Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution
Abstract
1. Introduction
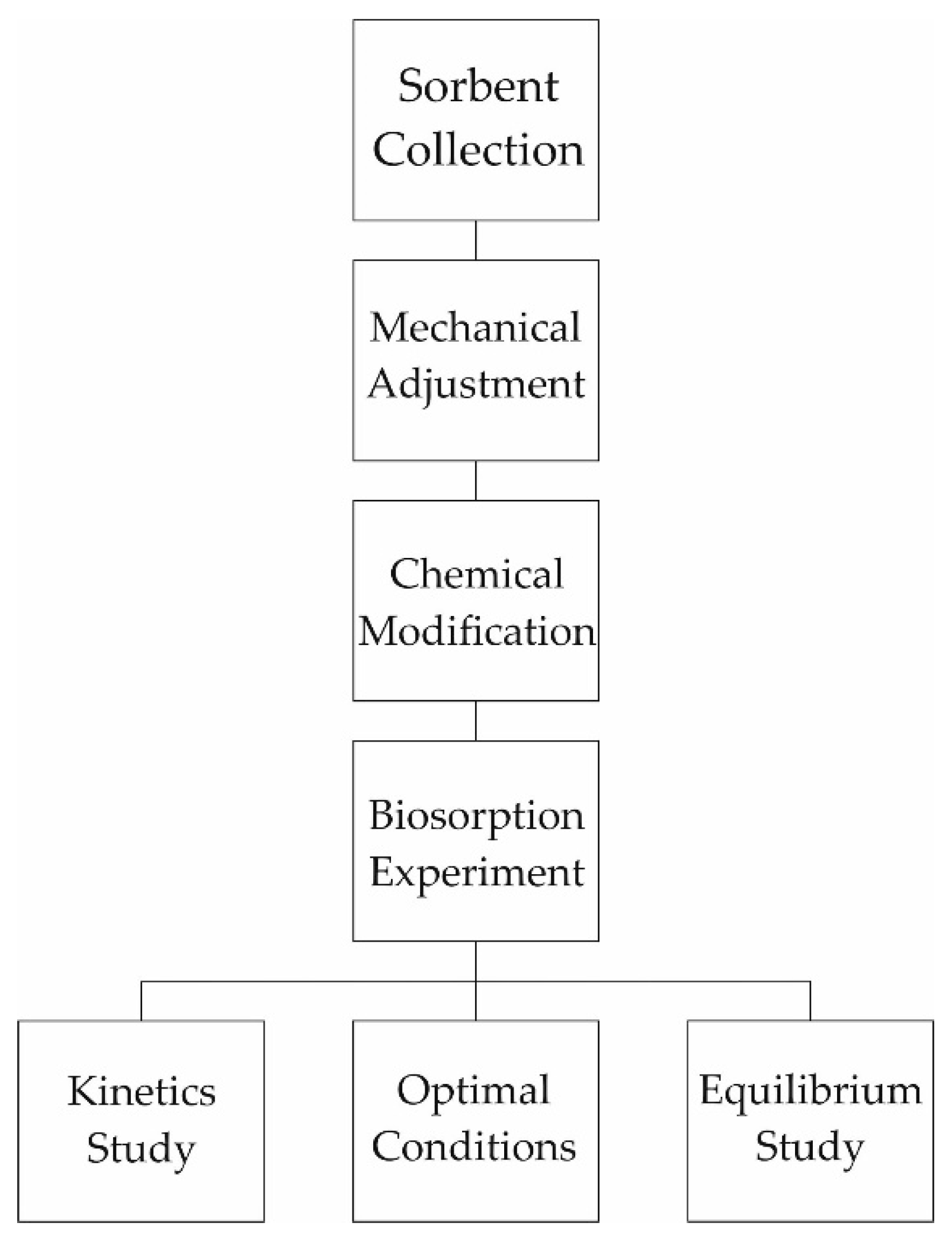
2. Materials and Methods
2.1. Biosorbent Preparation Methodology
2.2. Methodology of the Biosorption Modeling Process
2.2.1. Adsorption Kinetics Modeling
- q
- adsorption capacity of the biosorbent, the amount of solute adsorbed at equilibrium per unit of adsorbent weight (mg·g−1),
- ci
- initial concentration of adsorbate in the solution (mg·L−1),
- ce
- equilibrium concentration of adsorbate in the solution (mg·L−1),
- S
- sorbent weight (g),
- V
- sorbate solution volume (L).
- q
- adsorption capacity, the amount of solute adsorbed at equilibrium per unit of adsorbent weight, (mg·g−1),
- qt
- amount of solute adsorbed at each time t (min) per unit of adsorbent weight, (mg·g−1),
- k1
- equilibrium speed constant of the first order pseudo-equation, (min−1),
2.2.2. Optimal Conditions
- R
- universal gas constant (8.314 J·mol−1·K−1),
- T
- thermodynamic temperature (K),
- K, K1, and K2
- equilibrium constants at absolute temperatures of T, T1, and T2 (K).
