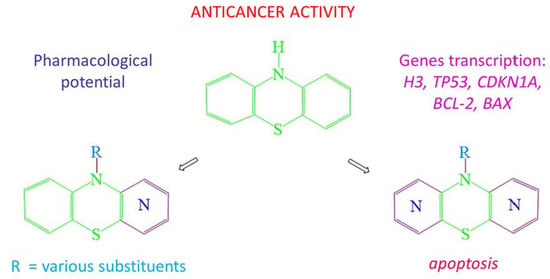
Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents
Abstract
:1. Introduction
2. Pyridobenzothiazines
2.1. 1-Azaphenothiazines (Pyrido[3,2,-b]benzo[1,4]thiazines)
2.2. 2-Azaphenothiazines (Pyrido[4,3-b]benzo[1,4]thiazines)
2.3. 3-Azaphenothiazines (Pyrido[3,4-b]benzo[1,4]thiazines)
2.4. 4-Azaphenothiazines (Pyrido[2,3-b]benzo[1,4]thiazines)
3. Dipyridothiazines
3.1. 1,6-Diazaphenothiazines (Dipyrido[2,3-b;2’,3’-e][1,4]thiazines)
3.2. 1,8-Diazaphenothiazines (Dipyrido[2,3-b;3’,4’-e][1,4]thiazines)
3.3. 1,9-Diazaphenothiazines (Dipyrido[2,3-b;2’,3’-e][1,4]thiazines).
3.4. 2,7-Diazaphenothiazines (Dipyrido[3,4-b;3’,4’-e][1,4]thiazines)
3.5. 3,6-Diazaphenothiazines (Dipyrido[2,3-b;4’,3’-e][1,4]thiazines)
3.6. 3,7-Diazaphenothiazines (Dipyrido[3,4-b;4’,3’-e][1,4]thiazines)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reinhardt, C.; Travis, A.S. Heinrich Caro and the Creation of Modem Chemical Industry, Chemists and Chemistry; Springer Science + Business Media: Dordrecht, The Netherlands, 1998; Volume 19, pp. 242–245. [Google Scholar]
- Bernthsen, A. Ű̈ber das Methylenblau. Ber. Dtsch. Chem. Ges. 1883, 16, 2896–2904. [Google Scholar] [CrossRef]
- Gupta, R.R.; Kumar, M. Synthesis, properties and reactions of phenothiazines. In Phenothiazine and 1,4-Benzothiazines—Chemical and Biological Aspect; Gupta, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 1–161. [Google Scholar]
- Ohlow, M.J.; Moosmann, B. Foundation review: Phenothiazine: The seven lives of pharmacology’s first lead structure. Drug Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, D.; Liu, Z.; Liu, F. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett. 2018, 419, 257–265. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J.M.; Jeon, B.; Elkamhawy, A.; Paik, S.; Hong, J.; Oh, S.-J.; Paek, S.H.; Lee, C.J.; Hassan, A.H.E.; et al. Repositioning of the antipsychotic trifluoperazine: Synthesis, biological evaluation and in silico study of trifluoperazine analogs as anti-glioblastoma agents. Eur. J. Med. Chem. 2018, 151, 186–198. [Google Scholar] [CrossRef]
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chiang, J.-H.; Weng, J.-R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents—A drug repurposing strategy. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Dastidar, S.G.; Palchoudhuri, S.; Roy, D.S.; Das, S.; Hendricks, O.; Christensen, J.B. Phenothiazines as a solution for multidrug resistant tuberculosis: From the origin to present. Int. Microb. 2015, 18, 1–12. [Google Scholar] [CrossRef]
- Amaral, L.; Viveiros, M. Thioridazine: A non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, A. Phenothiazines as anti-tubercular agents: Mechanistic insights and clinical implications. Expert Opin. Investig. Drugs 2011, 20, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, S.; Bhat, M.F.; Kumar, A.; Singh, S.K. Phenothiazine: A better scaffold against tuberculosis. Mini-Rev. Med. Chem. 2017, 17, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Csonka1, Á.; Molnár, J.; Amaral, L. The anticancer activity of the old neuroleptic phenothiazine-type drug thioridazine. Anticancer Res. 2016, 36, 5701–5706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible biological and clinical applications of phenothiazines. Anticancer Res. 2017, 37, 5983–5993. [Google Scholar] [CrossRef] [Green Version]
- Sudeshna, G.; Parimal, K. Muliple non-psychiatric effect of phenothiazines: A review. Eur. J. Pharmacol. 2010, 648, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Huang, D.; Qin, L.; Zheng, Z.; Hua, L.; Wang, G.; Huang, J.; Huang, H. Targeting lung cancer stem cells with antipsychological drug thioridazine. BioMed Res. Int. 2016. [Google Scholar] [CrossRef] [Green Version]
