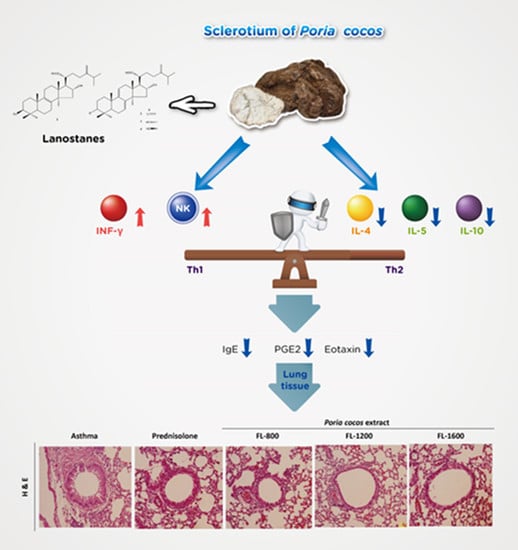
Poria cocos Modulates Th1/Th2 Response and Attenuates Airway Inflammation in an Ovalbumin-Sensitized Mouse Allergic Asthma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Poria cocos Extract (Lipucan®)
2.2. Animals
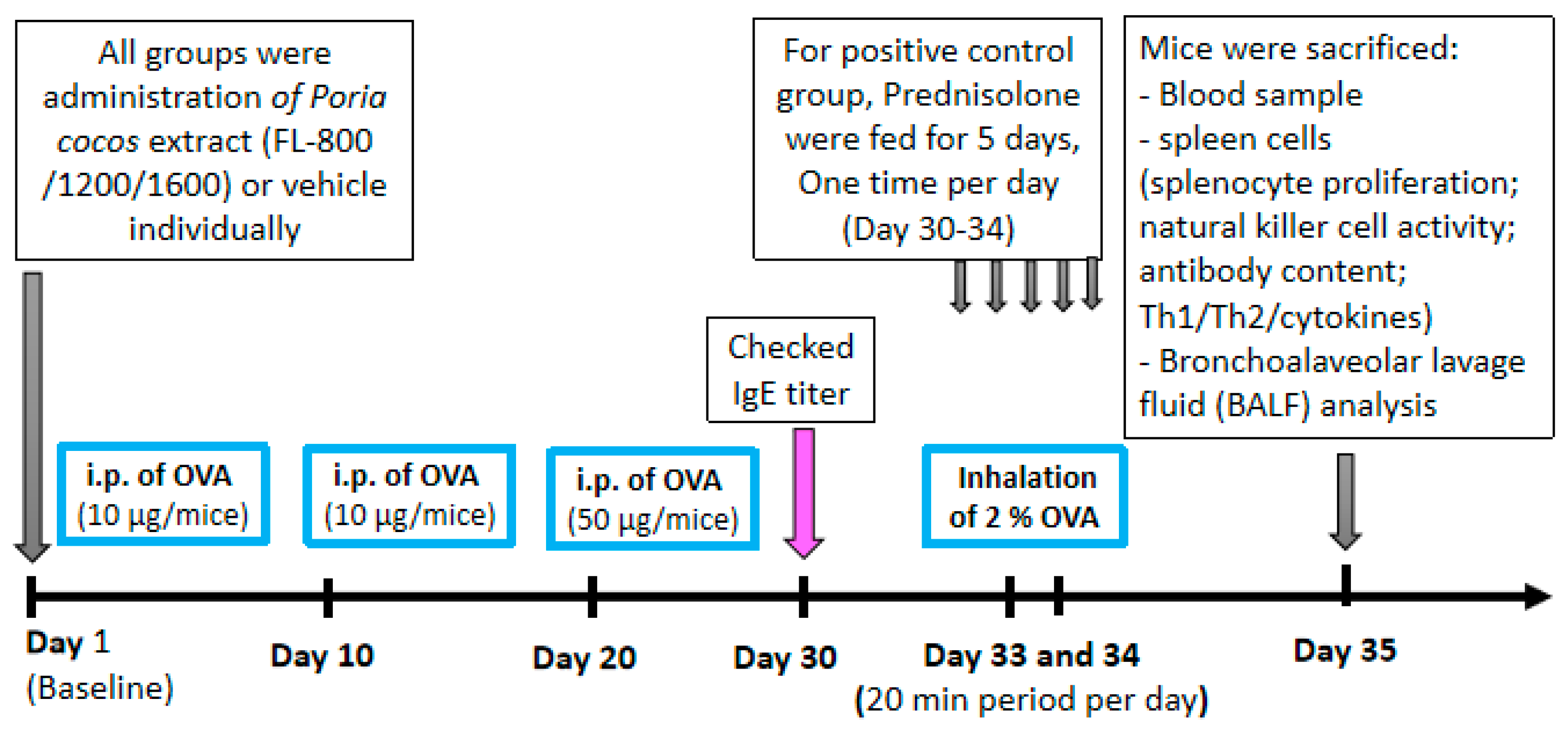
2.3. Establishment of the Experimental Animal Model of Asthma and Treatments
2.4. BronchoalveolarLavage Fluid (BALF) Analysis and Histological Analysis
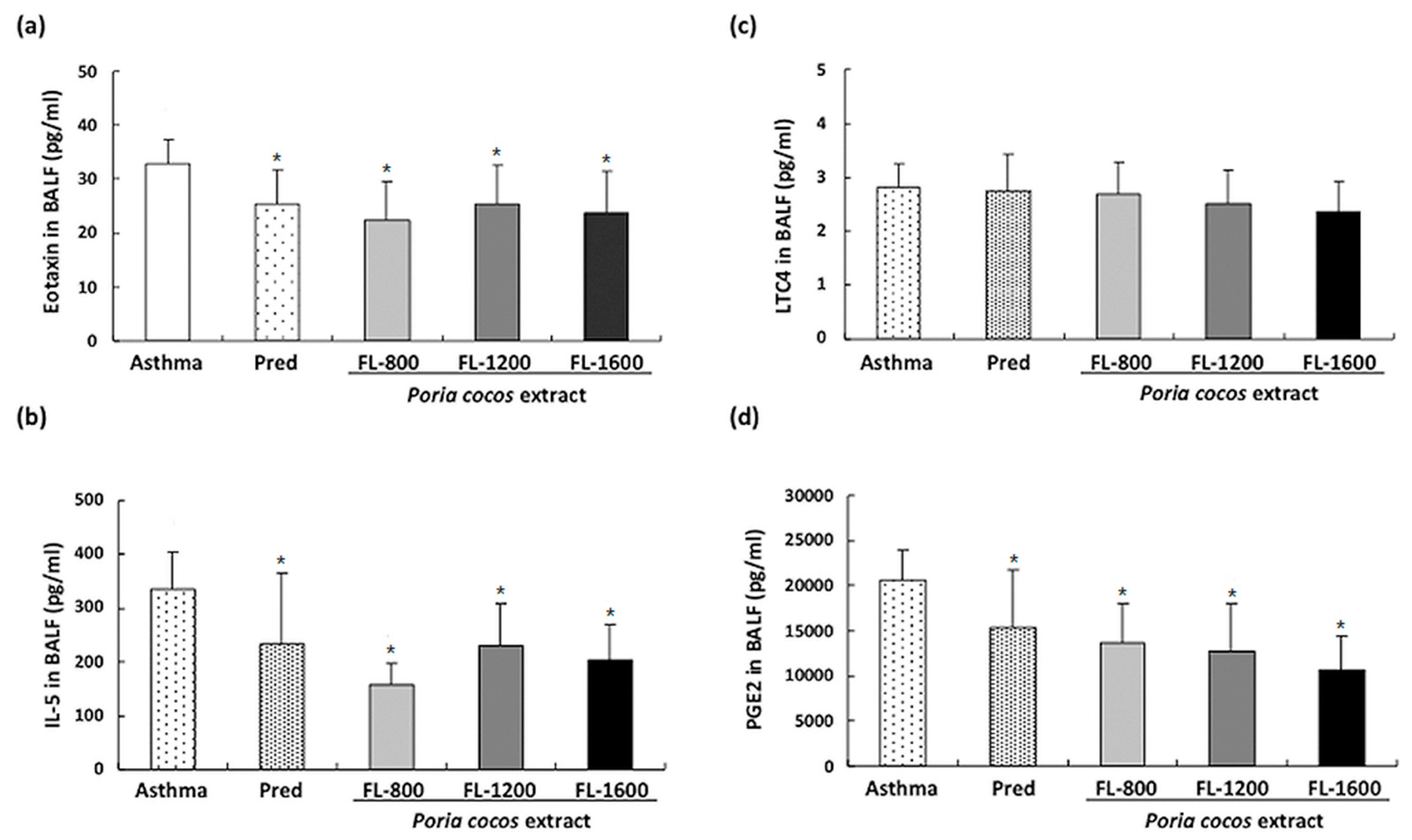
2.5. Determination of Eotaxin, IL-5, LTC4, and PGE2 in BALF
2.6. Determination of Total IgE, IgA, IgM, IgG, and OVA-Specific IgE
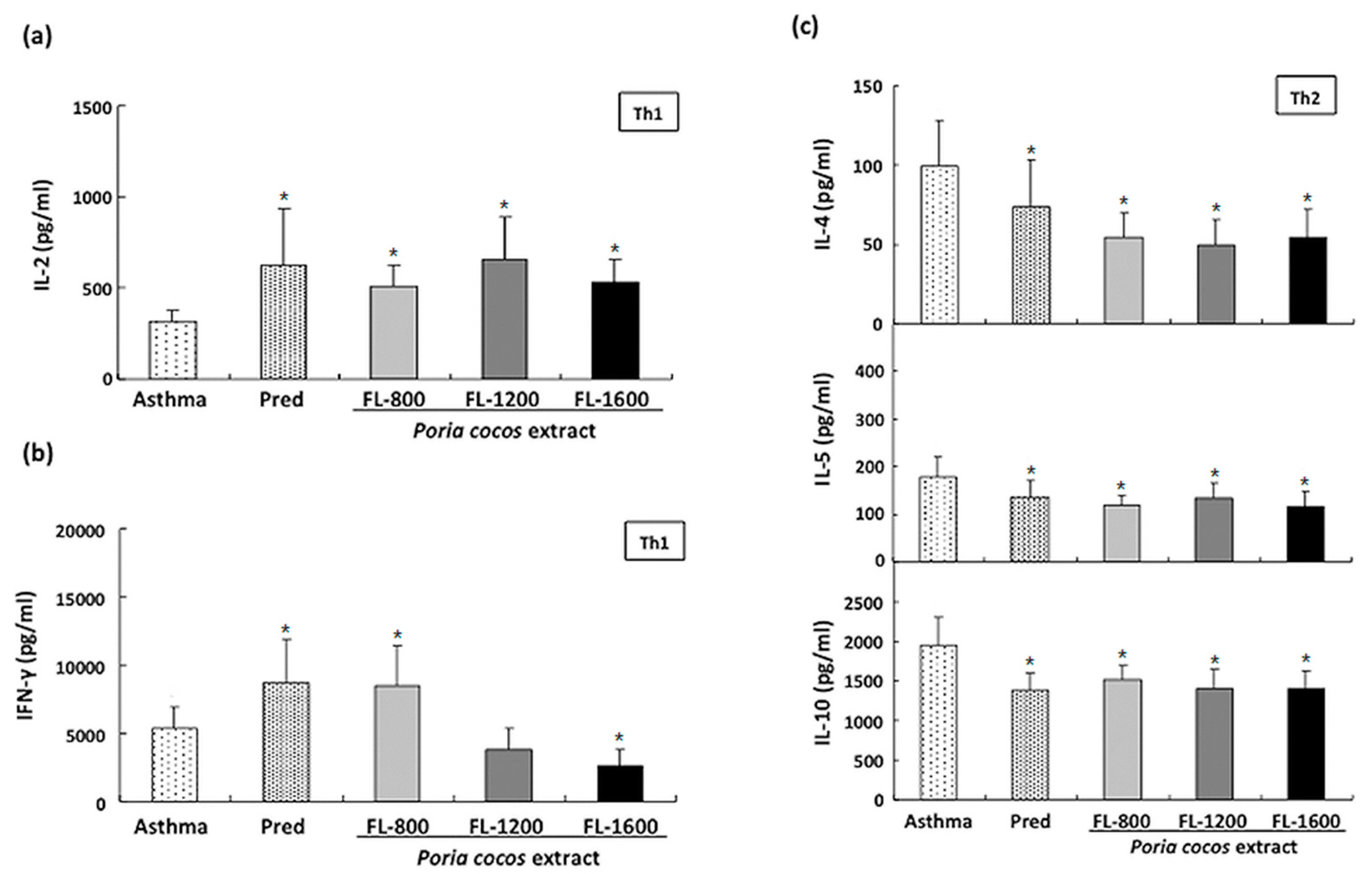
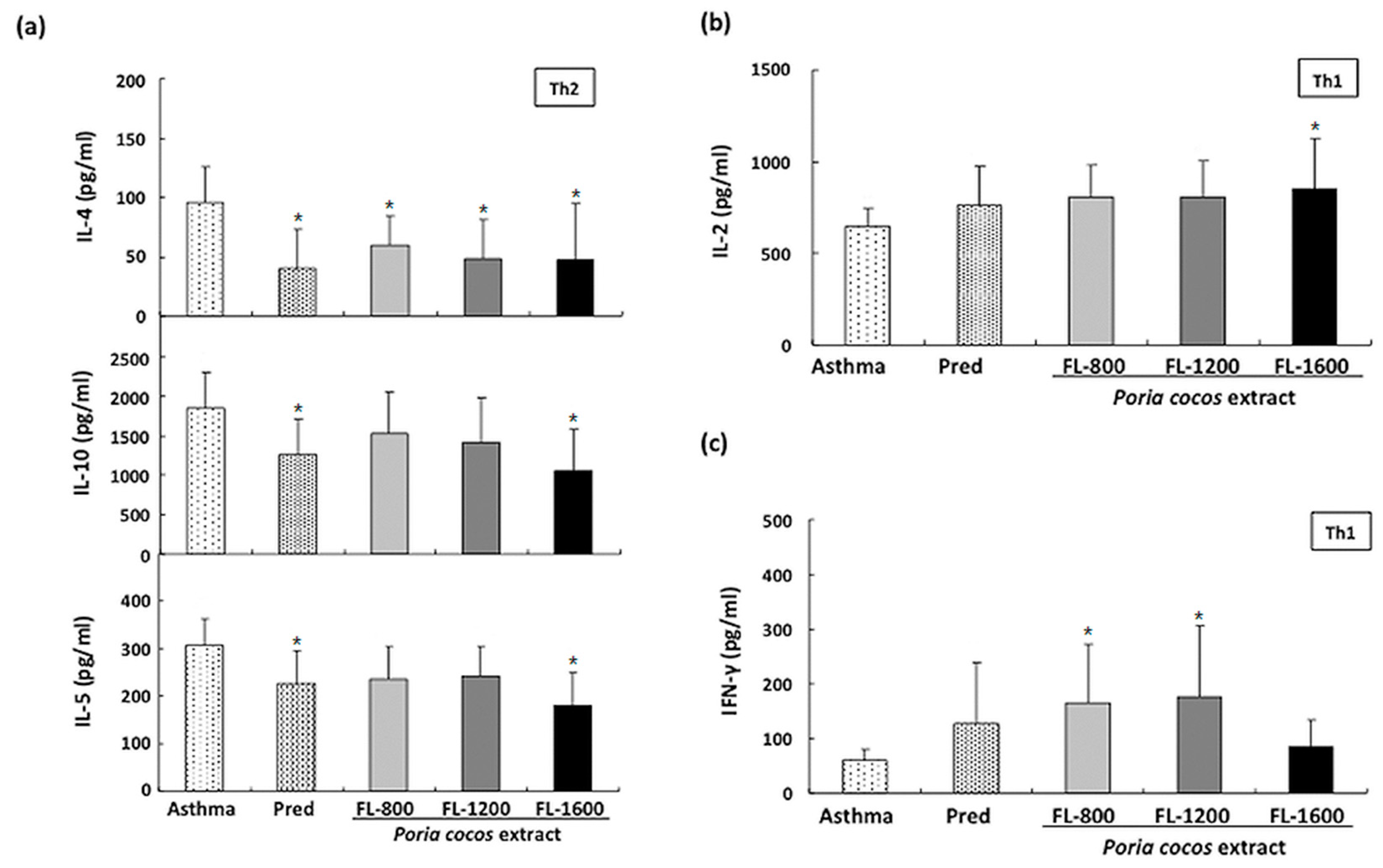
2.7. Cytokine Levels in Splenocytesand BALF Cultured Supernatants
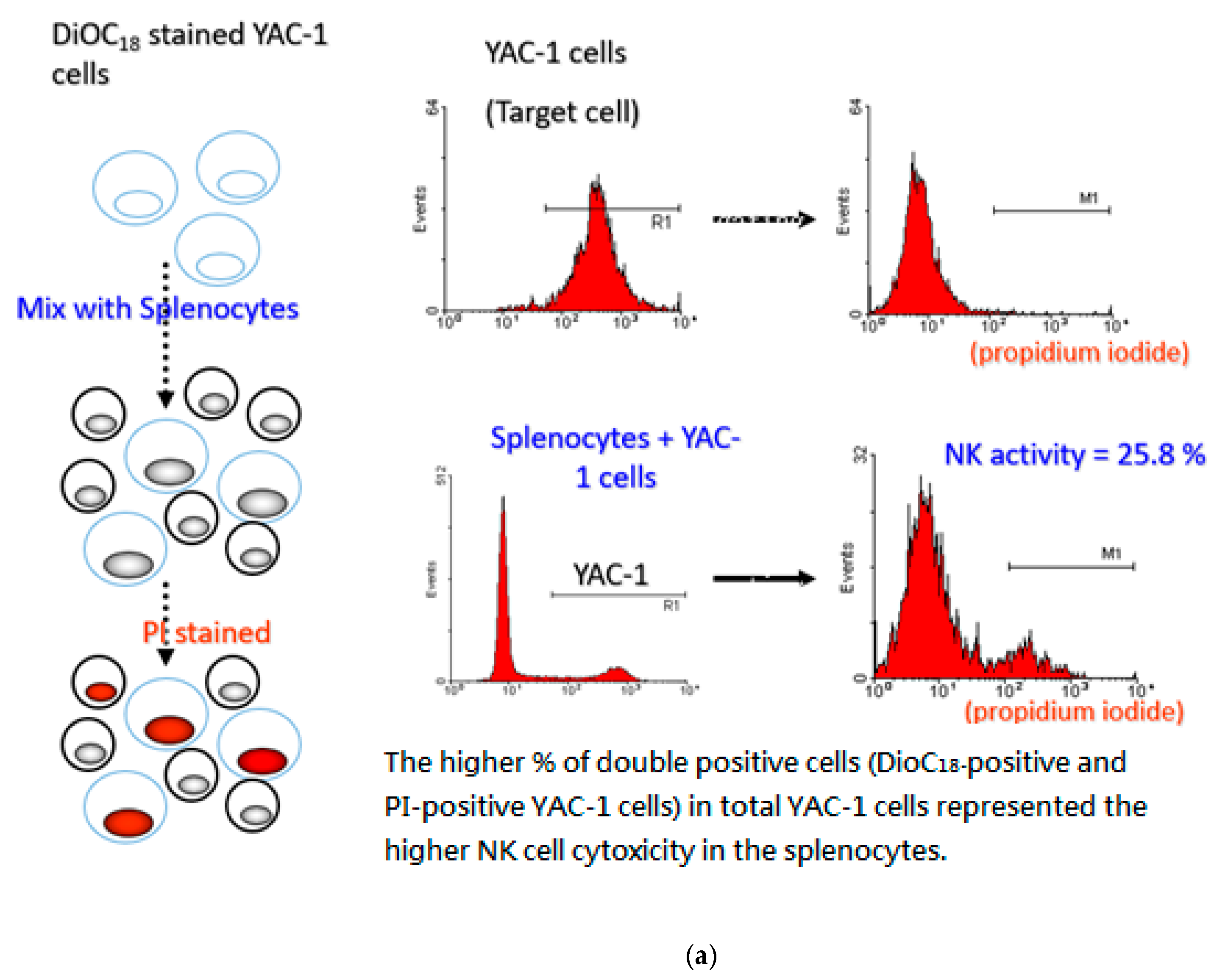
2.8. Evaluation of NK Cell Activity
2.9. Statistics
3. Results
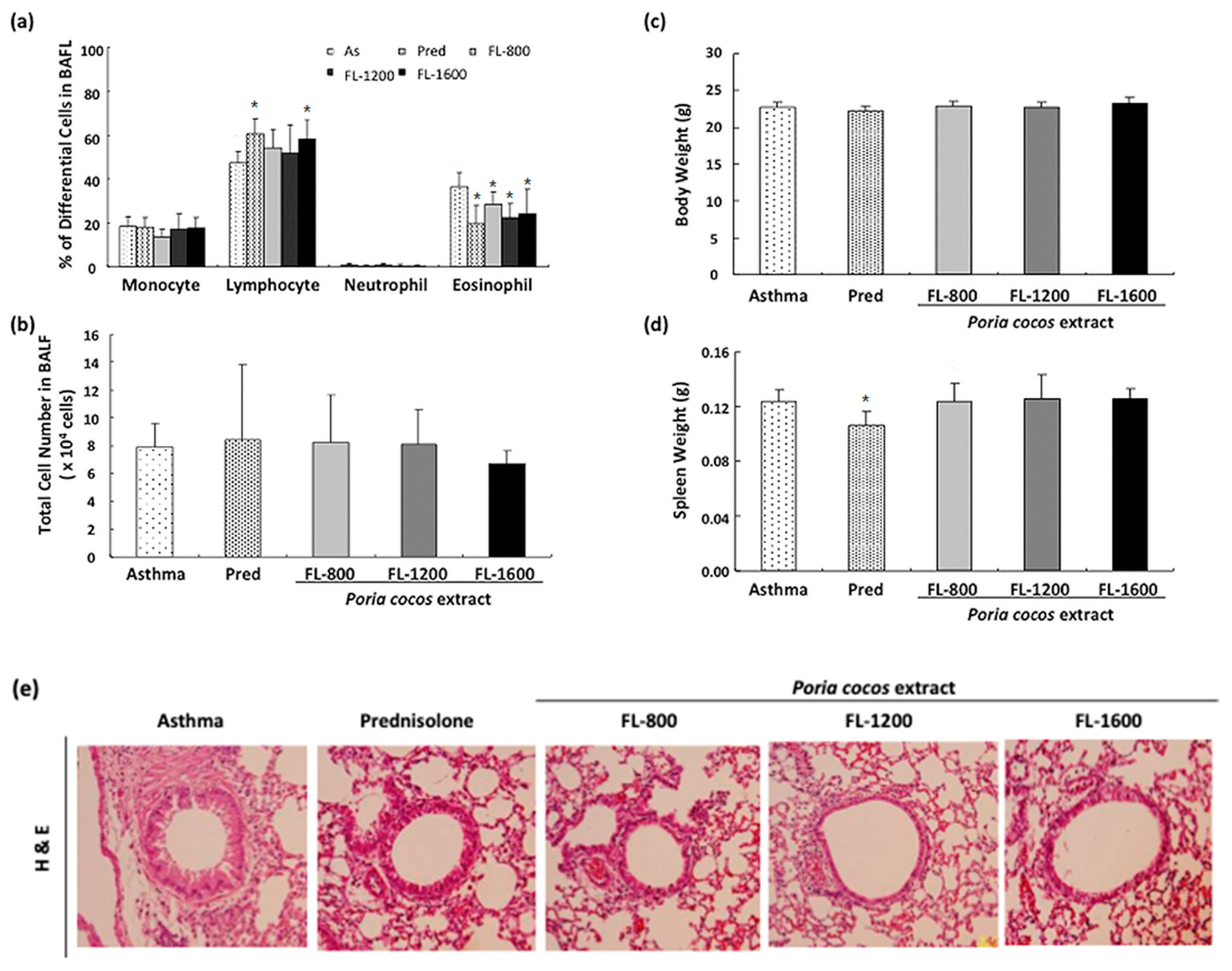
3.1. P. cocos Reduced Inflammation in BALF and Lung Tissues in OVA-Sensitized Mice
3.2. P. cocos Suppressed the Release of Total IgE in Blood Serum
3.3. P. cocos Suppressed OVA-Induced Chemokine and Cytokine Secretion in BALF
