DNA Methylation Change Profiling of Colorectal Disease: Screening towards Clinical Use
Abstract
:1. Introduction
2. Epigenetic Regulation in Human Cancers
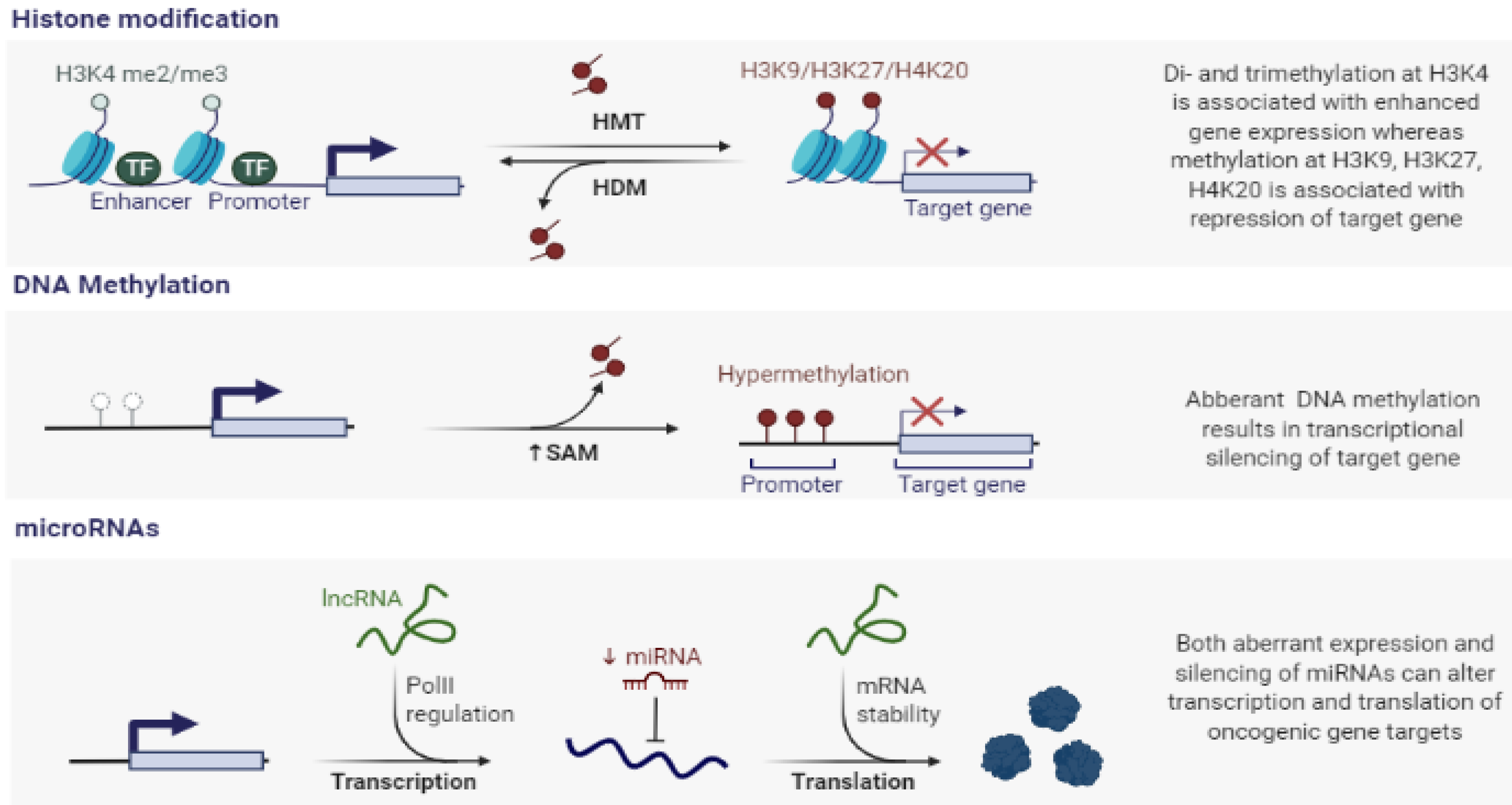
2.1. Histone Modification in Cancer
2.2. DNA Methylation in Cancer
2.3. miRNAs in Cancer
3. Promoter DNA Hypermethylation as a Biomarker for Clinical Use

4. DNA Methylation in IBD
| Diseases Types | Gene | Samples for Study | Methylation Sensitivity | References |
|---|---|---|---|---|
| CRC | TFPI2 | Tumor tissue | 99% | Grockner et al., 2009 [51] |
| Stool | 73% | Grockner et al., 2009 [51] | ||
| FBN2 | Tumor tissue | 86% | Yi et al., 2012 [52] | |
| TCERG1L | Tumor tissue | 99% | Yi et al., 2012 [52] | |
| SEPT9 | Plasma | 69% | Lofton-Day et al., 2008 [53] | |
| p16 | Serum | 70% | Nakayama et al., 2008 [54] | |
| EVL | Tumor tissue | 60% | Yi et al., 2011 [36] | |
| IGFBP3 | Tumor tissue | 25% | Yi et al., 2011 [36] | |
| NDRG4 | Tumor tissue | 86% | Melotte et al., 2009 [55] | |
| Stool | 61% | Melotte et al., 2009 [55] | ||
| UC | FAM217B, | Tissues | 62% | Kang et al., 2016 [56] |
| KIAA1614 | Tissues | 64% | Kang et al., 2016 [56] | |
| RIB2 | Tissues | 91% | Kang et al., 2016 [56] | |
| SYNE1 | Tissues | 80% | Papadia et al., 2014 [57] | |
| FOXE1 | Serum | 60% | Papadia et al., 2014 [57] | |
| CD | TCERG1L | Serum | 57% | Bae et al., 2014 [50] |
| FHIT | Tissues | 71% | Kim et al., 2020 [58] | |
| IBD | TGFB2 | Tissues | 30% | Azuara et al., 2013 [59] |
| SLIT2 | Tissues | 65% | Azuara et al., 2013 [59] | |
| TMEFF2 | Tissues | 25% | Azuara et al., 2013 [59] | |
| ITGA4 | Tissues | 80% | Gerecke et al., 2015 [60] | |
| TFPI2 | Tissues | 30% | Gerecke et al., 2015 [60] |
5. Early Detection Methylation Biomarkers in CRC
5.1. TFPI2
5.2. FBN2 and TCERG1L
6. Prognostic Methylation Biomarkers in CRC
6.1. EVL and IGFBP3
6.2. SEPT9
6.3. Vimentin
6.4. NDRG4
7. Methylation Biomarkers in IBD
7.1. FOXE1 and SYNE1
7.2. TCERG1L
7.3. FAM217B, KIAA1614, and RIB2
7.4. FHIT
8. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, P.A.; Baylin, S.B. The Epigenomics of Cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Schneider, R.; Myers, F.A.; Thorne, A.W.; Crane-Robinson, C.; Kouzarides, T. Spatial Distribution of Di- and Tri-methyl Lysine 36 of Histone H3 at Active Genes *. J. Biol. Chem. 2005, 280, 17732–17736. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Lin, J.C.Y.; Wei, V.; Yoo, C.; Cheng, J.C.; Nguyen, C.T.; Weisenberger, D.J.; Egger, G.; Takai, D.; Gonzales, F.A.; et al. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc. Natl. Acad. Sci. USA 2004, 101, 7357. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.H.; Ciccone, D.N.; Morshead, K.B.; Oettinger, M.A.; Struhl, K. Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: A potential mechanism for position-effect variegation. Proc. Natl. Acad. Sci. USA 2003, 100, 1820. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.H.; Mermoud, J.E.; O’Carroll, D.; Pagani, M.; Schweizer, D.; Brockdorff, N.; Jenuwein, T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat. Genet. 2002, 30, 77–80. [Google Scholar] [CrossRef]