- Nagy, S.; Argyelan, G.; Molnár, J.; Kawase, M.; Motohashi, N. Antitumor activity of phenothiazine-related compounds. Anticancer Res. 1996, 16, 1915–1918. [Google Scholar]
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E.; Puente, J.; Valenzuela, M.A. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am. J. Therapeut. 2006, 13, 261–273. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M.; Zaczyńska, E. Azaphenothiazines a promising phenothiazine derivatives. An insight into nomenclature, synthesis, structure elucidation and biological properties. Eur. J. Med. Chem. 2017, 138, 774–806. [Google Scholar] [CrossRef]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. J. Heterocycl. Chem. 2009, 46, 355–391. [Google Scholar] [CrossRef]
- Motohashi, N. Antitumor activity of phenothiazines (phenothiazine oncology). In Phenothiazines and 1,4-Benzothiazines. Chemical and Biological Aspects; Gupta, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 705–770. [Google Scholar]
- Sakagami, H.; Takahashi, H.; Yoshida, H.; Yamamura, M.; Fukuchi, K.; Gomi, K.; Motohashi, N.; Takeda, M. Induction of DNA fragmentation in human Myelogenous Leukaemic cell lines by phenothiazine-related compounds. Anticancer Res. 1995, 15, 2533–2540. [Google Scholar] [PubMed]
- Motohashi, N.; Sakagami, H.; Kamata, K.; Yamamoto, Y. Cytotoxicity and differentiation-inducing activity of phenothiazine and benzo[a]phenothiazine derivatives. Anticancer Res. 1991, 11, 1933–1937. [Google Scholar] [PubMed]
- Wuonola, M.A.; Palfreyman, M.G.; Motohashi, N.; Kawase, M.; Gabay, S.; Gupta, R.R.; Molnár, J. The primary in vitro anticancer activity of “half-mustard type” phenothiazines in NCI’s revised anticancer screening paradigm. Anticancer Res. 1998, 18, 337–348. [Google Scholar] [PubMed]
- Motohashi, N.; Kurihara, T.; Sakagami, H.; Szabo, D.; Csuri, K.; Molnár, J. Chemical structure and tumor type specificity of “half-mustard type” phenothiazines. Anticancer Res. 1999, 19, 1859–1864. [Google Scholar]
- Motohashi, N.; Kawase, M.; Kurihara, T. Synthesis and antitumor activity of 1-[2(chloroethyl)-3-(-substituted-10H-phenothiazin-10-yl)]alkyl-1-ureas as potent anticancer agents. Anticancer Res. 1996, 16, 2525–2532. [Google Scholar]
- Motohashi, N.; Kawase, M.; Saito, S. Synthesis and biological activity of N-acylphenothiazines. Int. J. Antimicrob. Agents. 2000, 14, 203–207. [Google Scholar] [CrossRef]
- Motohashi, N.; Kawase, M.; Saito, S.; Sakagami, H. Antitumor potential and possible targets of phenothiazine-related compounds. Curr. Drug Targets 2000, 1, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Kawase, M.; Satoh, K.; Sakagami, H. Cytotoxic potential of phenothiazines. Curr. Drug Targets 2006, 7, 1055–1066. [Google Scholar] [CrossRef]
- Gaye-Seye, M.D.; Aaron, J.J.; Parkanyi, C.; Motohashi, N. Luminescence and photophysical properties of benzo[a]phenothiazines-therapeutic, physico-chemical and analytical applications. Curr. Drug Targets 2006, 7, 1083–1093. [Google Scholar] [CrossRef]
- Bisi, A.; Meli, M.; Gobbi, S.; Rampa, A.; Tolomeo, M.; Dusonchet, L. Multidrug resistance reverting activity and antitumor profile of new phenothiazine derivatives. Bioorg. Med. Chem. 2008, 16, 6474–6482. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Yadav, A.; Gautam, N.; Gautam, D.C. Study and synthesis of biologically active phenothiazines, their sulfones, and ribofuranosides. Nucleos. Nucleot. Nucl. Acids 2012, 31, 680–691. [Google Scholar] [CrossRef]
- Gautam, N.; Garg, A.; Lal, T.; Gautam, D.C.; Joshi, J. Synthesis and antimicrobial assessment of new substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides. Heterocycl. Commun. 2014, 20, 343–349. [Google Scholar] [CrossRef]
