Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Naphthalene SOA By-Products
2.3. Measurements of Cytotoxicity
2.3.1. Effects on Cell Proliferation
2.3.2. Quantitative Phase Imaging
2.4. Statistical Analysis
3. Results
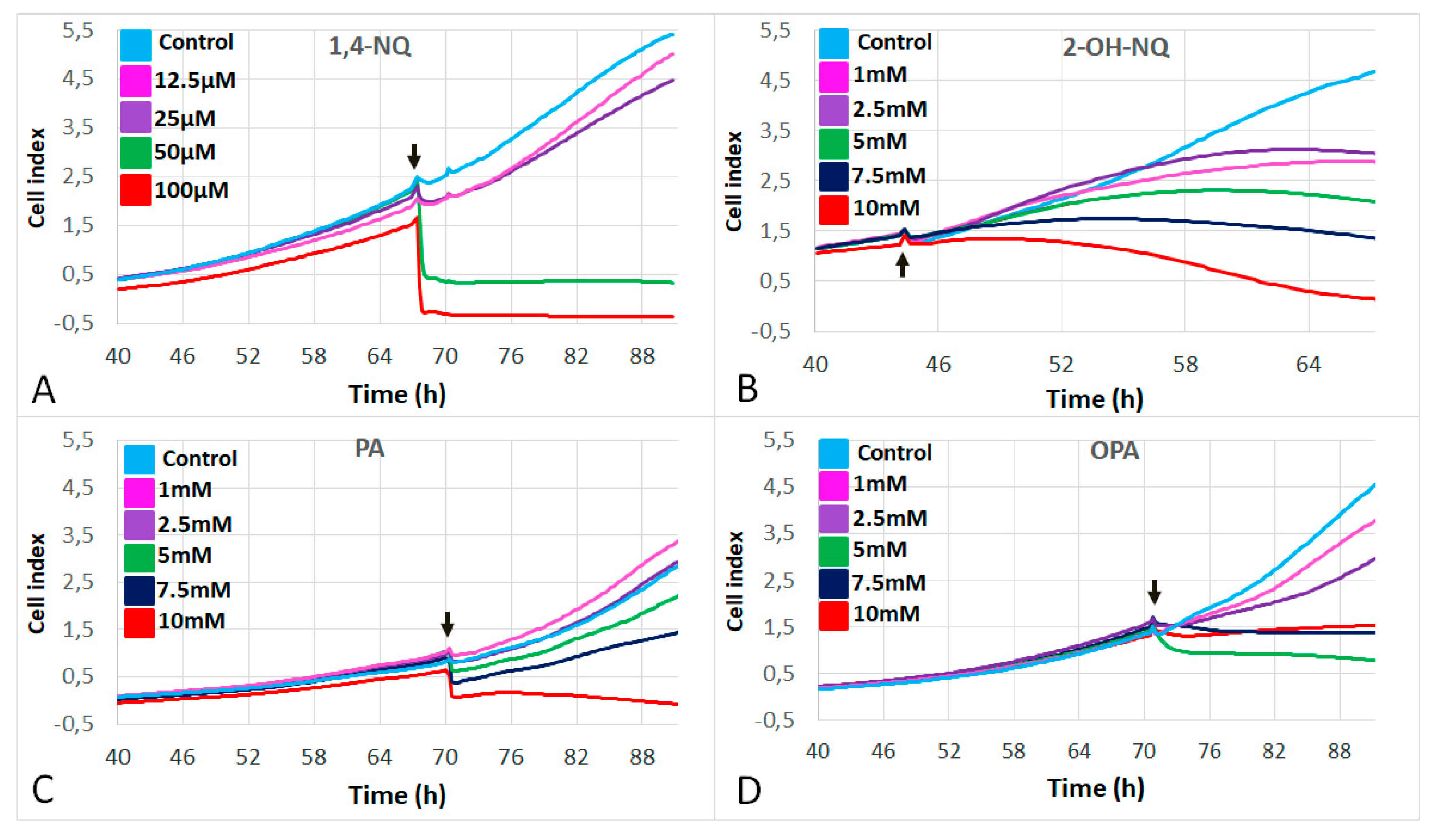
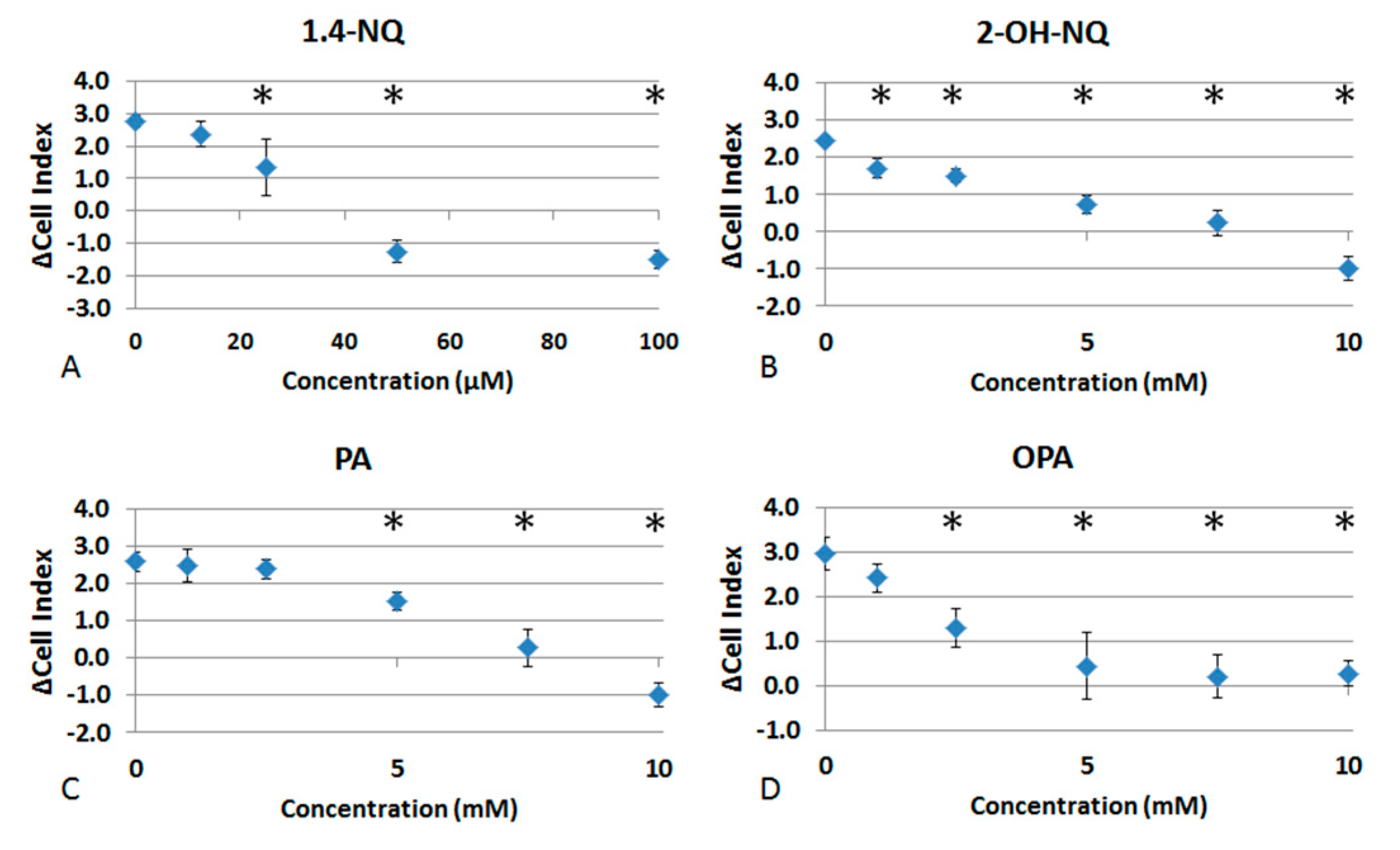
3.1. Effects of Naphthalene SOA By-Products on A549 Proliferation
