SOX11, SOX10 and MITF Gene Interaction: A Possible Diagnostic Tool in Malignant Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Histological Assessment and Tissue Microarray Construction
2.3. Immunohistochemistry—Technical Data
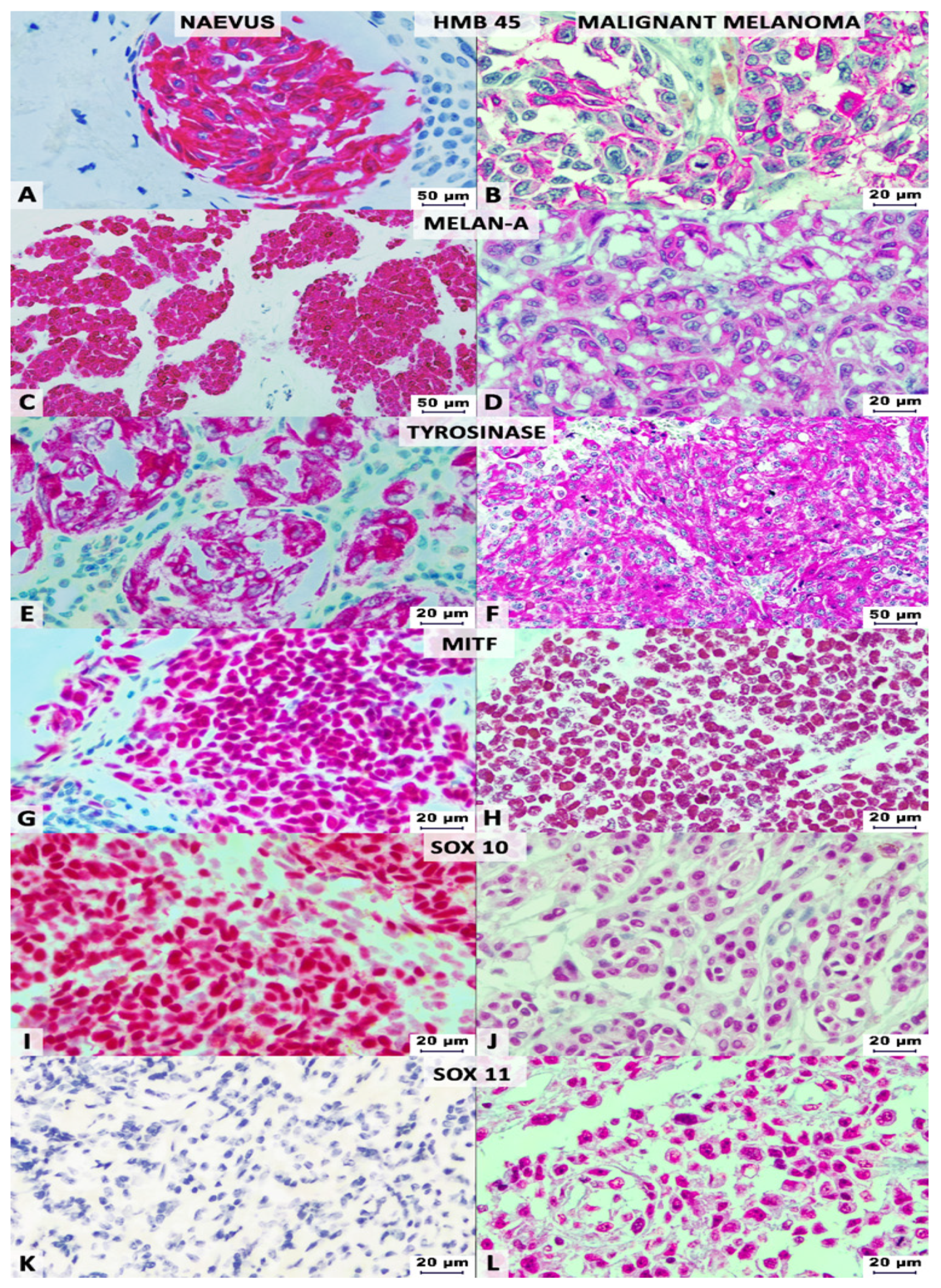
2.4. Interpretation of the IHC Stains
2.5. Statistical Analysis and Survival Curves
2.6. Gene Expression Levels, Survival Analysis, and Interactions in MMs, in Public Databases
3. Results
3.1. Naevi–Clinicopathological Factors
3.2. MMs—Clinicopathological Factors and IHC Assessment
3.3. IHC-Panel in MMs vs. Naevi
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Sommer, L. Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Maldonado, K.; Vega-López, G.A.; Aybar, M.J.; Velasco, I. Neurogenesis From Neural Crest Cells: Molecular Mechanisms in the Formation of Cranial Nerves and Ganglia. Front. Cell Dev. Biol. 2020, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Park, H.Y. Melanocytes: A window into the nervous system. J. Invest. Dermatol. 2012, 132, 835–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef]
- Orchard, G. Evaluation of melanocytic neoplasms: Application of a pan-melanoma antibody cocktail. Br. J. Biomed. Sci. 2002, 59, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, J.; Joseph, C.; Dawlett, M.; Guo, M.; Gong, Y. Reliability of immunostaining using pan-melanoma cocktail, SOX10, and microphthalmia transcription factor in confirming a diagnosis of melanoma on fine-needle aspiration smears. Cancer Cytopathol. 2014, 122, 779–785. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Aithal, C.; Kraechan, A.; Heise, C.; Cadilha, B.L.; Zhang, J.; Duewell, P.; Ballotti, R.; Endres, S.; Bertolotto, C.; et al. Microphthalmia-Associated Transcription Factor (MITF) Regulates Immune Cell Migration into Melanoma. Transl. Oncol. 2019, 12, 350–360. [Google Scholar] [CrossRef]
- Mohamed, A.; Gonzalez, R.S.; Lawson, D.; Wang, J.; Cohen, C. SOX10 expression in malignant melanoma, carcinoma, and normal tissue. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 506–510. [Google Scholar] [CrossRef]
- Ladstein, R.G.; Bachmann, I.M.; Straume, O.; Akslen, L.A. Ki67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer 2010, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Jay, P.; Goze, C.; Marsollier, C.; Taviaux, S.; Hardelin, J.P.; Koopman, P.; Berta, P. The human SOX11 gene: Cloning, chromosomal assignment and tissue expression. Genomics 1995, 29, 541–545. [Google Scholar] [CrossRef]