- Peters, A.H.; Kubicek, S.; Mechtler, K.; O’Sullivan, R.J.; Derijck, A.A.; Perez-Burgos, L.; Kohlmaier, A.; Opravil, S.; Tachibana, M.; Shinkai, Y.; et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 2003, 12, 1577–1589. [Google Scholar] [CrossRef]
- Sims, J.K.; Houston, S.I.; Magazinnik, T.; Rice, J.C. A Trans-tail Histone Code Defined by Monomethylated H4 Lys-20 and H3 Lys-9 Demarcates Distinct Regions of Silent Chromatin *. J. Biol. Chem. 2006, 281, 12760–12766. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, J.; Rice, J.C.; Strahl, B.D.; Allis, C.D.; Grewal, S.I. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 2001, 292, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Santenard, A.; Ziegler-Birling, C.; Koch, M.; Tora, L.; Bannister, A.J.; Torres-Padilla, M.-E. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nat. Cell Biol. 2010, 12, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Wissmann, M.; Yin, N.; Müller, J.M.; Greschik, H.; Fodor, B.D.; Jenuwein, T.; Vogler, C.; Schneider, R.; Günther, T.; Buettner, R.; et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat. Cell Biol. 2007, 9, 347–353. [Google Scholar] [CrossRef]
- Yamane, K.; Toumazou, C.; Tsukada, Y.; Erdjument-Bromage, H.; Tempst, P.; Wong, J.; Zhang, Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 2006, 125, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Cheung, P.; Lau, P. Epigenetic Regulation by Histone Methylation and Histone Variants. Mol. Endocrinol. 2005, 19, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.H.; Bird, A. DNA methylation and chromatin modification. Curr. Opin. Genet. Dev. 1999, 9, 158–163. [Google Scholar] [CrossRef]
- Lao, V.V.; Grady, W.M. Epigenetics and colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 686–700. [Google Scholar] [CrossRef]
- Nan, X.; Ng, H.H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef]
- Jones, P.A.; Laird, P.W. Cancer epigenetics comes of age. Nat.Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef]
- Baylin, S.; Bestor, T.H. Altered methylation patterns in cancer cell genomes: Cause or consequence? Cancer Cell 2002, 1, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Watkins, D.N.; Jair, K.W.; Schuebel, K.E.; Markowitz, S.D.; Chen, W.D.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; Van Engeland, M.; et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004, 36, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Garcia-Foncillas, J.; Andion, E.; Goodman, S.N.; Hidalgo, O.F.; Vanaclocha, V.; Baylin, S.B.; Herman, J.G. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 2000, 343, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS Is Regulated by the let-7 MicroRNA Family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The Consensus Coding Sequences of Human Breast and Colorectal Cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Schuebel, K.E.; Chen, W.; Cope, L.; Glöckner, S.C.; Suzuki, H.; Yi, J.M.; Chan, T.A.; Neste, L.V.; Criekinge, W.V.; Bosch, S.V.; et al. Comparing the DNA Hypermethylome with Gene Mutations in Human Colorectal Cancer. PLoS Genet. 2007, 3, 1709–1723. [Google Scholar] [CrossRef]