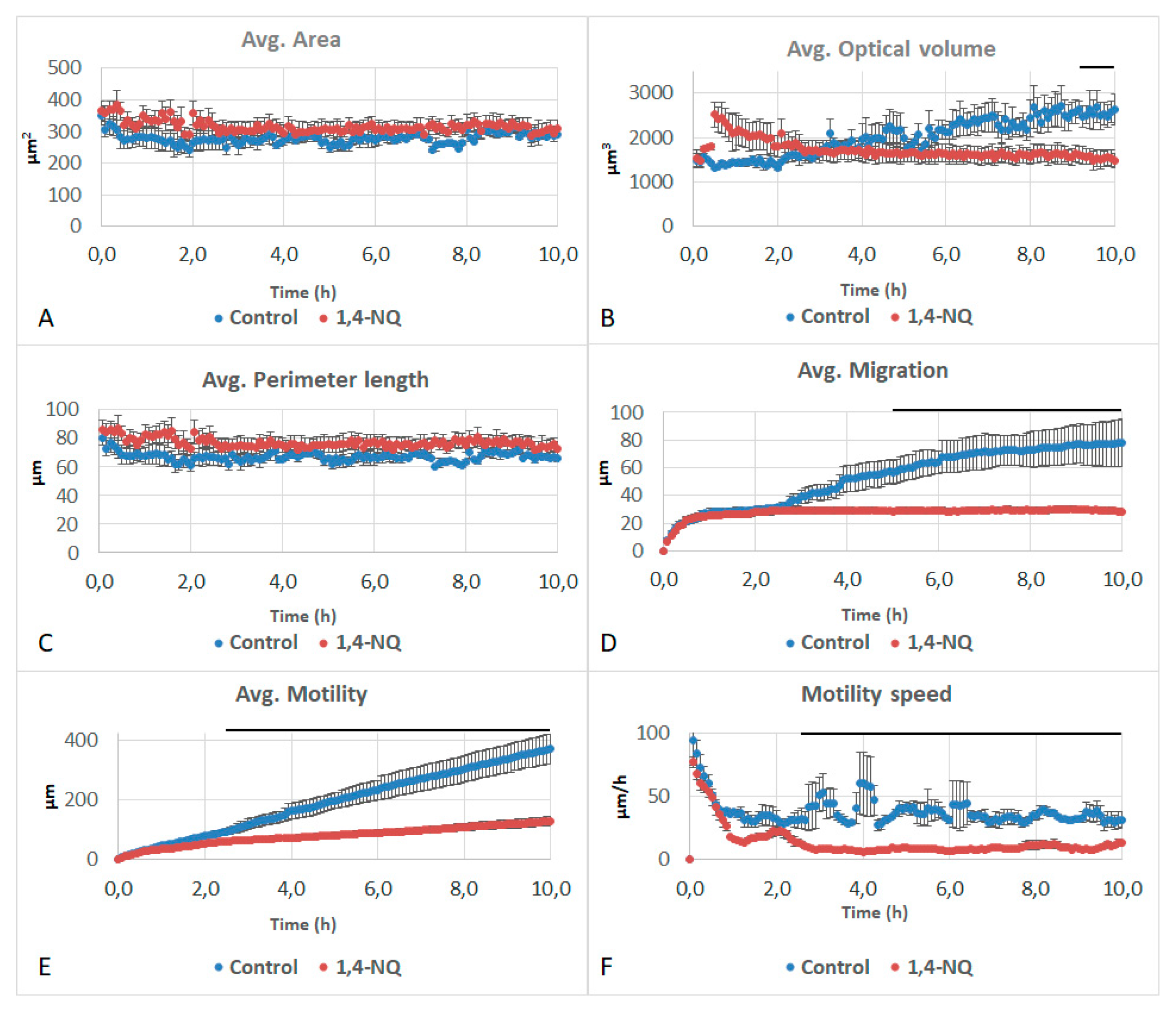
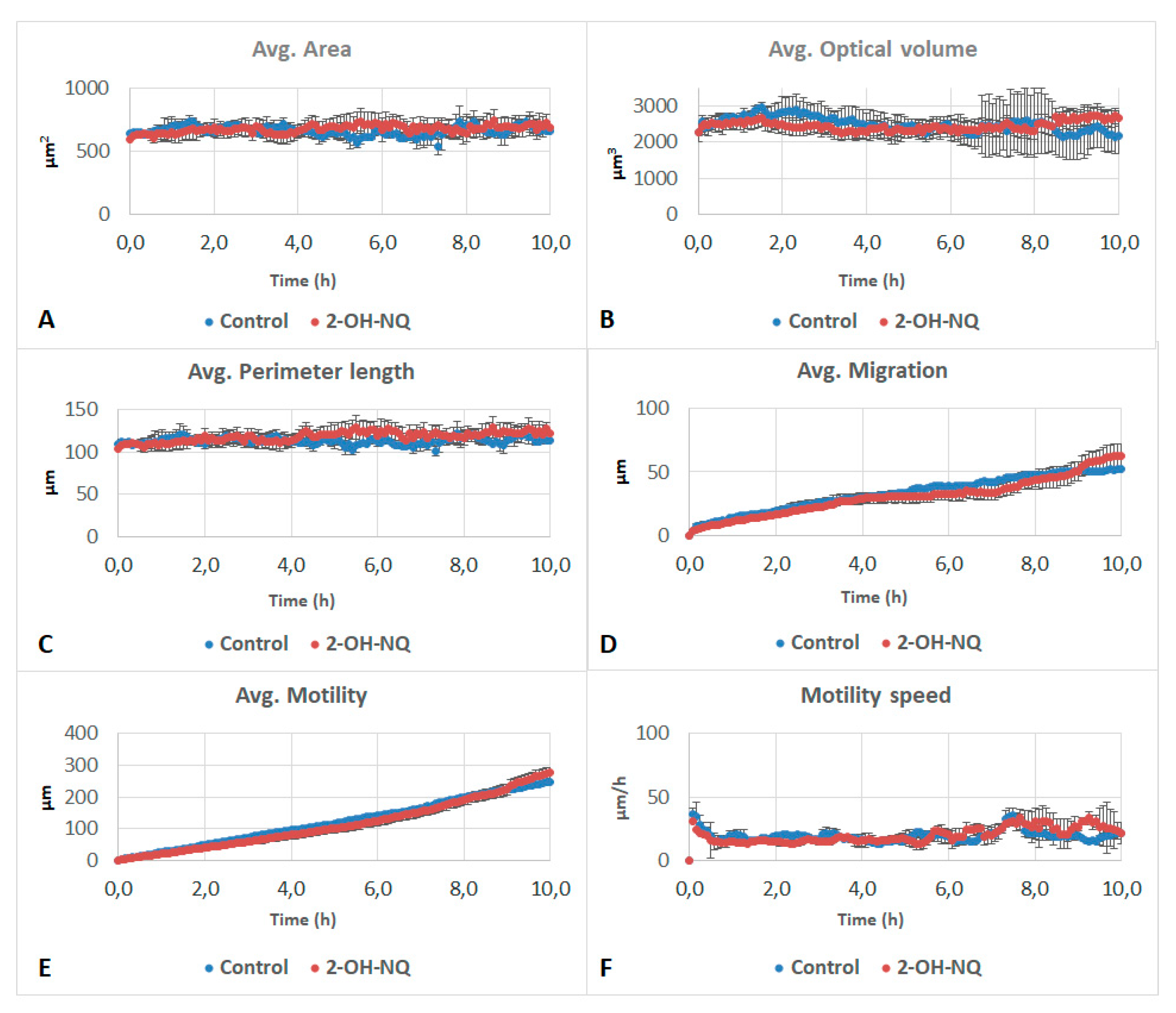
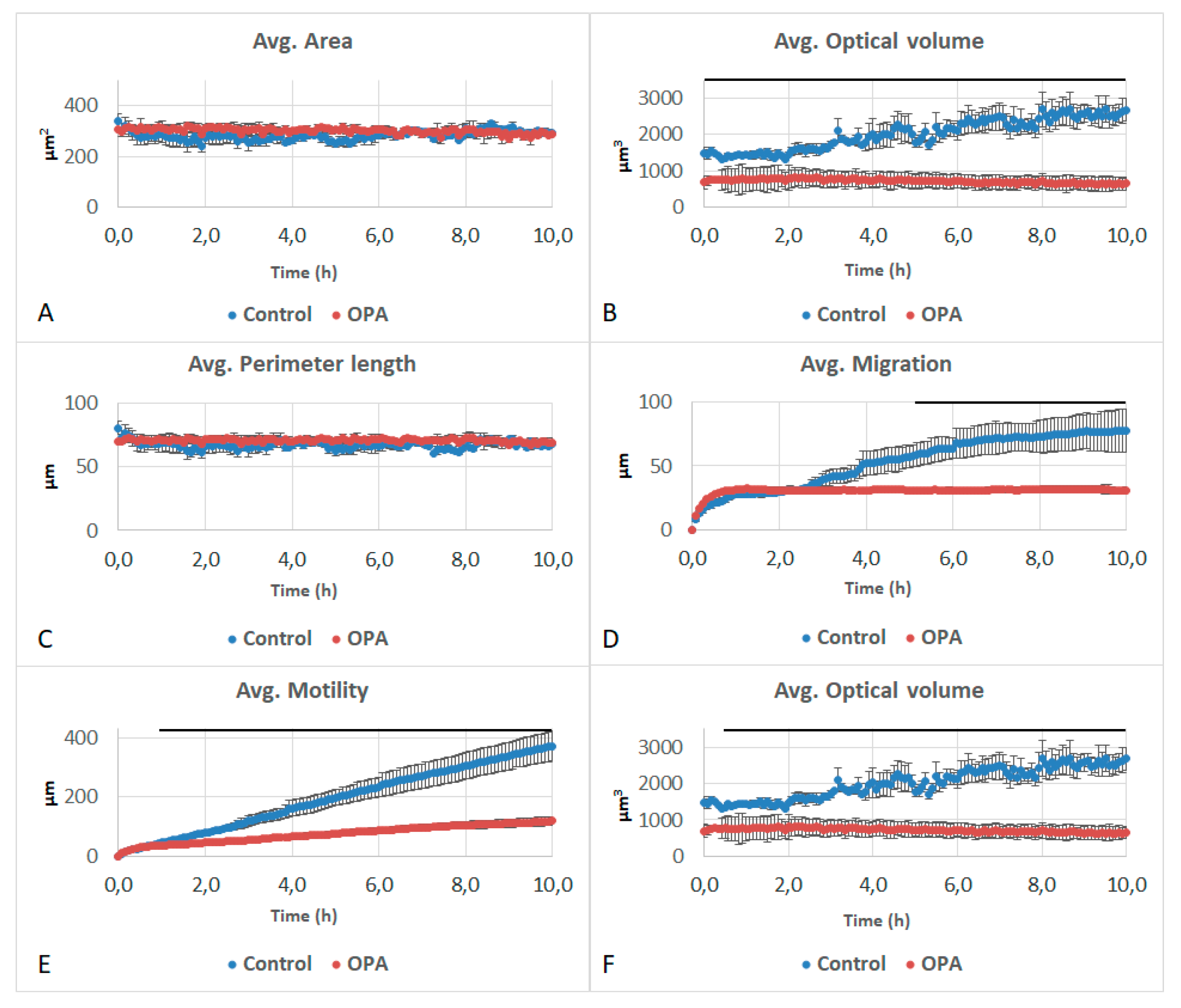
3.2. Effects of Naphthalene SOA By-Products on A549 Behavioral and Structural Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buckpitt, A.; Kephalopoulos, S.; Koistinen, K.; Kotzias, D.; Morawska, L.; Sagunski, H. Naphthalene. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK138704/ (accessed on 24 February 2021).
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air Particulate Matter and Cardiovascular Disease: The Epidemiological, Biomedical and Clinical Evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [CrossRef]
- World Health Organization. 9 Out of 10 People Worldwide Breathe Polluted Air, but More Countries Are Taking Action. Available online: https://www.who.int/news/item/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 24 February 2021).
- US Enviromental Protection Agency. Compendium Method TO-14A. Available online: https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-14ar.pdf (accessed on 24 February 2021).
- Menezes, H.C.; Paulo, B.P.; Costa, N.T.; Cardeal, Z.L. New Method to Determination of Naphthalene in Ambient Air Using Cold Fiber-Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry. Microchem. J. 2013, 109, 93–97. [Google Scholar] [CrossRef]
- US Health and Human Services. Toxicological Profile for Naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp67.pdf (accessed on 24 February 2021).