- Orqueda, A.J.; Gatti, C.R.; Ogara, M.F.; Falzone, T.L. SOX-11 regulates LINE-1 retrotransposon activity during neuronal differentiation. FEBS Lett. 2018, 592, 3708–3719. [Google Scholar] [CrossRef] [Green Version]
- Tsang, S.M.; Oliemuller, E.; Howard, B.A. Regulatory roles for SOX11 in development, stem cells and cancer. Semin. Cancer Biol. 2020, 67, 3–11. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R. Melanoma of the skin, In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 563–585. [Google Scholar]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. (Eds.) WHO Classification of Skin Tumors, 4th ed.; IARC, World Health Organization of Tumors: Lyon, France, 2018; Volume 11. [Google Scholar]
- Gurzu, S.; Bara, T.; Sincu, M.; Gabos, S.; Vlad, D.M.; Bara, T., Jr.; Beres, H.; Jung, I. Solid pseudopapillary neoplasm of pancreas: Two case reports. Med. Baltim. 2019, 98, e16455. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Xia, J. miRNet-Functional Analysis and Visual Exploration of miRNA-Target Interactions in a Network Context. Methods Mol. Biol. 2018, 1819, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Palla, B.; Su, A.; Binder, S.; Dry, S. SOX10 expression distinguishes desmoplastic melanoma from its histologic mimics. Am. J. Derm. 2013, 35, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Johnson, G.; Wang, J.; Cohen, C. SOX10: A useful marker for identifying metastatic melanoma in sentinel lymph nodes. Appl. Immunohistochem. Mol. Morphol. 2015, 3, 109–112. [Google Scholar] [CrossRef]
- Karamchandani, J.R.; Nielsen, T.O.; van de Rijn, M.; West, R.B. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Cheung, M.; Abu-Elmagd, M.; Clevers, H.; Scotting, P.J. Roles of Sox4 in central nervous system development. Brain Res. Mol. Brain Res. 2000, 79, 180–191. [Google Scholar] [CrossRef]
- Miao, Q.; Hill, M.C.; Chen, F.; Mo, Q.; Ku, A.T.; Ramos, C.; Sock, E.; Lefebvre, V.; Nguyen, H. SOX11 and SOX4 drive the reactivation of an embryonic gene program during murine wound repair. Nat. Commun. 2019, 10, 4042. [Google Scholar] [CrossRef] [Green Version]