- Prinz, H.; Ridder, A.-K.; Vogel, K.; Böhm, K.J.; Ivanov, I.; Ghasemi, J.B.; Aghaee, E.; Müller, K. N-Heterocyclic (4-phenylpiperazin-1-yl)methanones derived from phenoxazine and phenothiazine as highly potent inhibitors of tubulin polymerization. J. Med. Chem. 2017, 60, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Guo, J.-M.; Zhang, T.-T.; Lin, H.-J.; Qi, N.-S.; Li, Z.-G.; Zhou, J.-C.; Zhang, Z.-Z. Antiproliferative phenothiazine hybrids as novel apoptosis inducers against MCF-7 breast cancer. Molecules 2018, 23, 1288. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, K.G.; Kumar, C.U.; Lim, W.-M.; Mai, C.-W.; Thanikachalam, P.V.; Ramalingan, C. Novel cyanoacetamide integrated phenothiazines: Synthesis, characterization, computational studies and in vitro antioxidant and anticancer evaluations. J. Mol. Struct. 2020, 1199, 127037. [Google Scholar] [CrossRef]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 2011, 46, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Gopi, C.; Dhanaraju, M.D. Recent progress in synthesis, structure and biological activities of phenothiazine derivatives. Rev. J. Chem. 2019, 9, 95–126. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, T.-Y.; Bai, W.-F.; Bai, C.-G. Design, synthesis and evaluation of novel phenothiazine derivatives as inhibitors of breast cancer stem cells. Eur. J. Med. Chem. 2019, 183, 111692. [Google Scholar] [CrossRef] [PubMed]
- Yale, H.L.; Bernstein, J. Azaphenothiazine Compound and Their Preparation. U.S. Patent 2943086, 6 May 1960. [Google Scholar]
- Uhrig, S.; Hirth, N.; Broccoli, L.; Von Wilmsdorff, M.; Bauer, M.; Sommer, C.; Zink, M.; Steiner, J.; Frodl, T.; Malchow, B.; et al. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: A post-mortem study. Schizophr. Res. 2016, 177, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.; Pjrek, E.; Lanzenberger, R.; Baldinger, P.; Eitel, D.; Kasper, S.; Frey, R. Actigraphy in patients with treatment-resistant depression undergoing electroconvulsive therapy. J. Psychiatr. Res. 2014, 57, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kleinmann, A.; Schrader, V.; Stübner, S.; Greil, W.; Kahl, K.G.; Bleich, S.; Grohmann, R.; Frieling, H.; Toto, S. Psychopharmacological treatment of 1650 in-patients with acute mania-data from the AMSP study. J. Affect. Disord. 2016, 191, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Scharfetter, J.; Fischer, P. QTc prolongation induced by intravenous sedation with Haloperidol, Prothipendyl and Lorazepam. Neuropsychiatrie 2014, 28, 1–5. [Google Scholar] [CrossRef]
- Declercq, T.; Petrovic, M.; Azermani, M.; Vander Stichle, R.; De Sutter, A.I.; Van Driel, M.L.; Christiaens, T. Withdrawal versous continuation of chronic antipsychotic drugs for behavioral and psycholofical symptoms in older people with dementia. Cochrane Database Syst. Rev. 2013, 28, 1–95. [Google Scholar] [CrossRef] [Green Version]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis and anticancer and lipophilic properties of 10-dialkylaminobutynyl derivatives of 1,8- and 2,7-diazaphenothiazines. J. Enzym. Inhib. Med. Chem. 2016, 31, 1132–1138. [Google Scholar] [CrossRef] [Green Version]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Suwińska, K.; Jeleń, M.; Kuśmierz, D. 3,6-Diazaphenothiazines as potential lead molecules—Synthesis, characterization and anticancer activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 1512–1519. [Google Scholar] [CrossRef] [Green Version]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M. Synthesis, spectroscopic characterization, and anticancer activity of new 10-substituted 1,6-diazaphenothiazines. Med. Chem. Res. 2016, 25, 2425–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, K.; Kaushik, N.K.; Kaushik, N.; Chand, M.; Kaushik, R.; Ha Choi, E.; Jain, S.C. Novel aminoalkylated azaphenothiazines as potential inhibitors of T98G, H460 and SNU80 cancer cell lines in vitro. Bioorg. Med. Chem. Lett. 2016, 26, 2237–2244. [Google Scholar] [CrossRef]