- McConkey, B.J.; Hewitt, L.M.; Dixon, D.G.; Greenberg, B.M. Natural sunlight induced photooxidation of naphthalene in aqueous solution. Water Air Soil Pollut. 2002, 136, 347–359. [Google Scholar] [CrossRef]
- Nishino, N.; Arey, J.; Atkinson, R. 2-Formylcinnamaldehyde Formation Yield from the OH Radical-Initiated Reaction of Naphthalene: Effect of NO2 Concentration. Environ. Sci. Technol. 2012, 46, 8198–8204. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Shao, M.; Li, Y.; Chen, Q.; Zheng, Y.; Wu, Z.; Liu, Y.; Wu, Y.; Hu, M.; et al. Potentially Important Contribution of Gas-Phase Oxidation of Naphthalene and Methylnaphthalene to Secondary Organic Aerosol during Haze Events in Beijing. Environ. Sci. Technol. 2019, 53, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Panchagnula, S.; Sanz, M.E.; Pérez, C.; Evangelisti, L.; Pate, B.H. Structural Changes Induced by Quinones: High-Resolution Microwave Study of 1,4-Naphthoquinone. ChemPhysChem 2020, 21, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, D.; Kukshal, V.; Laubenthal, J.; Kumar, A.; Pandey, A.; Tripathi, S.; Arora, A.; Jain, S.K.; Ramachandran, R.; Anderson, D.; et al. Mechanism of Inhibition of the ATpase Domain of Human Topoisomerase IIα by 1,4-Benzoquinone, 1,2-Naphthoquinone, 1,4-Naphthoquinone, and 9,10-Phenanthroquinone. Toxicol. Sci. 2012, 126, 372–390. [Google Scholar] [CrossRef]
- Kumagai, Y.; Shinkai, Y.; Miura, T.; Cho, A.K. The Chemical Biology of Naphthoquinones and Its Environmental Implications. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 221–247. [Google Scholar] [CrossRef]
- De Sena Pereira, V.S.; Silva de Oliveira, C.B.; Fumagalli, F.; da Silva Emery, F.; da Silva, N.B.; de Andrade-Neto, V.F. Cytotoxicity, Hemolysis and in Vivo Acute Toxicity of 2-Hydroxy-3-Anilino-1,4-Naphthoquinone Derivatives. Toxicol. Rep. 2016, 3, 756–762. [Google Scholar] [CrossRef]
- Sreelatha, T.; Kandhasamy, S.; Dinesh, R.; Shruthy, S.; Shweta, S.; Mukesh, D.; Karunagaran, D.; Balaji, R.; Mathivanan, N.; Perumal, P.T. Synthesis and SAR Study of Novel Anticancer and Antimicrobial Naphthoquinone Amide Derivatives. Bioorganic Med. Chem. Lett. 2014, 24, 3647–3651. [Google Scholar] [CrossRef]
- da Costa, E.C.B.; Amorim, R.; da Silva, F.C.; Rocha, D.R.; Papa, M.P.; de Arruda, L.B.; Mohana-Borges, R.; Ferreira, V.F.; Tanuri, A.; da Costa, L.J.; et al. Synthetic 1,4-Pyran Naphthoquinones Are Potent Inhibitors of Dengue Virus Replication. PLoS ONE 2013, 8, e82504. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.G.; Santos, I.O.; Schmidt, T.J.; Borborema, S.E.T.; Ferreira, V.F.; Rocha, D.R.; Tempone, A.G. Potential of 2-Hydroxy-3-Phenylsulfanylmethyl-[1,4]-Naphthoquinones against Leishmania (L.) Infantum: Biological Activity and Structure-Activity Relationships. PLoS ONE 2014, 9, e105127. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, D.A.; Cesar-Rodo, E.; Bertrand, B.; Huang, H.-H.; Day, L.; Johann, L.; Elhabiri, M.; Becker, K.; Williams, D.L.; Davioud-Charvet, E. Synthesis and Biological Evaluation of 1{,}4-Naphthoquinones and Quinoline-5{,}8-Diones as Antimalarial and Schistosomicidal Agents. Org. Biomol. Chem. 2012, 10, 6375–6387. [Google Scholar] [CrossRef] [PubMed]
- David, C.C.; Lins, A.C.S.; Silva, T.M.S.; Campos, J.F.; Silva, T.G.; Militão, G.C.G.; Camara, C.A. Synthesis and Cytotoxicity Evaluation of a Series of 3-Alkenyl-2-Hydroxy-1,4-Naphthoquinones Obtained by an Efficient Knoevenagel Condensation. J. Braz. Chem. Soc. 2019, 30, 8–18. [Google Scholar] [CrossRef]
- Rudnicka, M.; Ludynia, M.; Karcz, W. A Comparison of the Effects of 1,4-Naphthoquinone and 2-Hydroxy-1,4-Naphthoquinone (Lawsone) on Indole-3-Acetic Acid (IAA)-Induced Growth of Maize Coleoptile Cells. Plant Growth Regul. 2018, 84, 107–122. [Google Scholar] [CrossRef]