- Jian, J.; Guoying, W.; Jing, Z. Increased expression of sex determining region Y-box 11 (SOX11) in cutaneous malignant melanoma. J. Int. Med. Res. 2013, 41, 1221–1227. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, S.; Lu, C.; Ji, T.; Yang, W.; Li, T.; Lv, J.; Hu, W.; Yang, Y.; Jin, Z. SOX11: Friend or foe in tumor prevention and carcinogenesis? Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Kuci, V.; Nordström, L.; Conrotto, P.; Ek, S. SOX11 and HIG-2 are cross-regulated and affect growth in mantle cell lymphoma. Leuk. Lymphoma. 2016, 57, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Gadi, J.; Jung, S.H.; Lee, M.J.; Jami, A.; Ruthala, K.; Kim, K.M.; Cho, N.H.; Jung, H.S.; Kim, C.H.; Lim, S.K. The transcription factor protein Sox11 enhances early osteoblast differentiation by facilitating proliferation and the survival of mesenchymal and osteoblast progenitors. J. Biol. Chem. 2013, 288, 25400–25413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomero, J.; Vegliante, M.C.; Rodríguez, M.L.; Eguileor, A.; Castellano, G.; Planas-Rigol, E.; Jares, P.; Ribera-Cortada, I.; Cid, M.C.; Campo, E.; et al. SOX11 promotes tumor angiogenesis through transcriptional regulation of PDGFA in mantle cell lymphoma. Blood 2014, 124, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Li, J.; Saksena, A.; Wang, S.A.; Shen, J.; Hu, Z.; Lin, P.; Tang, G.; Yin, C.C.; et al. SOX11-negative Mantle Cell Lymphoma: Clinicopathologic and Prognostic Features of 75 Patients. Am. J. Surg. Pathol. 2019, 43, 710–716. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Uray, I.P.; Mazumdar, A.; Tsimelzon, A.; Savage, M.; Hilsenbeck, S.G.; Brown, P.H. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget 2016, 7, 13106–13121. [Google Scholar] [CrossRef]
- Li, P.; Hu, Y.; Yi, J.; Li, J.; Yang, J.; Wang, J. Identification of potential markers to differentially diagnose solid pseudopapillary tumors and pancreatic malignancies via a gene regulatory network. J. Transl. Med. 2015, 13, 361. [Google Scholar] [CrossRef] [Green Version]
- Walter, R.F.; Mairinger, F.D.; Werner, R.; Ting, S.; Vollbrecht, C.; Theegarten, D.; Christoph, D.C.; Zarogoulidis, K.; Schmid, K.W.; Zarogoulidis, P.; et al. SOX4, SOX11 and PAX6 mRNA expression was identified as a (prognostic) marker for the aggressiveness of neuroendocrine tumors of the lung by using next-generation expression analysis (NanoString). Future Oncol. 2015, 11, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Beleaua, M.-A.; Jung, I. The role of tumor microenvironment in development and progression of malignant melanomas—A systematic review. Rom. J. Morphol. Embryol. 2018, 59, 23–28. [Google Scholar] [PubMed]