- Wu, M.C.; Joubert, B.R.; Kuan, P.-F.; Håberg, S.E.; Nystad, W.; Peddada, S.D.; London, S.J. A systematic assessment of normalization approaches for the Infinium 450K methylation platform. Epigenetics 2014, 9, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Widschwendter, M.; Teschendorff, A.E. A comparison of feature selection and classification methods in DNA methylation studies using the Illumina Infinium platform. BMC Bioinform. 2012, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, T.A.; Glockner, S.; Yi, J.M.; Chen, W.; Van Neste, L.; Cope, L.; Herman, J.G.; Velculescu, V.; Schuebel, K.E.; Ahuja, N.; et al. Convergence of Mutation and Epigenetic Alterations Identifies Common Genes in Cancer That Predict for Poor Prognosis. PLoS Med. 2008, 5, e114. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.M.; Dhir, M.; Van Neste, L.; Downing, S.R.; Jeschke, J.; Glöckner, S.C.; de Freitas Calmon, M.; Hooker, C.M.; Funes, J.M.; Boshoff, C.; et al. Genomic and Epigenomic Integration Identifies a Prognostic Signature in Colon Cancer. Clin. Cancer Res. 2011, 17, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar] [CrossRef]
- Laird, P.W. The power and the promise of DNA methylation markers. Nat. Rev. Cancer 2003, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef] [Green Version]
- Diehl, F.; Schmidt, K.; Durkee, K.H.; Moore, K.J.; Goodman, S.N.; Shuber, A.P.; Kinzler, K.W.; Vogelstein, B. Analysis of Mutations in DNA Isolated From Plasma and Stool of Colorectal Cancer Patients. Gastroenterology 2008, 135, 489–498.e487. [Google Scholar] [CrossRef] [Green Version]
- Bailey, V.J.; Easwaran, H.; Zhang, Y.; Griffiths, E.; Belinsky, S.A.; Herman, J.G.; Baylin, S.B.; Carraway, H.E.; Wang, T.-H. MS-qFRET: A quantum dot-based method for analysis of DNA methylation. Genome Res. 2009, 19, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Xavier, R.J.; Rioux, J.D. Genome-wide association studies: A new window into immune-mediated diseases. Nat. Rev. Immunol. 2008, 8, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 2008, 40, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glória, L.; Cravo, M.; Pinto, A.; de Sousa, L.S.; Chaves, P.; Leitão, C.N.; Quina, M.; Mira, F.C.; Soares, J. DNA hypomethylation and proliferative activity are increased in the rectal mucosa of patients with long-standing ulcerative colitis. Cancer 1996, 78, 2300–2306. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Klump, B.; Holzmann, K.; Borchard, F.; Gregor, M.; Porschen, R. Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Res. 1998, 58, 3942–3945. [Google Scholar] [PubMed]
- Kim, T.-O.; Park, J.; Kang, M.J.; Lee, S.H.; Jee, S.R.; Rye, D.Y.; Yang, K.; Yi, J.M. DNA hypermethylation of a selective gene panel as a risk marker for colon cancer in patients with ulcerative colitis. Int. J. Mol. Med. 2013, 31, 1255–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Kato, J.; Hiraoka, S.; Horii, J.; Suzuki, H.; Higashi, R.; Kaji, E.; Kondo, Y.; Yamamoto, K. DNA methylation of colon mucosa in ulcerative colitis patients: Correlation with inflammatory status. Inflamm. Bowel Dis. 2011, 17, 1955–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.; Park, J.; Yang, K.M.; Kim, T.; Yi, J.M. Detection