3.4. P. cocos Effects on Cytokine Release in ConA-Stimulated Cultured Splenocytes Derived from OVA-Sensitized Mice
3.5. P. cocos Effects on Cytokine Release in OVA-Stimulated Cultured Splenocytes Derived from OVA-Sensitized Mice
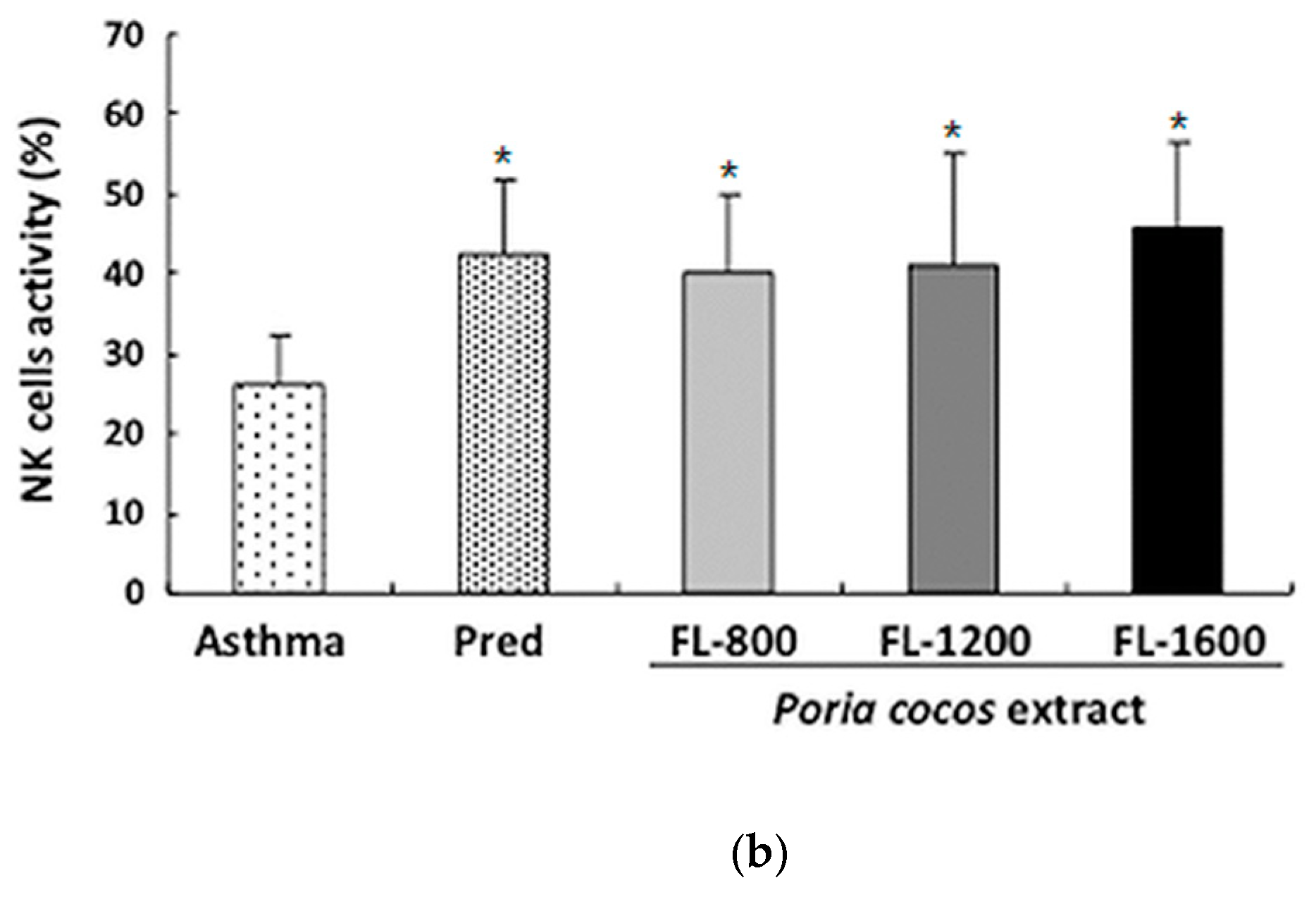
3.6. P. cocos Effects on NK Cell Activity in OVA-Sensitized Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Martin, B.L.; Muraro, A.M.; Wood, R.A.; Agache, I.O.; Ansotegui, I.J.; Ansotegui, I.J.; Casale, T.B.; Fleisher, T.A.; Hellings, P.W.; et al. The importance of allergic disease in public health: An iCAALL statement. World Allergy Organ. J. 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Immunologic influences on allergy and the TH1/TH2 balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Renauld, J.C. New insights into the role of cytokines in asthma. J. Clin. Pathol. 2001, 54, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Schuijs, M.J.; Willart, M.A.; Hammad, H.; Lambrecht, B.N. Cytokine targets in airway inflammation. Curr. Opin. Pharmacol. 2013, 13, 351–361. [Google Scholar] [CrossRef]
