu, S.-Y.; Kim, M.; Kwak, H.W. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina 2016, 36, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Channa, R.; The READ-2 Study Group; Sophie, R.; A Khwaja, A.; Do, D.V.; Hafiz, G.; Nguyen, Q.D.; A Campochiaro, P.; Abraham, P.; Green, B.; et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye 2013, 28, 269–278. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Wu, A.-L.; Chuang, C.-C.; Chen, S.-N. Factors influencing clinical outcomes in patients with diabetic macular edema treated with intravitreal ranibizumab: Comparison between responder and non-responder cases. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Yasuda, K.; Yasuda, M.; Nakazawa, T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina 2013, 33, 740–747. [Google Scholar] [CrossRef]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of diabetic macular edema with optical coherence tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- Murakami, T.; Nishijima, K.; Sakamoto, A.; Ota, M.; Horii, T.; Yoshimura, N. Foveal Cystoid Spaces Are Associated with Enlarged Foveal Avascular Zone and Microaneurysms in Diabetic Macular Edema. Ophthalmology 2011, 118, 359–367. [Google Scholar] [CrossRef]
- Singh, R.P.; Habbu, K.; Ehlers, J.P.; Lansang, M.C.; Hill, L.; Stoilov, I. The Impact of Systemic Factors on Clinical Response to Ranibizumab for Diabetic Macular Edema. Ophthalmology 2016, 123, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Wykoff, C.C.; Elman, M.J.; Regillo, C.D.; Ding, B.; Lu, N.; Stoilov, I. Predictors of Diabetic Macular Edema Treatment Frequency with Ranibizumab During the Open-Label Extension of the RIDE and RISE Trials. Ophthalmology 2016, 123, 1716–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.K.; Shin, H.Y.; Kim, S.Y.; Lee, Y.C.; Lee, M.Y. Factors Influencing Intravitreal Bevacizumab and Triamcinolone Treatment in Patients with Diabetic Macular Edema. Eur. J. Ophthalmol. 2017, 27, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Roh, M.; Kwon, O.W.; Koh, H.J. Effects of Macular Ischemia on the Outcome of Intravitreal Bevacizumab Therapy for Diabetic Macular Edema. Retina 2008, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Douvali, M.; Chatziralli, I.P.; Theodossiadis, P.G.; Chatzistefanou, K.I.; Giannakaki, E.; Rouvas, A.A. Effect of Macular Ischemia on Intravitreal Ranibizumab Treatment for Diabetic Macular Edema. Ophthalmology 2014, 232, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, P.; Kim, Y.; Yu, S.-Y.; Kwak, H.-W. Effect of Intravitreal Bevacizumab Based on Optical Coherence Tomography Patterns of Diabetic Macular Edema. Ophthalmology 2011, 226, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Tso, M.O. Pathology of Cystoid Macular Edema. Ophthalmology 1982, 89, 902–915. [Google Scholar] [CrossRef]