of DNA hypermethylation in sera of patients with Crohn’s disease. Mol. Med. Rep. 2014, 9, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Glockner, S.C.; Dhir, M.; Yi, J.M.; McGarvey, K.E.; Van Neste, L.; Louwagie, J.; Chan, T.A.; Kleeberger, W.; de Bruïne, A.P.; Smits, K.M.; et al. Methylation of TFPI2 in Stool DNA: A Potential Novel Biomarker for the Detection of Colorectal Cancer. Cancer Res. 2009, 69, 4691–4699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.M.; Dhir, M.; Guzzetta, A.A.; Iacobuzio-Donahue, C.A.; Heo, K.; Yang, K.M.; Suzuki, H.; Toyota, M.; Kim, H.-M.; Ahuja, N. DNA methylation biomarker candidates for early detection of colon cancer. Tumor Biol. 2012, 33, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofton-Day, C. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin. Chem. 2008, 54, 414–423. [Google Scholar] [CrossRef]
- Nakayama, H.; Hibi, K.; Taguchi, M.; Takase, T.; Yamazaki, T.; Kasai, Y.; Ito, K.; Akiyama, S.; Nakao, A. Molecular detection of p16 promoter methylation in the serum of colorectal cancer patients. Cancer Lett. 2002, 188, 115–119. [Google Scholar] [CrossRef]
- Melotte, V.; Lentjes, M.H.F.M.; van den Bosch, S.M.; Hellebrekers, D.M.E.I.; de Hoon, J.P.J.; Wouters, K.A.D.; Daenen, K.L.J.; Partouns-Hendriks, I.E.J.M.; Stessels, F.; Louwagie, J.; et al. N-Myc Downstream-Regulated Gene 4 (NDRG4): A Candidate Tumor Suppressor Gene and Potential Biomarker for Colorectal Cancer. J. Natl. Cancer Inst. 2009, 101, 916–927. [Google Scholar] [CrossRef]
- Kang, K.; Bae, J.-H.; Han, K.; Kim, E.S.; Kim, T.-O.; Yi, J.M. A Genome-Wide Methylation Approach Identifies a New Hypermethylated Gene Panel in Ulcerative Colitis. Int. J. Mol. Sci. 2016, 17, 1291. [Google Scholar] [CrossRef]
- Papadia, C.; Louwagie, J.; Del Rio, P.; Grooteclaes, M.; Coruzzi, A.; Montana, C.; Novelli, M.; Bordi, C.; de’Angelis, G.L.; Bassett, P.; et al. FOXE1 and SYNE1 genes hypermethylation panel as promising biomarker in colitis-associated colorectal neoplasia. Inflamm. Bowel Dis. 2014, 20, 271–277. [Google Scholar] [CrossRef]
- Kim, T.-O.; Park, D.-I.; Han, Y.K.; Kang, K.; Park, S.-G.; Park, H.R.; Yi, J.M. Genome-Wide Analysis of the DNA Methylation Profile Identifies the Fragile Histidine Triad (FHIT) Gene as a New Promising Biomarker of Crohn’s Disease. J. Clin. Med. 2020, 9, 1338. [Google Scholar] [CrossRef]
- Azuara, D.; Rodriguez-Moranta, F.; de Oca, J.; Sanjuan, X.; Guardiola, J.; Lobaton, T.; Wang, A.; Boadas, J.; Piqueras, M.; Monfort, D.; et al. Novel methylation panel for the early detection of neoplasia in high-risk ulcerative colitis and Crohn’s colitis patients. Inflamm. Bowel Dis 2013, 19, 165–173. [Google Scholar] [CrossRef]
- Gerecke, C.; Scholtka, B.; Löwenstein, Y.; Fait, I.; Gottschalk, U.; Rogoll, D.; Melcher, R.; Kleuser, B. Hypermethylation of ITGA4, TFPI2 and VIMENTIN promoters is increased in inflamed colon tissue: Putative risk markers for colitis-associated cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 2097–2107. [Google Scholar] [CrossRef]
- Laird, P.W. Oncogenic mechanisms mediated by DNA methylation. Mol. Med. Today 1997, 3, 223–229. [Google Scholar] [CrossRef]