- Lozza, L.; Moura-Alves, P.; Domaszewska, T.; Lage Crespo, C.; Streata, I.; Kreuchwig, A.; Puyskens, A.; Bechtle, M.; Klemm, M.; Zedler, U.; et al. The Henna Pigment Lawsone Activates the Aryl Hydrocarbon Receptor and Impacts Skin Homeostasis. Sci. Rep. 2019, 9, 1–21. [Google Scholar] [CrossRef]
- Sauriasari, R.; Wang, D.H.; Takemura, Y.; Tsutsui, K.; Masuoka, N.; Sano, K.; Horita, M.; Wang, B.L.; Ogino, K. Cytotoxicity of Lawsone and Cytoprotective Activity of Antioxidants in Catalase Mutant Escherichia Coli. Toxicology 2007, 235, 103–111. [Google Scholar] [CrossRef]
- Phthalic Acid and Its Isomers (Isophthalic Acid and Terephthalic Acid) [MAK Value Documentation, 2009]. The MAK-Collection for Occupational Health and Safety; American Cancer Society: Atlanta, GA, USA, 2012; pp. 194–221. [Google Scholar] [CrossRef]
- Smith, P.W.G.; Tatchell, A.R. Chapter VIII—Aromatic Carboxylic Acids; Pergamon: Oxford, UK, 1969; pp. 176–195. [Google Scholar] [CrossRef]
- Lorz, P.M.; Towae, F.K.; Enke, W.; Jäckh, R.; Bhargava, N.; Hillesheim, W. Phthalic Acid and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 15 July 2007. [Google Scholar] [CrossRef]
- Catlin, N.R.; Willson, C.J.; Stout, M.; Kissling, G.E.; Waidyanatha, S.; Baker, G.L.; Hayden, B.K.; Wyde, M. Evaluation of the Respiratory Tract Toxicity of Ortho-Phthalaldehyde, a Proposed Alternative for the Chemical Disinfectant Glutaraldehyde. Inhal. Toxicol. 2017, 29, 414–427. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4807, o-Phthalaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/o-Phthalaldehyde (accessed on 24 February 2021).
- Masarik, M.; Gumulec, J.; Hlavna, M.; Sztalmachova, M.; Babula, P.; Raudenska, M.; Pavkova-Goldbergova, M.; Cernei, N.; Sochor, J.; Zitka, O.; et al. Monitoring of the Prostate Tumour Cells Redox State and Real-Time Proliferation by Novel Biophysical Techniques and Fluorescent Staining. Integr. Biol. 2012, 4, 672–684. [Google Scholar] [CrossRef]
- Molder, A.; Sebesta, M.; Gustafsson, M.; Gisselson, L.; Wingren, A.G.; Alm, K. Non-Invasive, Label-Free Cell Counting and Quantitative Analysis of Adherent Cells Using Digital Holography. J. Microsc. 2008, 232, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The XCELLigence System for Real-Time and Label-Free Monitoring of Cell Viability. In Mammalian Cell Viability: Methods and Protocols; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 33–43. [Google Scholar] [CrossRef]
- Brussol, C.; Duane, M.; Carlier, P.; Kotzias, D. Photo-Induced OH Reactions of Naphthalene and Its Oxidation Products on SiO2. Environ. Sci. Pollut. Res. 1999, 6, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Stern, A. In Vitro Cytotoxicities of 1,4-Naphthoquinone and Hydroxylated 1,4-Naphthoquinones to Replicating Cells. J. Appl. Toxicol. 1993, 13, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Fan, L.; Feng, J.; Lv, S.; Wu, M.; Li, B.; Zang, Y.S. Genotoxic and Inflammatory Effects of Organic Extracts from Traffic-Related Particulate Matter in Human Lung Epithelial A549 Cells: The Role of Quinones. Toxicol. Vitr. 2013, 27, 922–931. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, L.; Jiang, Y.; Li, Y.; Lu, P. Airborne Quinones Induce Cytotoxicity and DNA Damage in Human Lung Epithelial A549 Cells: The Role of Reactive Oxygen Species. Chemosphere 2014, 100, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From Oxidative Damage to Cellular and Inter-Cellular Signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef]
- Gurbani, D.; Bharti, S.K.; Kumar, A.; Pandey, A.K.; Ana, G.R.E.E.; Verma, A.; Khan, A.H.; Patel, D.K.; Mudiam, M.K.R.; Jain, S.K.; et al. Polycyclic Aromatic Hydrocarbons and Their Quinones Modulate the Metabolic Profile and Induce DNA Damage in Human Alveolar and Bronchiolar Cells. Int. J. Hyg. Environ. Health 2013, 216, 553–565. [Google Scholar] [CrossRef]