- q
- adsorption capacity, amount of solute adsorbed at equilibrium per unit of adsorbent weight (mg·g−1),
- ce
- equilibrium concentration of adsorbate in solution (mg·L−1).
- KF
- Freundlich constant, also known as Freundlich capacity (mg·g−1),
- 1/n
- Freundlich constant, indicates the intensity of adsorption,
- q
- amount of solute adsorbed per unit of adsorbent weight (mg·g−1),
- ce
- equilibrium concentration of the solute in the solution volume (mg·L−1).
- q
- adsorption capacity, amount of solute adsorbed per unit of adsorbent weight (mg·g−1),
- Qmax
- maximum metal adsorption under constant conditions (mg·g−1);
- KL
- Langmuir constant related to metal–sorbent affinity;
- ce
- equilibrium concentration of adsorbate in solution (mg·L−1).
- b
- parameter from the straight-line equation,
- ci
- initial metal concentration in solution (mg·L−1).
2.3. Cr (VI) Analysis Methodology
3. Results and Discussion
- External diffusion-transport of adsorbate from the solution by means of a liquid film to the outer surface of the sorbent.
- Internal diffusion-transport of adsorbate from the outer surface of the adsorbent to the pores of the sorbent.
- The adsorbate is adsorbed to the active groups on the inner and outer surface of the adsorbent.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yeon Park, J.; Yoo, Y.J.; Kwak, J.W. Removal of Lead Using Xanthated Marine Brown Alga, Undaria Pinnatifida. Process Biochem. 1999, 34, 647–652. [Google Scholar] [CrossRef]
- Gürişik, E.; Arica, M.Y.; Bektaş, S.; Genç, Ö. Comparison of the Heavy Metal Biosorption Capacity of Active, Heat-Inactivated and NaOH-Treated Phanerochaete Chrysosporium Biosorbents. Eng. Life Sci. 2004, 4, 86–89. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Park, J.M. Reduction of Hexavalent Chromium with the Brown Seaweed Ecklonia Biomass. Environ. Sci. Technol. 2004, 38, 4860–4864. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B. Sorption and Biosorption, 1st ed.; BV-Sorbex, Inc. St. Lambert: Montreal, QC, Canada, 2003; ISBN 0-9732983-0-8. [Google Scholar]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy Metal Adsorption by Modified Oak Sawdust: Thermodynamics and Kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of Heavy Metals from Aqueous Solutions by Chemically Modified Orange Peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Effective Removal of Heavy Metals from Aqueous Solutions by Orange Peel Xanthate. Trans. Nonferrous Met. Soc. China 2010, 20, s187–s191. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cao, X.; Lu, D.; Luo, F.; Shao, W. Preparation and Evaluation of Orange Peel Cellulose Adsorbents for Effective Removal of Cadmium, Zinc, Cobalt and Nickel. Colloids Surf. Physicochem. Eng. Asp. 2008, 317, 512–521. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; ul Haq, A. Removal of Lead From Aqueous Media Using Carbonized and Acid Treated Orange Peel. Tenside Surfactants Deterg. 2014, 51, 240–246. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/Desorption of Cd(II), Cu(II) and Pb(II) Using Chemically Modified Orange Peel: Equilibrium and Kinetic Studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Lugo-Lugo, V.; Hernández-López, S.; Barrera-Díaz, C.; Ureña-Núñez, F.; Bilyeu, B. A Comparative Study of Natural, Formaldehyde-Treated and Copolymer-Grafted Orange Peel for Pb(II) Adsorption under Batch and Continuous Mode. J. Hazard. Mater. 2009, 161, 1255–1264. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X. Characterization of Adsorptive Capacity and Mechanisms on Adsorption of Copper, Lead and Zinc by Modified Orange Peel. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Tian, Q. Adsorption of Pb2+ and Zn2+ from Aqueous Solutions by Sulfured Orange Peel. Desalination 2011, 275, 212–216. [Google Scholar] [CrossRef]