- Wong, C.-M.; Ng, Y.-L.; Lee, J.M.-F.; Wong, C.C.-L.; Cheung, O.-F.; Chan, C.-Y.; Tung, E.K.-K.; Ching, Y.-P.; Ng, I.O.-L. Tissue factor pathway inhibitor-2 as a frequently silenced tumor suppressor gene in hepatocellular carcinoma. Hepatology 2007, 45, 1129–1138. [Google Scholar] [CrossRef]
- Hibi, K.; Goto, T.; Shirahata, A.; Saito, M.; Kigawa, G.; Nemoto, H.; Sanada, Y. Detection of TFPI2 Methylation in the Serum of Gastric Cancer Patients. Anticancer Res. 2011, 31, 3835–3838. [Google Scholar]
- Wu, D.; Xiong, L.; Wu, S.; Jiang, M.; Lian, G.; Wang, M. TFPI-2 methylation predicts poor prognosis in non-small cell lung cancer. Lung Cancer 2012, 76, 106–111. [Google Scholar] [CrossRef]
- Hong, S.-M.; Kelly, D.; Griffith, M.; Omura, N.; Li, A.; Li, C.-P.; Hruban, R.H.; Goggins, M. Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod. Pathol. 2008, 21, 1499–1507. [Google Scholar] [CrossRef]
- Kim, S.Y.; Han, Y.K.; Song, J.M.; Lee, C.H.; Kang, K.; Yi, J.M.; Park, H.R. Aberrantly hypermethylated tumor suppressor genes were identified in oral squamous cell carcinoma (OSCC). Clin. Epigenetics 2019, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Yang, Y.; Brock, M.V.; Cao, B.; Zhan, Q.; Li, Y.; Yu, Y.; Herman, J.G.; Guo, M. Methylation of TFPI-2 is an early event of esophageal carcinogenesis. Epigenomics 2012, 4, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.-K.; Sun, Q.; Fan, Y.-C.; Gao, S.; Zhao, J.; Li, F.; Jia, Y.-B.; Liu, C.; Wang, L.-Y.; Li, X.-Y.; et al. Methylation of tissue factor pathway inhibitor 2 as a prognostic biomarker for hepatocellular carcinoma after hepatectomy. J. Gastroenterol. Hepatol. 2016, 31, 484–492. [Google Scholar] [CrossRef]
- Ahmed, D.; Danielsen, S.A.; Aagesen, T.H.; Bretthauer, M.; Thiis-Evensen, E.; Hoff, G.; Rognum, T.O.; Nesbakken, A.; Lothe, R.A.; Lind, G.E. A Tissue-Based Comparative Effectiveness Analysis of Biomarkers for Early Detection of Colorectal Tumors. Clin. Transl. Gastroenterol. 2012, 3, e27. [Google Scholar] [CrossRef]
- Tänzer, M.; Balluff, B.; Distler, J.; Hale, K.; Leodolter, A.; Röcken, C.; Molnar, B.; Schmid, R.; Lofton-Day, C.; Schuster, T.; et al. Performance of Epigenetic Markers SEPT9 and ALX4 in Plasma for Detection of Colorectal Precancerous Lesions. PLoS ONE 2010, 5, e9061. [Google Scholar] [CrossRef] [Green Version]
- deVos, T.; Tetzner, R.; Model, F.; Weiss, G.; Schuster, M.; Distler, J.; Steiger, K.V.; Grützmann, R.; Pilarsky, C.; Habermann, J.K.; et al. Circulating Methylated SEPT9 DNA in Plasma Is a Biomarker for Colorectal Cancer. Clin. Chem. 2009, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, F.J.; Azuara, D.; Berenguer-Llergo, A.; Fernández, A.F.; Biondo, S.; de Oca, J.; Rodriguez-Moranta, F.; Salazar, R.; Villanueva, A.; Fraga, M.F.; et al. DNA Methylation Biomarkers for Noninvasive Diagnosis of Colorectal Cancer. Cancer Prev. Res. 2013, 6, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Chen, W.-D.; Papadopoulos, N.; Goodman, S.N.; Bjerregaard, N.C.; Laurberg, S.; Levin, B.; Juhl, H.; Arber, N.; Moinova, H.; et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat. Biotechnol. 2009, 27, 858. [Google Scholar] [CrossRef] [Green Version]