- Lavrich, K.S.; Corteselli, E.M.; Wages, P.A.; Bromberg, P.A.; Simmons, S.O.; Gibbs-flournoy, E.A.; Samet, J.M. Investigating Mitochondrial Dysfunction in Human Lung Cells Exposed to Redox-Active PM Components Katelyn. Toxicol. Appl. Pharmacol. 2018, 342, 99–107. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Cavalcanti Chipoline, I.; da Fonseca, A.; da Costa, G.; de Souza, M.; Won-Held Rabelo, V.; de Queiroz, L.N.; de Souza, T.; de Almeida, E.; Alvarez Abreu, P.; Pontes, B.; et al. Molecular Mechanism of Action of New 1,4-Naphthoquinones Tethered to 1,2,3-1H-Triazoles with Cytotoxic and Selective Effect against Oral Squamous Cell Carcinoma. Bioorg. Chem. 2020, 101, 103984. [Google Scholar] [CrossRef]
- Fowler, P.; Meurer, K.; Honarvar, N.; Kirkland, D. A Review of the Genotoxic Potential of 1,4-Naphthoquinone. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 834, 6–17. [Google Scholar] [CrossRef]
- Sheng, K.; Lu, J. Typical Airborne Quinones Modulate Oxidative Stress and Cytokine Expression in Lung Epithelial A549 Cells. J. Environ. Sci. Health Part A 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Mucenski, M.L.; Thitoff, A.R.; Le Cras, T.D.; Park, K.S.; Ikegami, M.; Müller, W.; Whitsett, J.A. GP130-STAT3 Regulates Epithelial Cell Migration and Is Required for Repair of the Bronchiolar Epithelium. Am. J. Pathol. 2008, 172, 1542–1554. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.H.; Shen, G.N.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Wang, J.R.; Feng, Y.C.; Li, J.Q.; Zhang, Y.; et al. Two Novel 1,4-Naphthoquinone Derivatives Induce Human Gastric Cancer Cell Apoptosis and Cell Cycle Arrest by Regulating Reactive Oxygen Species-Mediated MAPK/Akt/STAT3 Signaling Pathways. Mol. Med. Rep. 2019, 20, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Miyawaki, E.; Harazono, A.; Kawashima, K. Developmental Toxicity Evaluation of Phthalic Acid, One of the Metabolites of Phthalic Acid Esters, in Rats. Toxicol. Lett. 1997, 93, 109–115. [Google Scholar] [CrossRef]
- Kwack, S.J.; Lee, B.M. Comparative Cytotoxicity and Sperm Motility Using a Computer-Aided Sperm Analysis System (CASA) for Isomers of Phthalic Acid, a Common Final Metabolite of Phthalates. J. Toxicol. Environ. Health Part A Curr. Issues 2015, 78, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Suzukawa, M.; Komiya, A.; Koketsu, R.; Kawakami, A.; Kimura, M.; Nito, T.; Yamamoto, K.; Yamaguchi, M. Three Cases of Ortho-Phthalaldehyde-Induced Anaphylaxis after Laryngoscopy: Detection of Specific IgE in Serum. Allergol. Int. 2007, 56, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Horikiri, M.; Park, S.; Matsui, T.; Suzuki, K.; Matsuoka, T. Ortho-Phthalaldehyde-Induced Skin Mucous Membrane Damage from Inadequate Washing. BMJ Case Rep. 2011, 2011, bcr0220102709. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima de Albuquerque, Y.; Berger, E.; Tomaz, S.; George, C.; Géloën, A. Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols. Life 2021, 11, 319. https://doi.org/10.3390/life11040319
Lima de Albuquerque Y, Berger E, Tomaz S, George C, Géloën A. Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols. Life. 2021; 11(4):319. https://doi.org/10.3390/life11040319
Chicago/Turabian StyleLima de Albuquerque, Yuri, Emmanuelle Berger, Sophie Tomaz, Christian George, and Alain Géloën. 2021. "Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols" Life 11, no. 4: 319. https://doi.org/10.3390/life11040319
APA StyleLima de Albuquerque, Y., Berger, E., Tomaz, S., George, C., & Géloën, A. (2021). Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols. Life, 11(4), 319. https://doi.org/10.3390/life11040319