- Kaur, P.; Hann Chu, J.J. Chikungunya virus: An update on antiviral development and challenges. Drug Discov. Today 2013, 18, 969–983. [Google Scholar] [CrossRef]
- Hagerdorn, H.W.; Zuck, S.; Schulz, R. Prothipendyl: Detection and elimination in the horse—A casereport. Dtsch Tierarztl Wochenschr 1996, 103, 125–127. [Google Scholar]
- Martindale, the Extra Pharmacopoeia, 29th ed.; Reynolds, J.E.F. (Ed.) Pharmaceutical Press: London, UK, 1989. [Google Scholar]
- Shaikh, S.M.T.; Seetharamappa, J.; Kandagal, P.B.; Ashoka, S. Binding of the bioactive component isothipendyl hydrochloride with bovine serum albumin. J. Mol. Struct. 2006, 786, 46–52. [Google Scholar] [CrossRef]
- Moreau, A.; Dompmartin, A.; Dubreuil, A.; Leroy, D. Phototoxic and photoprotective effects of topical isothipendyl. Photodermatol. Photoimmunol. Photomed. 1995, 11, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Bibas, N.; Sartor, V.; Bulai Livideanu, C.; Bagheri, H.; Nougue, J.; Giordano- Labadie, F.; Maza, A.; Paul, C.; Chouini-Lalanne, N.; Marguery, M.C. Contact photoallergy to isothipendyl chlorohydrate. Dermatology 2012, 224, 289–291. [Google Scholar] [CrossRef]
- Amin, A.S.; El-Sheikh, R.; Zahran, F.; El-fetough Gouda, A.A. Spectrophotometric determination of pipazethate HCl, dextromethorphan HBr and drotaverine HCl in their pharmaceutical preparations. Spectrochim. Acta A 2007, 67, 1088–1093. [Google Scholar] [CrossRef]
- Atkinson, E.R.; Russ, P.L.; Tucker, M.T. Neuropharmacological profile of 1-azaphenothiazine-10-thiolcarboxylates. J. Med. Chem. 1971, 14, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tyagi, E. Synthesis of some substituted pyrido[3,2-b][1,4]benzothiazines and their antibacterial activity. Pharmazie 1991, 46, 746–747. [Google Scholar]
- Swati, S.S.; Mishira, A.K.; Prakash, L. Synthesis of some novel 1-azaphenothiazines and their mesoionic as analogues of popent CNS-depressants. Phosphorus Sulfur Silicon Relat. Elem. 1996, 117, 111–120. [Google Scholar] [CrossRef]
- Agrawal, H.; Yador, A.K.; Prakash, L. An elegant synthesis of some new potential biologically active pyrido[3,3-b][1,4]benzothiazine derivatives and their nucleosides by phase transfer catalysis. Heterocycl. Commun. 1998, 4, 589–594. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, G.; Khatoon, S.; Yadav, A. Synthesis and antimicrobial activities of novel 10H-pyrido[3,2-b][1,4]benzo[b]thiazine ribofuranosides. Indian J. Chem. B 2003, 42, 2015–2018. [Google Scholar] [CrossRef]
- Madrid, P.; Polgar, W.; Toll, L.; Tanga, M. Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonin receptors. Bioorg. Med. Chem. Lett. 2007, 17, 3014–3017. [Google Scholar] [CrossRef]
- Saggiomo, A.; Craig, P.; Gordon, M. Synthesis of 2-aza- and 8-chloro-2-aza-phenothiazine. J. Org. Chem. 1958, 23, 1906–1909. [Google Scholar] [CrossRef]
- Okafor, C.O. Studies in the heterocyclic series. III. The Chemistry of azaphenothiazine compounds. Int. J. Sulfur Chem. B 1971, 6, 239–265. [Google Scholar]
- Zirkle, C.L.; Kaiser, C. Antipsychotic agents (tricyclic), In Psychopharmacological Agents; Gordon, M., Ed.; Academic Press: New York, NY, USA, 1974; Volume 3, pp. 39–128. [Google Scholar]
- Clarke, F.H.; Silverman, G.B.; Watnick, C.M.; Sperber, N. 3-Azaphenothiazine and dialkylaminoalkyl derivatives. J. Org. Chem. 1961, 26, 1126–1132. [Google Scholar] [CrossRef]
- Chorvat, R.J.; Desai, B.N.; Radak, S.E.; Bloss, J.; Hirsch, J.; Tenen, S. Synthesis, benzodiazepine receptor binding, and anticonvulsant activity of 2,3-dihydro-3-oxo-5H-pyrido[3,4-b][1,4]benzothiazine-4-carbonitriles. J. Med. Chem. 1983, 26, 845–850. [Google Scholar] [CrossRef]
- Rhone-Poulenc, Azaphenothiazines and Intermediates. British Patent 791190, 26 February 1958.