- Gleich, G.J. Mechanisms of eosinophil-associated inflammation. J. Allergy. Clin. Immunol. 2000, 105, 651–663. [Google Scholar] [CrossRef]
- Erle, D.J.; Sheppard, D. The cell biology of asthma. J. Cell Biol. 2014, 205, 621–631. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma-present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
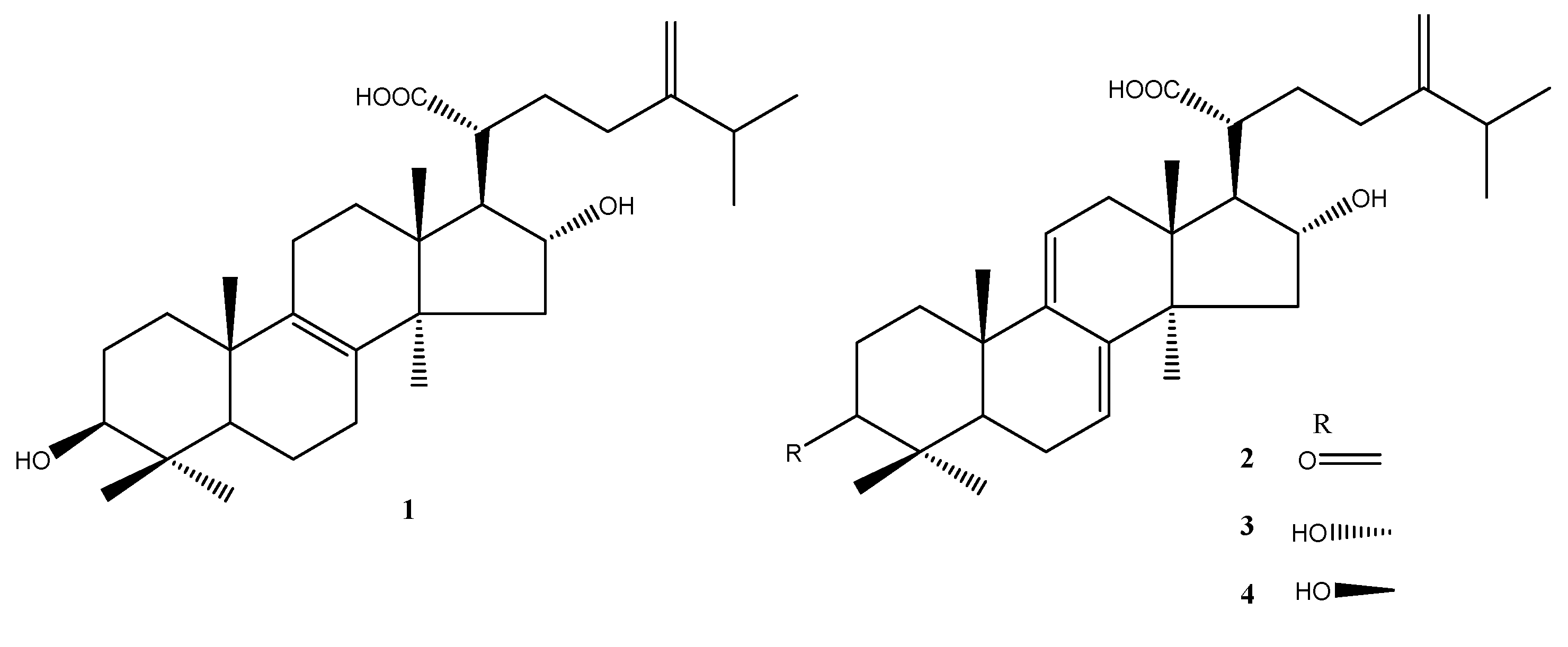
- Rios, J.L. Chemical constituents and pharmacological properties of Poriacocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef]
- Esteban, C.I. Medicinal interest of Poriacocos (= Wolfiporiaextensa). RevistaIberoamericana de Micologia 2009, 26, 103–107. [Google Scholar] [CrossRef][Green Version]
- Cuella, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Manez, S.; Rios, J.L. Two fungal lanostane derivatives as phospholipase A2 inhibitors. J. Nat. Prod. 1996, 59, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Yu, B.Z.; Rogers, J.M.; Smith, A.E.; Boger, E.T.; Ostrander, R.L.; Rheingold, A.L. Specific competitive inhibitor of secreted phospholipase A2 from berries of Schinusterebinthifolius. Phytochemistry 1995, 39, 537–547. [Google Scholar] [CrossRef]