- Kumar, K.; Patavardhan, S.S.; Lobo, S.; Gonsalves, R. Equilibrium Study of Dried Orange Peel for Its Efficiency in Removal of Cupric Ions from Water. Int. J. Phytoremediat. 2018, 20, 593–598. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; Gonzalez-Gutierrez, L.V.; Baldenegro-Perez, L.A. Biosorbents Prepared from Orange Peels Using Instant Controlled Pressure Drop for Cu(II) and Phenol Removal. Ind. Crops Prod. 2016, 84, 344–349. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S. Adsorption Study of Copper (II) by Chemically Modified Orange Peel. J. Hazard. Mater. 2009, 164, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Khormaei, M.; Nasernejad, B.; Edrisi, M.; Eslamzadeh, T. Copper Biosorption from Aqueous Solutions by Sour Orange Residue. J. Hazard. Mater. 2007, 149, 269–274. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S. Enhanced Cu(II) Adsorption by Orange Peel Modified with Sodium Hydroxide. Trans. Nonferrous Met. Soc. China 2010, 20, s146–s152. [Google Scholar] [CrossRef]
- Lugo-Lugo, V.; Barrera-Díaz, C.; Ureña-Núñez, F.; Bilyeu, B.; Linares-Hernández, I. Biosorption of Cr(III) and Fe(III) in Single and Binary Systems onto Pretreated Orange Peel. J. Environ. Manag. 2012, 112, 120–127. [Google Scholar] [CrossRef]
- Pérez Marín, A.B.; Aguilar, M.I.; Meseguer, V.F.; Ortuño, J.F.; Sáez, J.; Lloréns, M. Biosorption of Chromium (III) by Orange (Citrus Cinensis) Waste: Batch and Continuous Studies. Chem. Eng. J. 2009, 155, 199–206. [Google Scholar] [CrossRef]
- Pakshirajan, K.; Worku, A.N.; Acheampong, M.A.; Lubberding, H.J.; Lens, P.N.L. Cr(III) and Cr(VI) Removal from Aqueous Solutions by Cheaply Available Fruit Waste and Algal Biomass. Appl. Biochem. Biotechnol. 2013, 170, 498–513. [Google Scholar] [CrossRef]
- López-Téllez, G.; Barrera-Díaz, C.E.; Balderas-Hernández, P.; Roa-Morales, G.; Bilyeu, B. Removal of Hexavalent Chromium in Aquatic Solutions by Iron Nanoparticles Embedded in Orange Peel Pith. Chem. Eng. J. 2011, 173, 480–485. [Google Scholar] [CrossRef]
- Pehlivan, E.; Pehlivan, E.; Tutar Kahraman, H. Hexavalent Chromium Removal by Osage Orange. Food Chem. 2012, 133, 1478–1484. [Google Scholar] [CrossRef]
- Ghimire, K.N.; Inoue, K.; Makino, K.; Miyajima, T. Adsorptive Removal of Arsenic Using Orange Juice Residue. Sep. Sci. Technol. 2002, 37, 2785–2799. [Google Scholar] [CrossRef]
- Khaskheli, M.I.; Memon, S.Q.; Siyal, A.N.; Khuhawar, M.Y. Use of Orange Peel Waste for Arsenic Remediation of Drinking Water. Waste Biomass Valorization 2011, 2, 423. [Google Scholar] [CrossRef]
- Farinella, N.V.; Matos, G.D.; Arruda, M.A.Z. Grape Bagasse as a Potential Biosorbent of Metals in Effluent Treatments. Bioresour. Technol. 2007, 98, 1940–1946. [Google Scholar] [CrossRef]
- Li, F.T.; Yang, H.; Zhao, Y.; Xu, R. Novel Modified Pectin for Heavy Metal Adsorption. Chin. Chem. Lett. 2007, 18, 325–328. [Google Scholar] [CrossRef]
- Cimino, G.; Passerini, A.; Toscano, G. Removal of Toxic Cations and Cr(VI) from Aqueous Solution by Hazelnut Shell. Water Res. 2000, 34, 2955–2962. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of Lead, Cadmium, Zinc, and Copper from Industrial Wastewater by Carbon Developed from Walnut, Hazelnut, Almond, Pistachio Shell, and Apricot Stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, V.S.; Cummings, G.; Maillacheruvu, K.Y.; Tang, W.Z. Food-Processing Wastes. Water Environ. Res. 2013, 85, 1501–1514. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption Studies on Ground Shells of Hazelnut and Almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, E.; Kobya, M.; Konukman, A.E.S. Error Analysis of Equilibrium Studies for the Almond Shell Activated Carbon Adsorption of Cr(VI) from Aqueous Solutions. J. Hazard. Mater. 2008, 154, 787–794. [Google Scholar] [CrossRef]
- Hashemian, S.; Salari, K.; Yazdi, Z.A. Preparation of Activated Carbon from Agricultural Wastes (Almond Shell and Orange Peel) for Adsorption of 2-Pic from Aqueous Solution. J. Ind. Eng. Chem. 2014, 20, 1892–1900. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. Biosorption of Chromium(VI) Ion from Aqueous Solutions Using Walnut, Hazelnut and Almond Shell. J. Hazard. Mater. 2008, 155, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wolfová, R.; Pertile, E.; Fečko, P. Removal of Lead from Aqueous Solution by Walnut Shell. J. Environ. Chem. Ecotoxicol. 2013, 5, 159–167. [Google Scholar] [CrossRef]