- Fine, B.S.; Brucker, A.J. Macular Edema and Cystoid Macular Edema. Am. J. Ophthalmol. 1981, 92, 466–481. [Google Scholar] [CrossRef]
- Rayess, N.; Rahimy, E.; Ying, G.-S.; Bagheri, N.; Ho, A.C.; Regillo, C.D.; Vander, J.F.; Hsu, J. Baseline Choroidal Thickness as a Predictor for Response to Anti–Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema. Am. J. Ophthalmol. 2015, 159, 85–91.e3. [Google Scholar] [CrossRef]
- Koyanagi, Y.; Yoshida, S.; Kobayashi, Y.; Kubo, Y.; Nakama, T.; Ishikawa, K.; Nakao, S.; Hisatomi, T.; Ikeda, Y.; Oshima, Y.; et al. Visual Outcomes Based on Early Response to Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema. Ophthalmology 2018, 239, 94–102. [Google Scholar] [CrossRef]
- Shah, A.R.; Yonekawa, Y.; Todorich, B.; Van Laere, L.; Hussain, R.; Woodward, M.A.; Abbey, A.M.; Wolfe, J.D. Prediction of Anti-VEGF Response in Diabetic Macular Edema After 1 Injection. J. Vitr. Dis. 2017, 1, 169–174. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, D.H.; Kim, M. Optical coherence tomography findings predictive of response to treatment in diabetic macular edema. J. Int. Med. Res. 2018, 46, 4455–4464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salz, D.A.; De Carlo, T.E.; Adhi, M.; Moult, E.M.; Choi, W.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Fujimoto, J.G.; Waheed, N.K. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared With Fluorescein Angiography and Normal Eyes. JAMA Ophthalmol. 2016, 134, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, M.B.; Kato, R.T.; Kniggendorf, V.F.; Cole, E.D.; Onal, S.; Torres, E.; Louzada, R.; Belfort, R.; Duker, J.S.; Novais, E.A.; et al. Comparison of Optical Coherence Tomography Angiography and Fluorescein Angiography for the Identification of Retinal Vascular Changes in Eyes With Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 1013–1019. [Google Scholar] [CrossRef]
- Lee, J.; Gil Moon, B.; Cho, A.R.; Yoon, Y.H. Optical Coherence Tomography Angiography of DME and Its Association with Anti-VEGF Treatment Response. Ophthalmology 2016, 123, 2368–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Total (N = 123 Eyes, 102 Patients) | Good Responders (N = 81 Eyes, 64 Patients) 1 | Poor Responders (N = 42 Eyes, 38 Patients) 2 | p-Value 3 |
|---|---|---|---|---|
| Age | 63.4 ± 10.8 | 62.1 ± 11.4 | 65.9 ± 9.2 | 0.061 |