- Yamada, K.; Miyamoto, M.; Tatsuya, T.; Sato, K.; Soejima, M.; Sato, T.; Kikuchi, K.; Yoshimura, H.; Moriya, K.; Sakuma, Y. Preparation of Heterocycle-Fused Benzothiazine Derivatives as Allergy Inhibitors. Japan Patent WO 9943683 A1 19990909, 2 September 1999. [Google Scholar]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents. Molecules 2019, 24, 4388. [Google Scholar] [CrossRef] [Green Version]
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1, 8-diazaphenothiazines. Med. Chem. Res. 2015, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, K.; Morak-Młodawska, B.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. 10H-1,8-Diazaphenothiazine, Its 10-Substituted Derivatives, Their Usage, the Way of Synthesis and Their Pharmaceutical Compositions. Polish Patent PL 227918 B1, 10 July 2013. [Google Scholar]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D.; Suwińska, K.; Shkurenko, A.; Czuba, Z.; Jurzak, M. 10H-1,9-diazaphenothiazine and its 10-derivatives: Synthesis, characterisation and biological evaluation as potential anticancer agents. J. Enzyme Inhib. Med. Chem. 2019, 34, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M. Anticancer activity of newly synthesized azaphenothiazines in NCI’s anticancer screening. Pharmacol. Rep. 2010, 62, 319–332. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Wenzhi, Z.; Okechukwu, P.N.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Md Akim, A.; Ang, K.-P.; Ooi, K.K. 10H-3,6-Diazaphenothiazines Induce G2/M Phase Cell Cycle Arrest, Caspase-dependent Apoptosis and Inhibits Cell Invasion of A2780 Ovarian Carcinoma Cells through Regulation on NF-κB and [BIRC6-XIAP] Complexes. Drug Des. Dev. Ther. 2017, 11, 3045–3063. [Google Scholar] [CrossRef] [Green Version]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis, anticancer activity and apoptosis induction of novel 3,6-diazaphenothiazines. Molecules 2019, 24, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodig, O.; Collier, R.; Schlatzer, R. Pyridine chemistry. II. Further studies on the Smiles rearrangement of the 3-amino-2,2‘-dipyridyl sulfide system. The synthesis of some 1,6-diazaphenothiazines. J. Med. Chem. 1965, 9, 116–120. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Lipophilicity of New Anticancer 1,6- and 3,6-diazaphenothiazines by of Use RP TLC and Different Computational Methods. J. Chrom. Sci. 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Estimation of the Lipophilicity of New Anticancer and Immunosuppressive 1,8-Diazaphenothiazine Derivatives. J. Chrom. Sci. 2015, 53, 462–466. [Google Scholar] [CrossRef] [Green Version]
- Rath, S. Dimethylaminopropyldipyridothiazine. U.S. Patent 2,789,978, 23 April 1957. [Google Scholar]
- Morak, B.; Pluta, K.; Suwińska, K. Unexpected simple route to novel dipyrido-1,4-thiazine system. Heterocyclic Commun. 2002, 8, 331–334. [Google Scholar] [CrossRef]
- Morak, B.; Pluta, K. Synthesis of novel dipyrido-1,4-thiazines. Heterocycles 2007, 71, 1347–1361. [Google Scholar]
- Zimecki, M.; Artym, J.; Kocięba, M.; Pluta, K.; Morak-Młodawska, B.; Jeleń, M. The immunosupressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell. Mol. Biol. Lett. 2009, 14, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B. Anticancer activity of selected dipyridothiazines and diquinothiazines determined in National Cancer Institute, in Bethesdzie, USA. Farm. Przegląd Nauk. 2009, 10, 26–29. [Google Scholar]
- Morak-Młodawska, B.; Pluta, K.; Matralis, A.N.; Kourounakis, A.P. Antioxidant activity of newly synthesized 2,7-diazaphenothiazines. Archiv. Pharm. Chem. Life Sci. 2010, 343, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Evaluation of the Lipophilicity of New Anticancer 1,2,3-Triazole-Dipyridothiazine Hybrids Using RP TLC and Different Computational Methods. Processes 2020, 8, 858. [Google Scholar] [CrossRef]
- Okafor, C. Studies in heterocyclic series. I. A novel diazaphenothiazine system. J. Org. Chem. 1967, 32, 2006–2007. [Google Scholar] [CrossRef]
- Okafor, C. Heterocyclic series. II. 3,6-Diazaphenothiazine sulfoxides and other potential antiparasitic and pesticidal agents. J. Chem. Eng. Data 1971, 16, 244–246. [Google Scholar] [CrossRef]
