Current Status of Brain Tumor in the Kingdom of Saudi Arabia and Application of Nanobiotechnology for Its Treatment: A Comprehensive Review
Abstract
:1. Introduction
2. Materials and Methods
3. Brain Cancer in Saudi Arabia
3.1. Incidences of Brain Cancer in Saudi Arabia
3.2. Treatment Strategy Followed for Brain Cancer in Saudi Arabia
3.3. Success and Failure Rate of Brain Cancer Treatment
3.4. Need for Alternate Treatment Strategy?
4. Scope and Application of Nanobiotechnology in Brain Cancer Treatment
4.1. Technological Advancements
4.2. Applicability of Nanobiotechnology in Brain Cancer
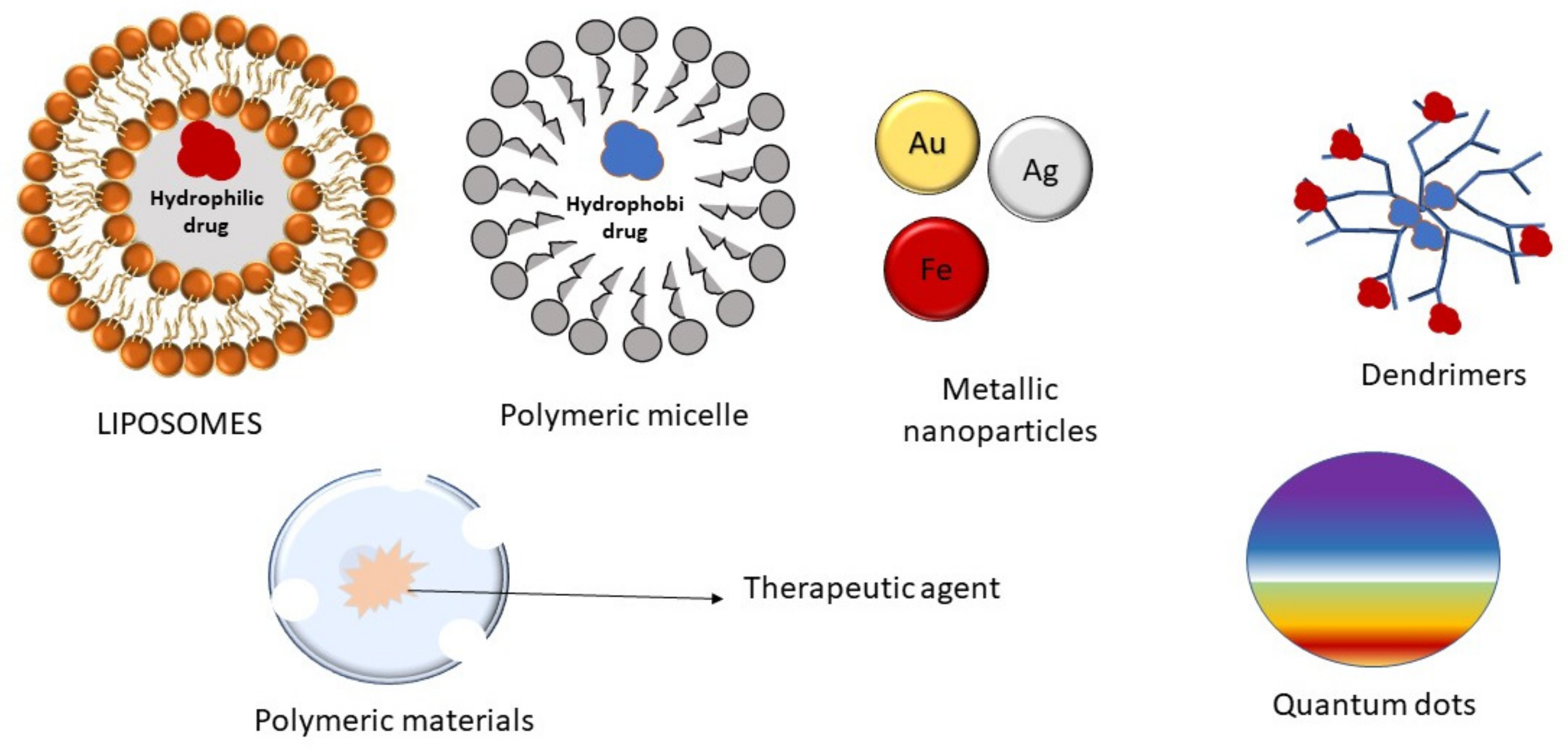
4.3. Nanoparticles
4.3.1. Magnetic Nanoparticles
4.3.2. Polymeric Nanoparticles
4.3.3. Liposomes
4.3.4. Polymeric Micelles
4.3.5. Dendrimers
4.3.6. Quantum Dots
4.4. Limitations of Nanobiotechnology Based Approaches
4.5. Can Nanobiotechnology Fulfill the Current Need for Alternate Brain Cancer Therapy?
4.6. Prospects of Nanobiotechnology
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaufmann, J.K.; Chiocca, E.A. Glioma Virus Therapies between Bench and Bedside. Neuro Oncol. 2014, 16, 334–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the Brain and CNS: Global Patterns and Trends in Incidence. Neuro Oncol. 2017, 19, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 8, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood Brain Tumors: Current Management, Biological Insights, and Future Directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Perkins, A.; Liu, G. Primary Brain Tumors in Adults: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 211–217. [Google Scholar]
- Fisusi, F.A.; Schätzlein, A.G.; Uchegbu, I.F. Nanomedicines in the Treatment of Brain Tumors. Nanomedicine 2018, 13, 579–583. [Google Scholar] [CrossRef]
- Rivkin, M.; Kanoff, R.B. Metastatic Brain Tumors: Current Therapeutic Options and Historical Perspective. J. Am. Osteopath. Assoc. 2013, 113, 418–423. [Google Scholar] [PubMed]
- Fokas, E.; Steinbach, J.P.; Rödel, C. Biology of Brain Metastases and Novel Targeted Therapies: Time to Translate the Research. Biochim. Biophys. Acta Rev. Cancer 2013, 1835, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Manchanda, P.; Vogelbaum, M.A.; Ohlfest, J.R.; Elmquist, W.F. Function of the Blood-Brain Barrier and Restriction of Drug Delivery to Invasive Glioma Cells: Findings in an Orthotopic Rat Xenograft Model of Glioma. Drug Metab. Dispos. 2013, 41, 33–39. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of Care for Treatment of Recurrent Glioblastoma-Are We There Yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, H.; Sharma, A.K.; Mahant, S.; Kapoor, D.N. Recent Advancements in Brain Tumor Targeting Using Magnetic Nanoparticles. Ther. Deliv. 