- Hajialigol, S.; Masoum, S. Optimization of Biosorption Potential of Nano Biomass Derived from Walnut Shell for the Removal of Malachite Green from Liquids Solution: Experimental Design Approaches. J. Mol. Liq. 2019, 286, 110904. [Google Scholar] [CrossRef]
- Lawal, O.S.; Ayanda, O.S.; Rabiu, O.O.; Adebowale, K.O. Application of Black Walnut (Juglans Nigra) Husk for the Removal of Lead (II) Ion from Aqueous Solution. Water Sci. Technol. 2017, 75, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Gondhalekar, S.C.; Shukla, S.R. Biosorption of Cadmium Metal Ions on Raw and Chemically Modified Walnut Shells. Environ. Prog. Sustain. Energy 2015, 34, 1613–1619. [Google Scholar] [CrossRef]
- Deniz, F. Effective Removal of Maxilon Red Grl from Aqueous Solutions by Walnut Shell: Nonlinear Kinetic and Equilibrium Models. Environ. Prog. Sustain. Energy 2014, 33, 396–401. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Szafran, R.G.; Modelski, S. Biosorption of Heavy Metals from Aqueous Solutions onto Peanut Shell as a Low-Cost Biosorbent. Desalination 2011, 265, 126–134. [Google Scholar] [CrossRef]
- Burevska, K.A.; Memedi, H.; Lisichkov, K.; Kuvendziev, S.; Marinkovski, M.; Ruseska, G.; Grozdanov, A. Biosorption of Nickel Ions from Aqueous Solutions by Natural and Modified Peanut Husks: Equilibrium and Kinetics. Water Environ. J. 2018, 32, 276–284. [Google Scholar] [CrossRef]
- Saha, A.; Bhaduri, D.; Pipariya, A.; Ghosh, R.K. Linear and Nonlinear Sorption Modelling for Adsorption of Atrazine onto Activated Peanut Husk. Environ. Prog. Sustain. Energy 2017, 36, 348–358. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of Lead(II) Ions from Aqueous Solution by Peanut Shells: Equilibrium, Thermodynamic and Kinetic Studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of Chromium(VI) from Industrial Wastewater onto Pistachio Hull Waste Biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Moradi, P.; Hayati, S.; Ghahrizadeh, T. Modeling and Optimization of Lead and Cobalt Biosorption from Water with Rafsanjan Pistachio Shell, Using Experiment Based Models of ANN and GP, and the Grey Wolf Optimizer. Chemom. Intell. Lab. Syst. 2020, 202, 104041. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sureshkumar, M.V. Removal of Chromium(VI) from Water and Wastewater Using Surfactant Modified Coconut Coir Pith as a Biosorbent. Bioresour. Technol. 2008, 99, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Pino, G.H.; Souza de Mesquita, L.M.; Torem, M.L.; Saavedra Pinto, G.A. Biosorption of Cadmium by Green Coconut Shell Powder. Miner. Eng. 2006, 19, 380–387. [Google Scholar] [CrossRef]
- Costa, A.W.M.C.; Guerhardt, F.; Júnior, S.E.R.R.; Cânovas, G.; Vanale, R.M.; de Coelho, D.F.; Ehrhardt, D.D.; Rosa, J.M.; BasileTambourgi, E.; Santana, J.C.C.; et al. Biosorption of Cr(VI) Using Coconut Fibers from Agro-Industrial Waste Magnetized Using Magnetite Nanoparticles. Environ. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- do Nascimento, J.M.; de Oliveira, J.D.; Leite, S.G.F. Chemical Characterization of Biomass Flour of the Babassu Coconut Mesocarp (Orbignya Speciosa) during Biosorption Process of Copper Ions. Environ. Technol. Innov. 2019, 16, 100440. [Google Scholar] [CrossRef]
- Gondhalekar, S.C.; Shukla, S.R. Enhanced Adsorption Performance of Oxidised Coconut Coir for Removal of Cd(II) Ions by Multi-Column Arrangement in Series. Environ. Sci. Pollut. Res. 2019, 26, 28022–28030. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.E.; Champagne, E.T. Agricultural Byproducts as Adsorbents for Metal Ions in Laboratory Prepared Solutions and in Manufacturing Wastewater. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1995, 30, 241–261. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, Z.Z.; Sun, C. Removal of Cr(VI) from Aqueous Solutions by Low-Cost Biosorbents: Marine Macroalgae and Agricultural by-Products. J. Hazard. Mater. 2008, 153, 1176–1184. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhan, Y.K.; Kaya, Y.; Cakici, A.; Faruk Algur, O. Biosorption of Chromium(VI) from Aqueous Solution by Cone Biomass of Pinus Sylvestris. Bioresour. Technol. 2002, 85, 155–158. [Google Scholar] [CrossRef]