- Scott, D.L.; White, S.P.; Otwinowski, Z.; Yuan, W.; Gelb, M.H.; Sigler, P.B. Interfacial catalysis: The mechanism of phospholipase A2. Science 1990, 250, 1541–1546. [Google Scholar] [CrossRef]
- Spelman, K.; Burns, J.; Nichols, D.; Winters, N.; Ottersberg, S.; Tenborg, M. Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Altern. Med. Rev. 2006, 11, 128–150. [Google Scholar]
- Yu, S.J.; Tseng, J.; Fu-Ling, A. Chinese herbal drug, modulates cytokine secretion by human peripheral blood monocytes. Int. J. Immunopharmacol. 1996, 18, 37–44. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Cheung, P.C. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1-->3)-d-glucan from Poriacocos. Int. Immunopharmacol. 2010, 10, 398–405. [Google Scholar] [CrossRef]
- Poon, T.Y.; Ong, K.L.; Cheung, B.M. Review of the effects of the traditional Chinese medicine Rehmannia Six Formula on diabetes mellitus and its complications. J. Diabetes 2011, 3, 184–200. [Google Scholar] [CrossRef]
- Nie, A.; Chao, Y.; Zhang, X.; Jia, W.; Zhou, Z.; Zhu, C. Phytochemistry and Pharmacological Activities of Wolfiporiacocos(F.A. Wolf) Ryvarden&Gilb. Front. Pharmacol. 2020, 11, 1399. [Google Scholar]