2020, 11, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to Curing Primary Brain Tumours. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Cerna, T.; Stiborova, M.; Adam, V.; Kizek, R.; Eckschlager, T. Nanocarrier Drugs in the Treatment of Brain Tumors. J. Cancer Metastasis Treat. 2016, 2, 407–416. [Google Scholar] [CrossRef]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016. [Google Scholar] [CrossRef] [Green Version]
- Raizer, J. Issues in Developing Drugs for Primary Brain Tumors: Barriers and Toxicities. Toxicol. Pathol. 2011. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of Molecularly Targeted Therapy to Malignant Glioma, a Disease of the Whole Brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef] [Green Version]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005. [Google Scholar] [CrossRef]
- Mohammed, A.; Hamdan, A.; Homoud, A. Histopathological Profile of Brain Tumors: A 12-Year Retrospective Study from Madinah, Saudi Arabia. Asian J. Neurosurg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Quader, S.; Kataoka, K. Nanomaterial-Enabled Cancer Therapy. Mol. Ther. 2017, 25, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Zottel, A.; Paska, A.V.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef] [Green Version]
- Karathanasis, E.; Ghaghada, K.B. Crossing the Barrier: Treatment of Brain Tumors Using Nanochain Particles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 678–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisusi, F.A.; Siew, A.; Chooi, K.W.; Okubanjo, O.; Garrett, N.; Lalatsa, K.; Serrano, D.; Summers, I.; Moger, J.; Stapleton, P.; et al. Lomustine Nanoparticles Enable Both Bone Marrow Sparing and High Brain Drug Levels-A Strategy for Brain Cancer Treatments. Pharm. Res. 2016. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. J. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef] [Green Version]
- Imbault, M.; Chauvet, D.; Gennisson, J.L.; Capelle, L.; Tanter, M. Intraoperative Functional Ultrasound Imaging of Human Brain Activity. Sci. Rep. 2017. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near Infrared Fluorescent Imaging of Brain Tumor with IR780 Dye Incorporated Phospholipid Nanoparticles. J. Transl. Med. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bette, S.; Wiestler, B.; Wiedenmann, F.; Kaesmacher, J.; Bretschneider, M.; Barz, M.; Huber, T.; Ryang, Y.M.; Kochs, E.; Zimmer, C.; et al. Safe Brain Tumor Resection Does Not Depend on Surgery Alone-Role of Hemodynamics. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutka, J.T.; Kim, B.; Etame, A.; Diaz, R.J. Nanosurgical Resection of Malignant Brain Tumors: Beyond the Cutting Edge. ACS Nano 2014, 8, 9716–9722. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Yu, J.B.; Piepmeier, J.M. Role of Neurosurgery and Radiation Therapy in the Management of Brain Tumors. Hematol. Oncol. Clin. N. Am. 2012, 26, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Zhong, J.; Dou, N.N.; Visocchi, M.; Gao, G. One-pot aqueous synthesization of near-infrared quantum dots for bioimaging and photodynamic therapy of gliomas. Acta Neurochir. Suppl. 2017, 124, 303–308. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Yang, W.; Chen, X.; Gao, J.; Gong, X.; Wang, H.; Duan, Y.; Wei, D.; Chang, J. Nanoparticle-Based Diagnostic and Therapeutic Systems for Brain Tumors. J. Mater. Chem. B 2019, 7, 4734–4750. [Google Scholar] [CrossRef]
- Piktel, E.; Niemirowicz, K.; Watek, M.; Wollny, T.; Deptuła, P.; Bucki, R. Recent Insights in Nanotechnology-Based Drugs and Formulations Designed for Effective Anti-Cancer Therapy. J. Nanobiotechnol. 2016, 14, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalatsa, A.; Lee, V.; Malkinson, J.P.; Zloh, M.; Schätzlein, A.G.; Uchegbu, I.F. A Prodrug Nanoparticle Approach for the Oral Delivery of a Hydrophilic Peptide, Leucine5-Enkephalin, to the Brain. Mol. Pharm. 2012, 9, 1665–1680. [Google Scholar] [CrossRef]
- Rex, S.; Heger, Z.; Kudr, J.; Vaculovicova, M.; Adam, V.; Stiborová, M. Apoferritin: Protein Nanocarrier for Targeted Delivery. In Nano Based Drug Delivery; IAPC Publishing: Zagreb, Croatia, 2015; pp. 217–233. ISBN 9789535694229. [Google Scholar] [CrossRef]
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for Cancer: Targeting and Multifunctionality in the Future of Cancer Therapies. ACS Biomater. Sci. Eng. 2015, 1, 64–78. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Almutrafi, A.; Bashawry, Y.; Alshakweer, W.; Al-Harbi, M.; Altwairgi, A.; Al-Dandan, S. The Epidemiology of Primary Central Nervous System Tumors at the National Neurologic Institute in Saudi Arabia: A Ten-Year Single-Institution Study. J. Cancer Epidemiol. 2020. [Google Scholar] [CrossRef]
- Al-Madouj, A.; Eldali, A.; Al-Zahrani, A.S.; Alsayyad, J.; Bazarbashi, S.; Al-Eid, H. Ten-Year Cancer Incidence among Nationals of the GCC States 1998—2007. Gulf Cent. Cancer Control Prev. 2011. [Google Scholar]
- Bangash, M.H. Incidence of Brain Tumours at an Academic Centre in Western Saudi Arabia. East Afr. Med. J. 2011, 88, 138–142. [Google Scholar]
- Taha, M.S.; Almsned, F.M.; Hassen, M.A.; Atean, I.M.; Alwbari, A.M.; Alharbi, Q.K.; Abdulkader, M.M.; Almuhaish, H.S. Demographic and Histopathological Patterns of Neuro-Epithelial Brain Tumors in Eastern Province of Saudi Arabia. Neurosciences 2018. [Google Scholar] [CrossRef] [Green Version]
- Altwairgi, A.K.; Alghareeb, W.A.; Yahya, G.M.; AlShakweer, W.; Elyamany, A. Outcome of Patients with Glioblastoma in Saudi Arabia: Single Center Experience. J. Clin. Oncol. 2015. [Google Scholar] [CrossRef]