| Variable | N = 105 (%) |
|---|---|
| Age (years) | 63.63 ± 14.48 (range 30–90) |
| Gender: Male: Female | 52:53 (1:1.01) |
| Histological type | |
| Nodular | 76 (72.37) |
| Superficial | 18 (17.15) |
| Lentiginous | 11 (10.48) |
| Thickness (Breslow) | (Median: 6.73 ± 8.25, range 0.4–60) |
| ≤1 mm | 20 (19.05) |
| >1 to ≤2 mm | 15 (14.29) |
| >2 to ≤4 mm | 14 (13.33) |
| >4 mm | 56 (53.33) |
| Ulceration | 74 (70.47) |
| Microsatellites | 19 (18.09) |
| Mitotic Rate (mm2) | (Median: 10.09 ± 11.9, range 0–57) |
| <5 | 42 (40) |
| ≥5 | 63 (60) |
| TILs (Tumor-Infiltrating Lymphocytes) | |
| Not identified | 30 (28.58) |
| Brisk | 19 (18.09) |
| Non-Brisk | 56 (53.33) |
| Lymphovascular Invasion | 23 (21.9) |
| Neurotropism | 9 (8.57) |
| Tumor regression | 36 (34.28) |
| Anatomic Level (Clark) | |
| I | 1 (0.95) |
| II | 11 (10.48) |
| III | 13 (12.38) |
| IV | 58 (55.24) |
| V | 22 (20.95) |
| Tumor location | |
| Anterior Trunk | 18 (17.15) |
| Posterior Trunk | 29 (27.61) |
| Head and Neck | 17 (16.19) |
| Superior Limb | 16 (15.24) |
| Inferior Limb | 25 (23.81) |
| Tumor satellites | 12 (11.42) |
| Tumor size (mm) | 23.45 ± 15.78 (range 6–110) |
| Tumor stage | |
| pT1 | 20 (19.05) |
| pT2 | 17 (16.19) |
| pT3 | 14 (13.33) |
| pT4 | 54 (51.43) |
| Deep Margin distance (mm) | 8.41 ± 5.21 (range 6–22.8) |
| Peripheral Margins distance (mm) | 7.67 ± 5.9 (range 1–35) |
| Growth Phase | |
| Radial | 20 (19.05) |
| Vertical | 85 (80.95) |
| Parameters | N | SOX11 | SOX10 | MITF | Conventional Cocktail | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | R | p | + | − | r | p | + | − | r | p | + | − | R | p | ||
| Gender | |||||||||||||||||
| Male | 52 | 29 | 23 | 0.12 | 0.2 | 51 | 1 | 0.09 | 0.32 | 39 | 13 | 0.09 | 0.64 | 45 | 7 | 0.02 | 0.81 |
| Female | 53 | 23 | 30 | 50 | 3 | 41 | 12 | 45 | 8 | ||||||||
| Age (years) | |||||||||||||||||
| ≤60 | 39 | 18 | 21 | 0.003 | 0.97 | 37 | 2 | 0.1 | 0.26 | 31 | 8 | −0.03 | 0.89 | 35 | 4 | −0.1 | 0.33 |