- Chao, C.L.; Huang, H.W.; Su, M.H.; Lin, H.C.; Wu, W.M. The Lanostane Triterpenoids in Poriacocos Play Beneficial Roles in Immunoregulatory Activity. Life 2021, 11, 111. [Google Scholar] [CrossRef]
- Kumar, R.K.; Herbert, C.; Foster, P.S. The “classical” ovalbumin challenge model of asthma in mice. Curr. Drug Targets 2008, 9, 485–494. [Google Scholar] [CrossRef]
- Hall, S.; Agrawal, D.K. Key mediators in the immunopathogenesis of allergic asthma. Int. Immunopharmacol. 2014, 23, 316–329. [Google Scholar] [CrossRef]
- Sy, L.B.; Wu, Y.L.; Chiang, B.L.; Wang, Y.H.; Wu, W.M. Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model. Int. Immunopharmacol. 2006, 6, 1053–1060. [Google Scholar] [CrossRef]
- Sakai, K.; Yokoyama, A.; Kohno, N.; Hiwada, K. Effect of different sensitizing doses of antigen in a murine model of atopic asthma. Clin. Exp. Immunol. 1999, 118, 9–15. [Google Scholar] [CrossRef]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Blumchen, K.; Gerhold, K.; Schwede, M.; Niggemann, B.; Avagyan, A.; Dittrich, A.M.; Wagner, B.; Breiteneder, H.; Hamelmann, E. Effects of established allergen sensitization on immune and airway responses after secondary allergen sensitization. J. Allergy Clin. Immunol. 