- Hellebrekers, D.M.E.I.; Melotte, V.; Viré, E.; Langenkamp, E.; Molema, G.; Fuks, F.; Herman, J.G.; Van Criekinge, W.; Griffioen, A.W.; van Engeland, M. Identification of Epigenetically Silenced Genes in Tumor Endothelial Cells. Cancer Res. 2007, 67, 4138–4148. [Google Scholar] [CrossRef] [Green Version]
- Planell, N.; Lozano, J.J.; Mora-Buch, R.; Masamunt, M.C.; Jimeno, M.; Ordás, I.; Esteller, M.; Ricart, E.; Piqué, J.M.; Panés, J.; et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut 2013, 62, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhir, M.; Yachida, S.; Neste, L.V.; Glöckner, S.C.; Jeschke, J.; Pappou, E.P.; Montgomery, E.A.; Herman, J.G.; Baylin, S.B.; Iacobuzio-Donahue, C.; et al. Sessile serrated adenomas and classical adenomas: An epigenetic perspective on premalignant neoplastic lesions of the gastrointestinal tract. Int. J. Cancer 2011, 129, 1889–1898. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Zhou, P.-Y.; Liu, P.; Zhang, Y. Abnormal FHIT protein expression may be correlated with poor prognosis in gastric cancer: A meta-analysis. Tumor Biol. 2014, 35, 6815–6821. [Google Scholar] [CrossRef]
- Guo, X.Q.; Wang, S.J.; Zhang, L.W.; Wang, X.L.; Zhang, J.H.; Guo, W. DNA methylation and loss of protein expression in esophageal squamous cell carcinogenesis of high-risk area. J. Exp. Clin. Cancer Res. 2007, 26, 587–594. [Google Scholar]
- Younes, S.F.; Aiad, H.A.; Asaad, N.Y.; Kandil, M.A.; Natkunam, Y.; Mokhtar, N.M. FHIT, EGFR, and MSH2: Possible Etiopathologic, Prognostic, and Predictive Role in Non–Small Cell Lung Carcinoma in Egyptian Patients. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 275–283. [Google Scholar] [CrossRef]
- Kapitanović, S.; Čačev, T.; Lončar, B.; Catela Ivković, T.; Križanac, Š.; Pavelić, K. Reduced FHIT expression is associated with tumor progression in sporadic colon adenocarcinoma. Exp. Mol. Pathol. 2014, 96, 92–97. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Li, C.; Li, X.; Zhang, Y.; Yu, Y.; Xia, W. Quantitative detection of methylation of FHIT and BRCA1 promoters in the serum of ductal breast cancer patients. Bio-Med Mater. Eng. 2015, 26 (Suppl 1), S2217–S2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Pu, W.; Tan, Y.; Lu, Z.; Wang, A.; Tan, L.; Chen, S.; Guo, S.; Wang, J.; Chen, X. Quantitative assessment of the diagnostic role of FHIT promoter methylation in non-small cell lung cancer. Oncotarget 2017, 8, 6845–6856. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.M. DNA Methylation Change Profiling of Colorectal Disease: Screening towards Clinical Use. Life 2021, 11, 412. https://doi.org/10.3390/life11050412
Yi JM. DNA Methylation Change Profiling of Colorectal Disease: Screening towards Clinical Use. Life. 2021; 11(5):412. https://doi.org/10.3390/life11050412
Chicago/Turabian StyleYi, Joo Mi. 2021. "DNA Methylation Change Profiling of Colorectal Disease: Screening towards Clinical Use" Life 11, no. 5: 412. https://doi.org/10.3390/life11050412
APA StyleYi, J. M. (2021). DNA Methylation Change Profiling of Colorectal Disease: Screening towards Clinical Use. Life, 11(5), 412. https://doi.org/10.3390/life11050412