| >60 | 66 | 34 | 32 | 64 | 2 | 49 | 17 | 55 | 11 | ||||||||
| Histologic type | |||||||||||||||||
| Nodular | 76 | 41 | 35 | −0.13 | 0.17 | 75 | 1 | −0.24 | 0.01 | 58 | 18 | 0.15 | 0.47 | 64 | 12 | 0.07 | 0.49 |
| Superficial | 18 | 7 | 11 | 15 | 3 | 14 | 4 | 16 | 2 | ||||||||
| Lentiginous | 11 | 4 | 7 | 11 | 0 | 8 | 3 | 10 | 1 | ||||||||
| Thickness (Breslow) | |||||||||||||||||
| ≤1 mm | 20 | 5 | 15 | 0.14 | 0.15 | 19 | 1 | 0.11 | 0.26 | 14 | 6 | −0.08 | 0.7 | 18 | 2 | −0.17 | 0.08 |
| >1 to ≤2 mm | 15 | 9 | 6 | 14 | 1 | 14 | 1 | 14 | 1 | ||||||||
| >2 to ≤4 mm | 14 | 8 | 6 | 13 | 1 | 10 | 4 | 13 | 1 | ||||||||
| >4 mm | 56 | 30 | 26 | 55 | 1 | 42 | 14 | 45 | 11 | ||||||||
| Ulceration | |||||||||||||||||
| Present | 74 | 40 | 34 | 0.13 | 0.17 | 72 | 2 | 0.09 | 0.37 | 59 | 15 | −0.01 | 0.96 | 62 | 12 | −0.09 | 0.37 |
| Absent | 31 | 12 | 19 | 29 | 2 | 21 | 10 | 28 | 3 | ||||||||
| Microsatellites | |||||||||||||||||
| Present | 19 | 12 | 7 | 0.13 | 0.18 | 18 | 1 | −0.03 | 0.72 | 16 | 3 | 0.18 | 0.37 | 14 | 5 | −0.16 | 0.1 |
| Absent | 86 | 40 | 46 | 83 | 3 | 64 | 22 | 76 | 10 | ||||||||
| Mitotic Rate (mm2) | |||||||||||||||||
| <5 | 42 | 19 | 23 | 0.1 | 0.3 | 39 | 3 | 0.14 | 0.17 | 33 | 9 | 0.1 | 0.6 | 37 | 5 | −0.06 | 0.56 |
| ≥5 | 63 | 33 | 30 | 62 | 1 | 47 | 16 | 53 | 10 | ||||||||
| TILs | |||||||||||||||||
| Not identified | 30 | 17 | 13 | −0.07 | 0.44 | 30 | 0 | −0.12 | 0.2 | 24 | 6 | 0.25 | 0.2 | 26 | 4 | −0.08 | 0.44 |
| Brisk | 19 | 10 | 9 | 19 | 0 | 18 | 1 | 18 | 1 | ||||||||
| Non-Brisk | 56 | 25 | 31 | 52 | 4 | 38 | 18 | 46 | 10 | ||||||||
| Lymphovascular Invasion | |||||||||||||||||
| Present | 23 | 16 | 7 | 0.21 | 0.02 | 23 | 0 | 0.1 | 0.28 | 19 | 4 | −0.06 | 0.77 | 21 | 2 | 0.09 | 0.38 |
| Absent | 82 | 36 | 46 | 78 | 4 | 61 | 21 | 69 | 13 | ||||||||
| Neurotropism | |||||||||||||||||
| Present | 9 | 7 | 2 | 0.17 | 0.07 | 9 | 0 | 0.06 | 0.53 | 7 | 2 | −0.02 | 0.92 | 8 | 1 | 0.03 | 0.77 |
| Absent | 96 | 45 | 51 | 92 | 4 | 73 | 23 | 82 | 14 | ||||||||
| Tumor regression | |||||||||||||||||
| Present | 36 | 17 | 19 | −0.02 | 0.79 | 34 | 2 | −0.06 | 0.51 | 27 | 9 | −0.15 | 0.44 | 31 | 5 | 0.01 | 0.91 |
| Absent | 69 | 35 | 34 | 67 | 2 | 53 | 16 | 59 | 10 | ||||||||
| Anatomic Level (Clark) | |||||||||||||||||
| I-III | 25 | 10 | 15 | 0.1 | 0.29 | 23 | 2 | 0.06 | 0.5 | 19 | 6 | −0.11 | 0.58 | 22 | 3 | 0.01 | 0.92 |