2006, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Vatrella, A.; Gallelli, L.; Renda, T.; Cazzola, M.; Maselli, R.; Marsico, S.A. Respiratory infections and asthma. Respir. Med. 2006, 100, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Randolph, D.A.; Stephens, R.; Carruthers, C.J.; Chaplin, D.D. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J. Clin. Investig. 1999, 104, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Shi, G.C.; Wan, H.Y.; Jiang, L.H.; Ai, X.Y.; Zhu, H.X.; Tang, W.; Ma, J.Y.; Jin, X.Y.; Zhang, B.Y. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with allergic asthma. Chin. Med. J. 2011, 124, 1951–1956. [Google Scholar] [PubMed]
- Gao, Y.; Zhao, C.; Wang, W.; Jin, R.; Li, Q.; Ge, Q.; Guan, Y.; Zhang, Y. Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci. Rep. 2016, 6, 20505. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]
- Epstein, M.M. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol. Ther. 2006, 109, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S. The role of the T cell in asthma. J. Allergy Clin. Immunol. 2010, 126, 1081–1093. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Henney, C.S.; Kuribayashi, K.; Kern, D.E.; Gillis, S. Interleukin-2 augments natural killer cell activity. Nature 1981, 291, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Forsythe, P. Natural killer cells in asthma. Front. Immunol. 2013, 21, 159. [Google Scholar] [CrossRef][Green Version]
- Pawankar, R.H.S.; Canonica, G.W.; Lockey, R.T.; Blaiss, M.S. WAO White Book on Allergy Update 2013. World Allergy Organ. 2013, 248, 11–19. [Google Scholar]
- Chen, F.; Lin, Z.; Chen, R.; Norback, D.; Liu, C.; Kan, H.; Deng, Q.; Huang, C.; Hu, Y.; Zou, Z.; et al. The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ. Pollut. 2018, 232, 329–337. [Google Scholar] [CrossRef]
- Sompornrattanaphan, M.; Thongngarm, T.; Ratanawatkul, P.; Wongsa, C.; Swigris, J.J. The contribution of particulate matter to respiratory allergy. Asian Pac. J. Allergy Immunol. 2020, 38, 19–28. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-L.; Wang, C.-J.; Huang, H.-W.; Kuo, H.-P.; Su, M.-H.; Lin, H.-C.; Teng, C.-W.; Sy, L.B.; Wu, W.