- Kopp, E.; Strell, M. Űber 2,7-diazaphenothiazin. Reaktionen in der Pyridinreihe, IV. Arch. Pharm. 1962, 295, 99–106. [Google Scholar] [CrossRef]
- Strell, M.; Kopp, E.; Janson, R. Verfahren zur Herstellung von 2,7-Diazaphenothiazinen. German Patent DE 1 147 235, 18 April 1963. [Google Scholar]
- Kopp, E. Tautomerie an einem 2, 7‐Diazaphenothiazinderivat. Reaktionen in der Pyridinreihe, V. Arch. Pharm. 1962, 295, 561–564. [Google Scholar] [CrossRef]
- Werle, E.; Kopp, E.; Leysath, G. Arzneim-Forsch Die Antihistaminwirkung von 2,7-Diazaphenothiazin und einiger seiner derivate. Arzneim-Forsch 1962, 4, 443–444. [Google Scholar]


















| Investigated Compounds | Substituents at the Thiazine Nitrogen Atom | Activity, IC50 [Ref] | Cancer Cell Lines |
|---|---|---|---|
| Pyridobenzothiazines | |||
| 1-Azaphenothiazine 3 | 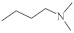 | 23.2 µg/mL [45,46,47] 28.1 µg/mL 32.3 µg/mL 36.6 µg/mL | C-32 MCF-7 T47D SNB-19 |
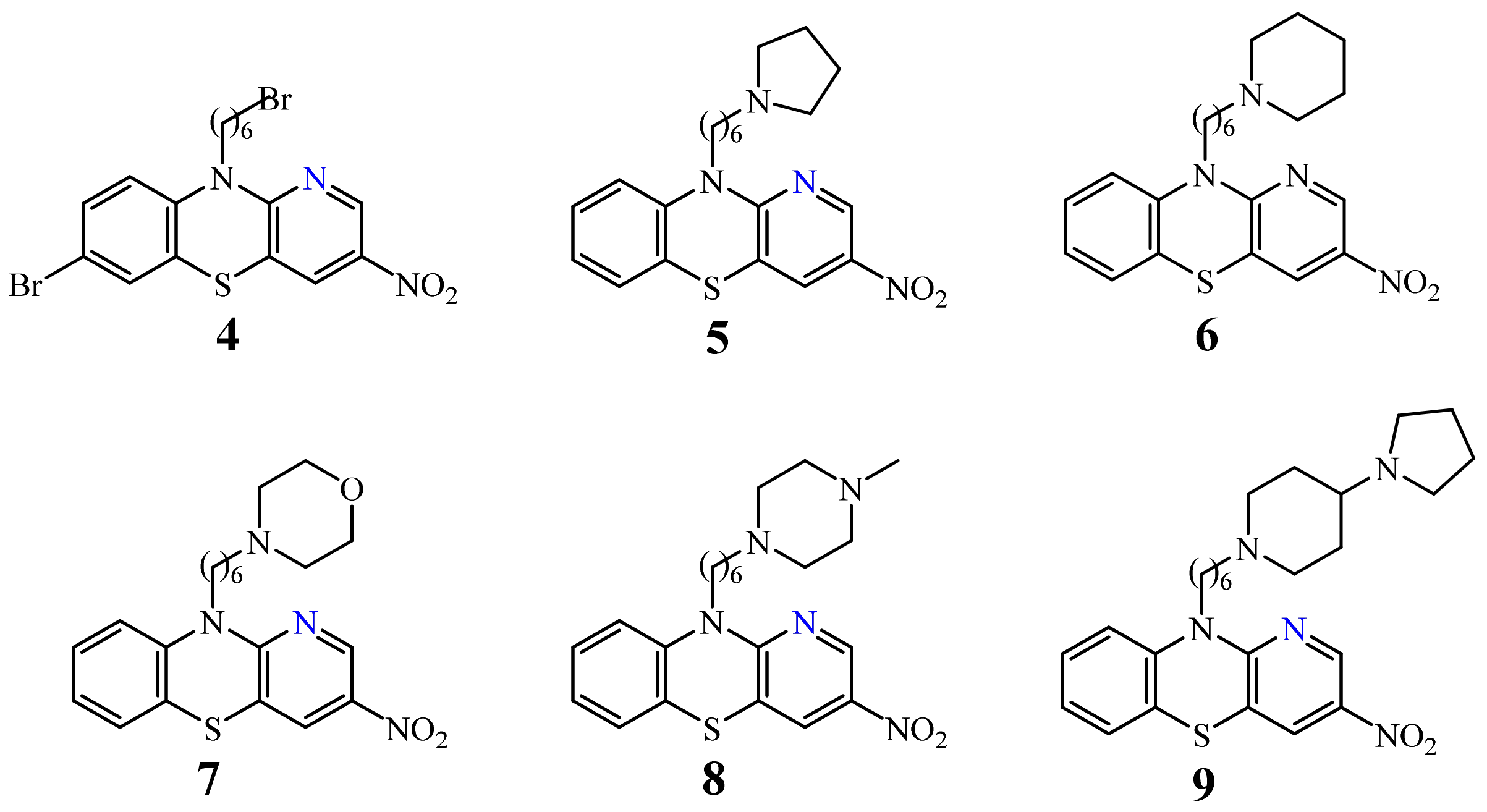
| 4 | 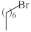 | 3.8–6.2 µg/mL [48] | H460,T98G, SNU80 |
| 6 | 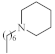 | 2.27–3.8 µg/mL [48] | H460, T98G, SNU80 |
| 7 | 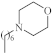 | 2.5 µg/mL [48] | H460 |
| 9 | 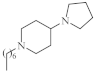 | 2.1–2.3 µg/mL [48] | H460, SNU80 |
| Dipyridothiazines | |||
| 1,6-Diazaphenothiazine 13 | H | 4.8 µg/mL [47] 7.5 µg/mL | MCF-7 C-32 |
| 14 |  | 3.9 µg/mL [47] | MCF-7 |
| 15 | 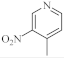 | 4.6 µg/mL [47] | MCF-7 |
| 16 | 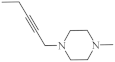 | 7.5 µg/mL [47] | MCF-7 |
| 17 | 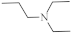 | 6.6 µg/mL [47] | C-32 |
| 18 |  | 18.9 µg/mL [47] | SNB-19 |
| 19 | 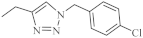 | 0.25-4.66 µM [69] | SNB-19, Caco-2, A549, MDA-MB231 |
| 1,8-Diazaphenothiazine | |||
| 20 | H | 5 µg/mL [70,71] 10 µg/mL | SW-948 L-1210. |