- Micales, J.A.; Han, J.S.; Davis, J.L.; Young, R.A. Chemical Composition and Fungitoxic Activities of Pine Cone Extractives. In Mycotoxins, Wood Decay, Plant Stress, Biocorrosion, and General Biodeterioration; Llewellyn, G.C., Dashek, W.V., O’Rear, C.E., Eds.; Biodeterioration Research; Springer: Boston, MA, USA, 1994; pp. 317–332. ISBN 978-1-4757-9450-2. [Google Scholar]
- Kim, N.; Park, M.; Park, D. A New Efficient Forest Biowaste as Biosorbent for Removal of Cationic Heavy Metals. Bioresour. Technol. 2015, 175, 629–632. [Google Scholar] [CrossRef]
- Değirmen, G.; Kılıç, M.; Çepelioğullar, Ö.; Pütün, A.E. Removal of Copper(II) and Cadmium(II) Ions from Aqueous Solutions by Biosorption onto Pine Cone. Water Sci. Technol. 2012, 66, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, G.; Martín-Lara, M.A.; Dionisio-Ruiz, E.; Tenorio, G.; Calero, M. Copper Biosorption by Pine Cone Shell and Thermal Decomposition Study of the Exhausted Biosorbent. J. Ind. Eng. Chem. 2012, 18, 1741–1750. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E. Removal of Copper(II) from Aqueous Solutions by Biosorption on the Cone Biomass of Thuja Orientalis. Process Biochem. 2003, 38, 1627–1631. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of Copper from Aqueous Solution by Chemically Activated Pine Cone: A Kinetic Study. Chem. Eng. J. 2011, 175, 260–270. [Google Scholar] [CrossRef]
- Ucun, H.; Aksakal, O.; Yildiz, E. Copper(II) and Zinc(II) Biosorption on Pinus Sylvestris L. J. Hazard. Mater. 2009, 161, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, M. Comment on “Biosorption of Chromium(VI) from Aqueous Solution by Cone Biomass of Pinus Sylvestris#x201D; Bioresour. Technol. 2003, 89, 317–318. [Google Scholar] [CrossRef]
- Can, M.Y.; Kaya, Y.; Algur, O.F. Response Surface Optimization of the Removal of Nickel from Aqueous Solution by Cone Biomass of Pinus Sylvestris. Bioresour. Technol. 2006, 97, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Almendros, A.I.; Martín-Lara, M.A.; Ronda, A.; Pérez, A.; Blázquez, G.; Calero, M. Physico-Chemical Characterization of Pine Cone Shell and Its Use as Biosorbent and Fuel. Bioresour. Technol. 2015, 196, 406–412. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhana, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of Lead (II) from Aqueous Solution by Cone Biomass of Pinus Sylvestris. Desalination 2003, 154, 233–238. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of Lead(II) onto Pine Cone Powder: Studies on Biosorption Performance and Process Design to Minimize Biosorbent Mass. Carbohydr. Polym. 2010, 82, 1031–1042. [Google Scholar] [CrossRef]
- Tsibranska, I.; Hristova, E. Modelling of Heavy Metal Adsorption into Activated Carbon from Apricot Stones in Fluidized Bed. Chem. Eng. Process. Process Intensif. 2010, 49, 1122–1127. [Google Scholar] [CrossRef]
- Petrović, M.S.; Šoštarić, T.D.; Pezo, L.L.; Stanković, S.M.; Lačnjevac, Č.M.; Milojković, J.V.; Stojanović, M.D. Usefulness of ANN-Based Model for Copper Removal from Aqueous Solutions Using Agro Industrial Waste Materials. Chem. Ind. Chem. Eng. Q. 2015, 21, 249–259. [Google Scholar] [CrossRef]
- Lopičić, Z.R.; Jelena, M.V.; Tatjana, Š.D.; Marija, P.S.; Marija, M.L.; Lačnjevac, Č.; Stojanović, M.D. Influence of PH Value on Cu (II) Biosorption by Lignocellulose Peach Shell Waste Material. Hem. Ind. 2013, 67, 1007–1015. [Google Scholar] [CrossRef]