-M. Poria cocos Modulates Th1/Th2 Response and Attenuates Airway Inflammation in an Ovalbumin-Sensitized Mouse Allergic Asthma Model. Life 2021, 11, 372. https://doi.org/10.3390/life11050372
Chao C-L, Wang C-J, Huang H-W, Kuo H-P, Su M-H, Lin H-C, Teng C-W, Sy LB, Wu W-M. Poria cocos Modulates Th1/Th2 Response and Attenuates Airway Inflammation in an Ovalbumin-Sensitized Mouse Allergic Asthma Model. Life. 2021; 11(5):372. https://doi.org/10.3390/life11050372
Chicago/Turabian StyleChao, Chien-Liang, Chao-Jih Wang, Hsin-Wen Huang, Han-Peng Kuo, Muh-Hwan Su, Hang-Ching Lin, Chia-Wen Teng, Leticia B. Sy, and Wen-Mein Wu. 2021. "Poria cocos Modulates Th1/Th2 Response and Attenuates Airway Inflammation in an Ovalbumin-Sensitized Mouse Allergic Asthma Model" Life 11, no. 5: 372. https://doi.org/10.3390/life11050372
APA StyleChao, C.-L., Wang, C.-J., Huang, H.-W., Kuo, H.-P., Su, M.-H., Lin, H.-C., Teng, C.-W., Sy, L. B., & Wu, W.-M. (2021). Poria cocos Modulates Th1/Th2 Response and Attenuates Airway Inflammation in an Ovalbumin-Sensitized Mouse Allergic Asthma Model. Life, 11(5), 372. https://doi.org/10.3390/life11050372