| 23 | 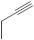 | 26-46 µg/mL [70] | SNB-19, C-32, T47D |
| 24 | 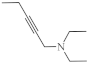 | 26.1 µg/mL [70] | C-32 |
| 25 | 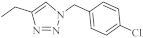 | 1.82 µM [69] | A549 |
| 1,9-Diazaphenothiazine | |||
| 26 | H | 3.83 µM [72] | C-32 |
| 27 | 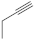 | 3.85 µM [72] 3.37 µM | SNB-19 C-32 |
| 28 | 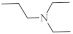 | 0.34 µM [72] 2.13 µM | SNB-19 MDA-MB-321 |
| 2,7-Diazaphenothiazine | |||
| 29 | H | 0.3 µg/mL [73] 1.7 µg/mL 2.4 µg/mL 3.6 µg/mL 3.1 µg/mL 3.9 µg/mL 5.5 µg/mL 4.1 µg/mL 5.9 µg/mL 6.5 µg/mL 6.8 µg/mL 7.1 µg/mL 8.4 µg/mL | HOP-62 HOP-92 colon 205 HTC-116 RXF 393 736-0 ACHN HL-60(TB) HS 578T M-14SF-539, SNB-19 OVCAR-8 PC-3 |
| 31 | 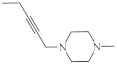 | 9.6 µg/mL [45] | T-47D |
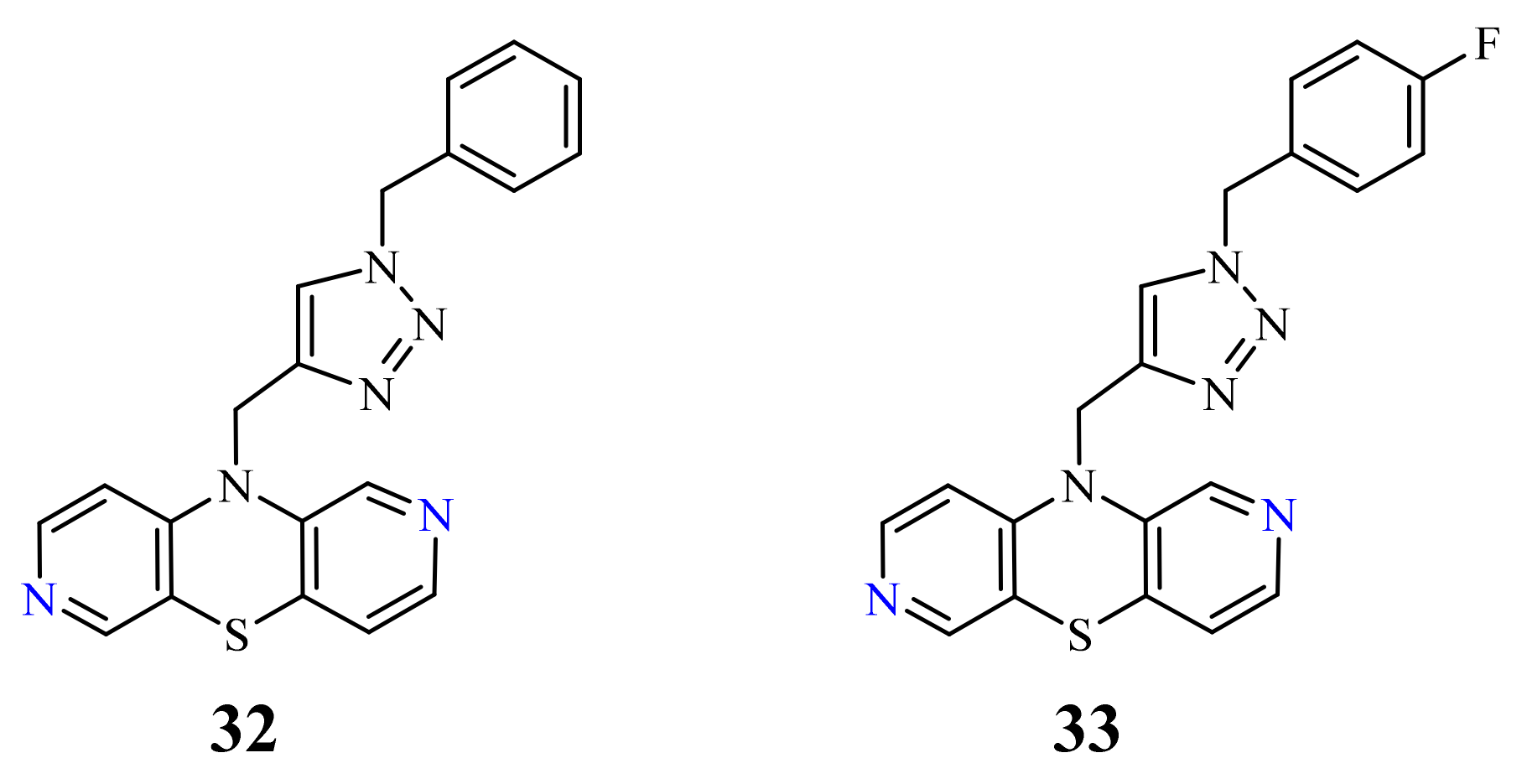
| 32 | 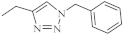 | 0.26 µM [69] 0.77 µM | Caco-2, A549 MDA-MB231 |
| 33 | 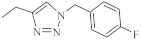 | 0.64 µM [69] 0.65 µM | MDA-MB231 A549 |
| 3,6-Diazaphenothiazine | |||
| 34 | H | 0.46 µg/mL [46] 0.72 µg/mL 0.62 µM [74] | SNB-19 C-32, MCF-7 A2780 |
| 35 | 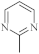 | 0.73 µg/mL [46] | MCF-7 |
| 36 | 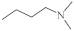 | 6.3 µg/mL [46] 11.3 µg/mL | C-32 MCF-7 |
| 37 | 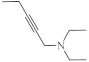 | 0.11 µg/mL [75] | SNB-19 |
| 38 | 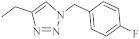 | 0.25 µM [69] | Caco-2, A549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morak-Młodawska, B.; Jeleń, M.; Pluta, K. Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents. Life 2021, 11, 206. https://doi.org/10.3390/life11030206
Morak-Młodawska B, Jeleń M, Pluta K. Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents. Life. 2021; 11(3):206. https://doi.org/10.3390/life11030206
Chicago/Turabian StyleMorak-Młodawska, Beata, Małgorzata Jeleń, and Krystian Pluta. 2021. "Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents" Life 11, no. 3: 206. https://doi.org/10.3390/life11030206