- Bouchalova, M.; Pertile, E.; Surovka, D.; Kučerová, R. Kinetics And Biosorption Efficiency Cr(Vi) By Using Peach Stones (Prunus Persica). In Proceedings of the 13th SGEM GeoConference on Ecology, Economics, Education And Legislation, Albena, Bulgaria, Bulgaria, 16–22 June 2013; SGEM World Science: Sofia, Bulgaria, 2013; Volume 1. [Google Scholar]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of Heavy Metal Ions from Aqueous Solutions by Activated Carbon Prepared from Apricot Stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Venkata Subbaiah, M.; Kalyani, S.; Sankara Reddy, G.; Boddu, V.M.; Krishnaiah, A. Biosorption of Cr(VI) from Aqueous Solutions Using Trametes Versicolor Polyporus Fungi. E-J. Chem. 2008, 5, 1–12. [Google Scholar] [CrossRef]
- Dursun, A.Y.; Uslu, G.; Tepe, O.; Cuci, Y.; Ekiz, H.İ. A Comparative Investigation on the Bioaccumulation of Heavy Metal Ions by Growing Rhizopus Arrhizus and Aspergillus Niger. Biochem. Eng. J. 2003, 15, 87–92. [Google Scholar] [CrossRef]
- Şatiroğlu, N.; Yalçınkaya, Y.; Denizli, A.; Arıca, M.Y.; Bektaş, S.; Genç, Ö. Application of NaOH Treated Polyporus Versicolor for Removal of Divalent Ions of Group IIB Elements from Synthetic Wastewater. Process Biochem. 2002, 38, 65–72. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of Heavy Metals with White-Rot Fungi. Enzyme Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Monier, M.; Nawar, N.; Abdel-Latif, D.A. Preparation and Characterization of Chelating Fibers Based on Natural Wool for Removal of Hg(II), Cu(II) and Co(II) Metal Ions from Aqueous Solutions. J. Hazard. Mater. 2010, 184, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, N.; Bektas, N. Chromium(VI) Sorption from Dilute Aqueous Solutions Using Wool. Desalin. Water Treat. 2009, 3, 43–49. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective Adsorption of Chromium(VI) in Industrial Wastewater Using Low-Cost Abundantly Available Adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) Removal from Aqueous Solution by Raw and Modified Lignocellulosic Materials: A Review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef]
- Lu, D.; Cao, Q.; Li, X.; Cao, X.; Luo, F.; Shao, W. Kinetics and Equilibrium of Cu(II) Adsorption onto Chemically Modified Orange Peel Cellulose Biosorbents. Hydrometallurgy 2009, 95, 145–152. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Kim, J.Y.; Park, J.M. How to Study Cr(VI) Biosorption: Use of Fermentation Waste for Detoxifying Cr(VI) in Aqueous Solution. Chem. Eng. J. 2008, 136, 173–179. [Google Scholar] [CrossRef]
- Namasivayam, C.; Ranganathan, K. Removal of Cd(II) from Wastewater by Adsorption on “Waste” Fe(III)Cr(III) Hydroxide. Water Res. 1995, 29, 1737–1744. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-Order Kinetic Model for the Sorption of Cadmium onto Tree Fern: A Comparison of Linear and Non-Linear Methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Djeribi, R.; Hamdaoui, O. Sorption of Copper(II) from Aqueous Solutions by Cedar Sawdust and Crushed Brick. Desalination 2008, 225, 95–112. [Google Scholar] [CrossRef]
- Fan, T.; Liu, Y.; Feng, B.; Zeng, G.; Yang, C.; Zhou, M.; Zhou, H.; Tan, Z.; Wang, X. Biosorption of Cadmium(II), Zinc(II) and Lead(II) by Penicillium Simplicissimum: Isotherms, Kinetics and Thermodynamics. J. Hazard. Mater. 2008, 160, 655–661. [Google Scholar] [CrossRef]
- Panayotova, M.I. Kinetics and Thermodynamics of Copper Ions Removal from Wastewater by Use of Zeolite. Waste Manag. 2001, 21, 671–676. [Google Scholar] [CrossRef]
- Aksu, Z.; Açıkel, Ü.; Kabasakal, E.; Tezer, S. Equilibrium Modelling of Individual and Simultaneous Biosorption of Chromium(VI) and Nickel(II) onto Dried Activated Sludge. Water Res. 2002, 36, 3063–3073. [Google Scholar] [CrossRef]
- Arfaoui, S.; Frini-Srasra, N.; Srasra, E. Modelling of the Adsorption of the Chromium Ion by Modified Clays. Desalination 2008, 222, 474–481. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Naiya, T.K.; Mandal, S.N.; Das, S.K. Adsorption, Kinetics and Equilibrium Studies on Removal of Cr(VI) from Aqueous Solutions Using Different Low-Cost Adsorbents. Chem. Eng. J. 2008, 137, 529–541. [Google Scholar] [CrossRef]