| IV-V | 80 | 42 | 38 | 78 | 2 | 61 | 19 | 68 | 12 | ||||||||
| UV exposure | |||||||||||||||||
| Present | 40 | 20 | 20 | 0.01 | 0.87 | 38 | 2 | −0.04 | 0.63 | 30 | 10 | −0.21 | 0.28 | 32 | 8 | −0.13 | 0.2 |
| Absent | 65 | 32 | 33 | 63 | 2 | 50 | 15 | 58 | 7 | ||||||||
| TNM stage | |||||||||||||||||
| pT1-pT2 | 37 | 15 | 22 | 0.13 | 0.18 | 35 | 2 | 0.09 | 0.32 | 29 | 8 | 0.01 | 0.96 | 34 | 3 | −0.14 | 0.15 |
| pT3-pT4 | 68 | 37 | 31 | 66 | 2 | 51 | 17 | 56 | 12 | ||||||||
| Ki67 Index | |||||||||||||||||
| ≤10 | 74 | 31 | 43 | 0.23 | 0.01 | 70 | 4 | 0.17 | 0.07 | 53 | 21 | 0.33 | 0.0005 | 64 | 10 | 0.34 | 0.72 |
| >10 | 31 | 21 | 10 | 31 | 0 | 27 | 4 | 26 | 5 | ||||||||
| IHC Marker | Melanoma | Naevi | Sensitivity | Specificity | |||
|---|---|---|---|---|---|---|---|
| + | − | + | − | % | % | p Value | |
| S100 | 105 | 0 | 44 | 0 | 100% | 0% | 0.99 |
| HMB-45 | 97 | 8 | 24 | 20 | 92.38% | 45.45% | <0.0001 |
| Melan-A | 97 | 8 | 37 | 7 | 92.38% | 15.91% | 0.14 |
| Tyrosinase | 96 | 9 | 26 | 18 | 91.43% | 40.91% | <0.0001 |
| MITF | 80 | 25 | 30 | 14 | 76.19% | 31.82% | 0.31 |
| SOX10 | 101 | 4 | 34 | 10 | 96.19% | 22.73% | 0.0008 |
| SOX11 | 52 | 53 | 0 | 44 | 49.52% | 100% | <0.0001 |
| Conventional cocktail 1 | 90 | 15 | 19 | 25 | 85.71% | 56.82% | <0.0001 |
| Conventional-adapted cocktail 2 | 90 | 15 | 17 | 27 | 85.71% | 61.36% | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleaua, M.-A.; Jung, I.; Braicu, C.; Milutin, D.; Gurzu, S. SOX11, SOX10 and MITF Gene Interaction: A Possible Diagnostic Tool in Malignant Melanoma. Life 2021, 11, 281. https://doi.org/10.3390/life11040281
Beleaua M-A, Jung I, Braicu C, Milutin D, Gurzu S. SOX11, SOX10 and MITF Gene Interaction: A Possible Diagnostic Tool in Malignant Melanoma. Life. 2021; 11(4):281. https://doi.org/10.3390/life11040281
Chicago/Turabian StyleBeleaua, Marius-Alexandru, Ioan Jung, Cornelia Braicu, Doina Milutin, and Simona Gurzu. 2021. "SOX11, SOX10 and MITF Gene Interaction: A Possible Diagnostic Tool in Malignant Melanoma" Life 11, no. 4: 281. https://doi.org/10.3390/life11040281
APA StyleBeleaua, M.-A., Jung, I., Braicu, C., Milutin, D., & Gurzu, S. (2021). SOX11, SOX10 and MITF Gene Interaction: A Possible Diagnostic Tool in Malignant Melanoma. Life, 11(4), 281. https://doi.org/10.3390/life11040281