| Gender (male:female) | 59:43 | 43:21 | 21:17 | 0.290 |
| HbA1c (%) | 7.2 ± 1.1 | 7.0 ± 1.2 | 7.5 ± 0.9 | 0.011 |
| Hypertension (yes:no) | 77:46 | 52:29 | 25:17 | 0.695 |
| Diastolic blood pressure (mmHg) | 135.9 ± 20.6 | 136.3 ± 22.3 | 135.1 ± 17.0 | 0.362 |
| Systolic blood pressure (mmHg) | 72.5 ± 12.4 | 73.2 ± 13.4 | 71.0 ± 10.1 | 0.350 |
| Nephropathy (yes:no) | 48:75 | 35:46 | 13:29 | 0.243 |
| Insulin therapy (yes:no) | 56:67 | 34:47 | 22:20 | 0.340 |
| Parameters | Total (N = 123) | Good Responders (N = 81) 1 | Poor Responders (N = 42) 2 | p-Value 3 | |
|---|---|---|---|---|---|
| LogMAR visual acuity (baseline) | 0.54 ± 0.31 | 0.52 ± 0.27 | 0.58 ± 0.37 | 0.416 | |
| PDR:NPDR | 28:95 | 22:59 | 6:36 | 0.119 | |
| PRP (yes:no) | 95:28 | 65:16 | 30:12 | 0.364 | |
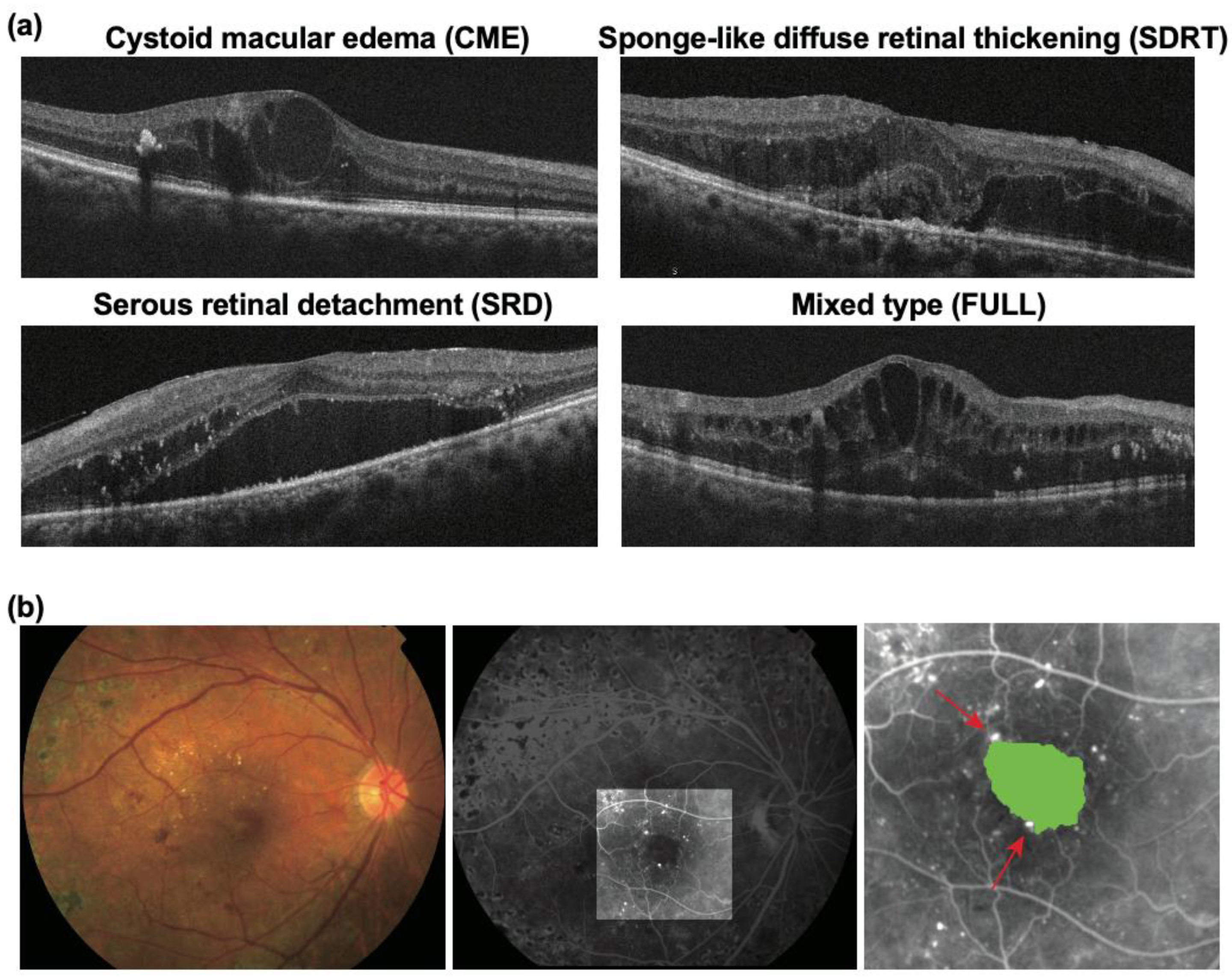
| OCT | CME | 45 (36.6%) | 25 (30.9%) | 20 (47.6%) | 0.060 |
| SDRT | 46 (37.4%) | 32 (39.5%) | 14 (33.3%) | ||
| SRD | 6 (4.9%) | 4 (4.9%) | 2 (4.8%) | ||
| FULL | 26 (21.1%) | 20 (24.7%) | 6 (14.3%) | ||
| FA | FAZ size (mm2) | 0.54 ± 0.28 | 0.47 ± 0.23 | 0.67 ± 0.33 | 0.0003 |
| Number of MAs in the PCN | 1.9 ± 2.2 | 1.4 ± 2.0 | 2.7 ± 2.2 | 0.0007 | |
| Parameter | Good Responders (N = 81) 1 | Poor Responders (N = 42) 2 | p-Value 3 |
|---|---|---|---|
| Ranibizumab: aflibercept (%) | 66.2:65.5 | 33.8:34.5 | >0.999 |
| Baseline CMT (μm) | 567.14 ± 164.31 | 517.54 ± 105.64 | 0.156 |
| CMT one month after anti-VEGF therapy (μm) | 314.07 ± 104.00 | 484.07 ± 98.92 | <0.0001 |
| % reduction | 43.00 ± 14.55 | 6.13 ± 8.93 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usui-Ouchi, A.; Tamaki, A.; Sakanishi, Y.; Tamaki, K.; Mashimo, K.; Sakuma, T.; Ebihara, N. Factors Affecting a Short-Term Response to Anti-VEGF Therapy in Diabetic Macular Edema. Life 2021, 11, 83. https://doi.org/10.3390/life11020083
Usui-Ouchi A, Tamaki A, Sakanishi Y, Tamaki K, Mashimo K, Sakuma T, Ebihara N. Factors Affecting a Short-Term Response to Anti-VEGF Therapy in Diabetic Macular Edema. Life. 2021; 11(2):83. https://doi.org/10.3390/life11020083
Chicago/Turabian StyleUsui-Ouchi, Ayumi, Asaka Tamaki, Yoshihito Sakanishi, Kazunori Tamaki, Keitaro Mashimo, Toshiro Sakuma, and Nobuyuki Ebihara. 2021. "Factors Affecting a Short-Term Response to Anti-VEGF Therapy in Diabetic Macular Edema" Life 11, no. 2: 83. https://doi.org/10.3390/life11020083
APA StyleUsui-Ouchi, A., Tamaki, A., Sakanishi, Y., Tamaki, K., Mashimo, K., Sakuma, T., & Ebihara, N. (2021). Factors Affecting a Short-Term Response to Anti-VEGF Therapy in Diabetic Macular Edema. Life, 11(2), 83. https://doi.org/10.3390/life11020083