- Debnath, S.; Ghosh, U.C. Kinetics, Isotherm and Thermodynamics for Cr(III) and Cr(VI) Adsorption from Aqueous Solutions by Crystalline Hydrous Titanium Oxide. J. Chem. Thermodyn. 2008, 40, 67–77. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, İ.; Tümsek, F.; Karabacakoğlu, B. Adsorption of Chromium(VI) from Aqueous Solution by Activated Carbon Derived from Olive Bagasse and Applicability of Different Adsorption Models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Adsorption of Nickel(II) from Aqueous Solution onto Activated Carbon Prepared from Coirpith. Sep. Purif. Technol. 2001, 24, 497–505. [Google Scholar] [CrossRef]
- ASTM International. ASTM International. ASTM D1687-17, (2017). In Standard Test Methods for Chromium in Water; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Flickinger, M.C. Encyclopedia of Industrial Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780471799306. [Google Scholar]
- Liu, T.; Li, H.; Li, Z.; Xiao, X.; Chen, L.; Deng, L. Removal of Hexavalent Chromium by Fungal Biomass of Mucor Racemosus: Influencing Factors and Removal Mechanism. World J. Microbiol. Biotechnol. 2007, 23, 1685–1693. [Google Scholar] [CrossRef]
- Park, D.; Lim, S.-R.; Yun, Y.-S.; Park, J.M. Development of a New Cr(VI)-Biosorbent from Agricultural Biowaste. Bioresour. Technol. 2008, 99, 8810–8818. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U. Activated Carbons and Low Cost Adsorbents for Remediation of Tri- and Hexavalent Chromium from Water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Hye Jo, J.; Park, J.M. Mechanism of Hexavalent Chromium Removal by Dead Fungal Biomass of Aspergillus Niger. Water Res. 2005, 39, 533–540. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Liu, Y.; Li, X.; Zeng, G.; Bao, Z.; Zeng, X.; Chen, A.; Long, F. Adsorption of Chromium (VI) by Ethylenediamine-Modified Cross-Linked Magnetic Chitosan Resin: Isotherms, Kinetics and Thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Holan, Z.R.; Volesky, B. Biosorption of Lead and Nickel by Biomass of Marine Algae. Biotechnol. Bioeng. 1994, 43, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Potential of Tea Factory Waste for Chromium(VI) Removal from Aqueous Solutions: Thermodynamic and Kinetic Studies. Sep. Purif. Technol. 2007, 54, 291–298. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Fungi as biosorbents. In Biosorbents for Metal Ions; d.a.j. Wase, J., Forster, C.H., Eds.; Taylor & Francis: London, UK, 1997; pp. 67–80. [Google Scholar]
- Dupont, L.; Guillon, E. Removal of Hexavalent Chromium with a Lignocellulosic Substrate Extracted from Wheat Bran. Environ. Sci. Technol. 2003, 37, 4235–4241. [Google Scholar] [CrossRef]
- Dubey, S.S.; Gupta, R.K. Removal Behavior of Babool Bark (Acacia Nilotica) for Submicro Concentrations of Hg2+ from Aqueous Solutions: A Radiotracer Study. Sep. Purif. Technol. 2005, 41, 21–28. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of Several Metal Ions onto a Low-Cost Biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and Plant Derived Biomass for Removal of Heavy Metals from Wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.K.; Kaur, M.P.; Sud, D.; Garg, V.K. Removal of Hexavalent Chromium from Aqueous Solution by Adsorption on Treated Sugarcane Bagasse Using Response Surface Methodological Approach. Desalination 2009, 249, 475–479. [Google Scholar] [CrossRef]
- Horsfall, M.; Abia, A.A. Sorption of Cadmium(II) and Zinc(II) Ions from Aqueous Solutions by Cassava Waste Biomass (Manihot Sculenta Cranz). Water Res. 2003, 37, 4913–4923. [Google Scholar] [CrossRef]
- Hong, H.; Kim, H.; Baek, K.; Yang, J.-W. Removal of Arsenate, Chromate and Ferricyanide by Cationic Surfactant Modified Powdered Activated Carbon. Desalination 2008, 223, 221–228. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E. Quaternized Agricultural By-Products as Anion Exchange Resins. J. Environ. Manag. 2006, 78, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ho Lee, S.; Hun Jung, C.; Chung, H.; Yeal Lee, M.; Yang, J.-W. Removal of Heavy Metals from Aqueous Solution by Apple Residues. Process Biochem. 1998, 33, 205–211. [Google Scholar] [CrossRef]
| Sorbent | HCl Concentration mol·L−1 | Activation Time min | Grain Size mm | qt mg·g−1 | Exposure Time (t) min |
|---|---|---|---|---|---|
| Orange peels | 1.0 | 30 | <0.5 | 3.81 | 120 |
| Fomitopsis pinicola | 1.0 | 60 | <0.5 | 5.07 | 60 |
| Mixture of cones | 2.0 | 60 | <0.5 | 4.70 | 40 |
| Peach stones | 1.0 | 30 | <0.5 | 1.83 | 180 |
| Apricot stones | 2.0 | 15 | <0.5 | 0.88 | 180 |
| Walnut shells | 1.0 | 30 | <0.5 | 2.74 | 180 |
| Fleece | 2.0 | 15 | x | 4.44 | 180 |
| Biosorbent | The Adsorption Capacity qt mg·g−1 pH = 1.1 | The Adsorption Capacity qt mg·g−1 without pH Modification |
|---|---|---|
| Orange peels | q40 = 5.0 | q40 = 1.7 |
| Fomitopsis pinicola pinicola | q10 = 5.0 | q10 = 2.4 |
| Mixture of cones | q10 = 5.1 | q10 = 3.5 |
| Peach stones | q180 = 4.6 | q180 = 1.8 |
| Apricot stones | q180 = 3.7 | q180 = 0.9 |
| Walnut shells | q60 = 5.0 | q60 = 2.0 |
| Fleece | q180 = 4.7 | q180 = 1.2 |
| Biosorbent | Without pH Modification | pH = 1.1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp | qtheor | k2 | h10 | R2 | qexp | qtheor | k2 | h10 | R2 | |
| Orange peel | 3.8 | 2.4 | 0.18 | 0.3 | 0.722 | 5.0 | 5.0 | 7.13 | 168 | 0.999 |
| Fomitopsis pinicola | 5.0 | 5.5 | 0.53 | 3.1 | 0.995 | 5.0 | 5.0 | 263 | 6190 | 1.000 |
| Mixture of cones | 4.7 | 5.3 | 0.68 | 3.7 | 1.000 | 5.1 | 5.1 | 55.2 | 1321 | 1.000 |
| Peach stones | 1.8 | 1.8 | 0.10 | 0.1 | 0.978 | 4.6 | 5.0 | 0.36 | 1.38 | 0.995 |
| Apricot stones | 0.9 | 1.1 | 0.02 | 0.0 | 0.968 | 3.7 | 3.8 | 0.38 | 1.18 | 0.999 |
| Walnut shells | 2.7 | 3.0 | 0.12 | 0.2 | 0.987 | 5.0 | 5.2 | 0.57 | 3.70 | 0.993 |
| Fleece | 4.4 | 4.8 | 0.20 | 0.3 | 0.993 | 4.7 | 4.8 | 0.74 | 10.86 | 0.999 |
| Biosorbent | T K | ΔG0 kJ·mol−1 | ΔH0 kJ·mol−1 | ΔS0 J·mol−1·K−1 | R2 |
|---|---|---|---|---|---|
| Orange peel | 293 | −5.46 | −74 | 18.6 | 0.902 |
| 303 | −3.57 | 11.8 | 1.000 | ||
| 313 | −7.53 | 24.1 | 0.897 | ||
| Fomitopsis pinicola | 293 | −4.18 | −179 | 14.3 | 1.000 |
| 303 | −6.33 | 20.9 | 0.998 | ||
| 313 | −9.00 | 28.8 | 1.000 | ||
| Mixture of cones | 293 | −4.45 | −62 | 15.2 | 0.995 |
| 303 | −5.43 | 17.9 | 0.975 | ||
| 313 | −9.47 | 30.3 | 0.926 | ||
| Peach stones | 293 | −5.57 | −61 | 19.0 | 0.949 |
| 303 | −5.63 | 18.6 | 0.933 | ||
| 313 | −12.35 | 39.4 | 0.998 | ||
| Apricot stones | 293 | −2.37 | −80 | 8.1 | 0.903 |
| 303 | −15.69 | 51.7 | 0.998 | ||
| 313 | −14.28 | 45.6 | 0.883 | ||
| Walnut shells | 293 | −6.63 | −992 | 22.6 | 0.952 |
| 303 | −10.76 | 35.5 | 1.000 | ||
| 313 | −9.54 | 30.5 | 0.923 | ||
| Fleece | 293 | −5.19 | −71 | 17.7 | 0.916 |
| 303 | −6.32 | 20.9 | 0.961 | ||
| 313 | −12.74 | 40.7 | 0.984 |
| Langmuir Model | Freundlich Model | |||||||
|---|---|---|---|---|---|---|---|---|
| qt mg·g−1 | Qmax Mg g−1 | KL L mg−1 | RL ci = 1000 mg·L−1 | R2 | KF | n | R2 | |
| Orange peel | 31.3 | 31.4 | 0.055 | 0.00 | 0.988 | 3.44 | 14.51 | 0.841 |
| Fomitopsis pinicola | 46.2 | 45.1 | 1.116 | 0.05 | 0.993 | 8.13 | 2.06 | 0.842 |
| Mixture of cones | 41.4 | 41.0 | 0.453 | 1.00 | 0.996 | 10.03 | 3.06 | 0.763 |
| Peach stones | 23.2 | 25.5 | 0.017 | 0.00 | 0.994 | 2.31 | 2.58 | 0.963 |
| Apricot stones | 10.0 | 10.4 | 0.020 | 0.00 | 0.992 | 1.97 | 3.95 | 0.829 |
| Walnut shells | 37.5 | 37.7 | 0.141 | 0.06 | 0.998 | 8.05 | 3.06 | 0.844 |
| Fleece | 36.5 | 40.3 | 0.036 | 0.01 | 0.994 | 3.86 | 2.26 | 0.977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertile, E.; Dvorský, T.; Václavík, V.; Heviánková, S. Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution. Life 2021, 11, 240. https://doi.org/10.3390/life11030240
Pertile E, Dvorský T, Václavík V, Heviánková S. Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution. Life. 2021; 11(3):240. https://doi.org/10.3390/life11030240
Chicago/Turabian StylePertile, Eva, Tomáš Dvorský, Vojtěch Václavík, and Silvie Heviánková. 2021. "Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution" Life 11, no. 3: 240. https://doi.org/10.3390/life11030240
APA StylePertile, E., Dvorský, T., Václavík, V., & Heviánková, S. (2021). Use of Different Types of Biosorbents to Remove Cr (VI) from Aqueous Solution. Life, 11(3), 240. https://doi.org/10.3390/life11030240