- Murshid, W.R.; Siquiera, E.; Rahm, B.; Kanaan, I. Brain Tumors in the First 2 Years of Life in Saudi Arabia. Child’s Nerv. Syst. 1994, 10, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma Multiforme: State of the Art and Future Therapeutics. Surg. Neurol. Int. 2014. [Google Scholar] [CrossRef]
- Ellor, S.V.; Pagano-Young, T.A.; Avgeropoulos, N.G. Glioblastoma: Background, Standard Treatment Paradigms, and Supportive Care Considerations. J. Law Med. Ethics 2014. [Google Scholar] [CrossRef]
- Hess, K.R. Extent of Resection as a Prognostic Variable in the Treatment of Gliomas. J. Neurooncol. 1999. [Google Scholar] [CrossRef]
- Hentschel, S.J.; Sawaya, R. Optimizing Outcomes with Maximal Surgical Resection of Malignant Gliomas. Cancer Control 2003, 10, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bafuer, M.; Buchfelder, M.; Nimsky, C. Correlation of the Extent of Tumor Volume Resection and Patient Survival in Surgery of Glioblastoma Multiforme with High-Field Intraoperative MRI Guidance. Neuro Oncol. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roder, C.; Bisdas, S.; Ebner, F.H.; Honegger, J.; Naegele, T.; Ernemann, U.; Tatagiba, M. Maximizing the Extent of Resection and Survival Benefit of Patients in Glioblastoma Surgery: High-Field IMRI versus Conventional and 5-ALA-Assisted Surgery. Eur. J. Surg. Oncol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Iacob, G.; Dinca, E.B. Current Data and Strategy in Glioblastoma Multiforme. J. Med. Life 2009, 2, 386. [Google Scholar]
- Zhao, S.; Wu, J.; Wang, C.; Liu, H.; Dong, X.; Shi, C.; Shi, C.; Liu, Y.; Teng, L.; Han, D.; et al. Intraoperative Fluorescence-Guided Resection of High-Grade Malignant Gliomas Using 5-Aminolevulinic Acid-Induced Porphyrins: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2013, 8, e63682. [Google Scholar] [CrossRef] [PubMed]
- Chandana, S.R.; Movva, S.; Arora, M.; Singh, T. Primary Brain Tumors in Adults. Am. Fam. Physician 2008, 77, 1423–1430. [Google Scholar]
- Mahmoud, B.S.; Alamri, A.H.; McConville, C. Polymeric Nanoparticles for the Treatment of Malignant Gliomas. Cancers 2020, 12, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma Survival in the United States before and during the Temozolomide Era. J. Neurooncol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Sahgal, A.; Sanghera, P.; Tsao, M.; Davey, P.; Lam, K.; Symons, S.; Aviv, R.; Perry, J. Glioblastoma: Patterns of Recurrence and Efficacy of Salvage Treatments. Can. J. Neurol. Sci. 2011. [Google Scholar] [CrossRef] [Green Version]
- Trogrlić, I.; Trogrlić, D.; Trogrlić, D.; Trogrlić, A.K. Treatment of Glioblastoma with Herbal Medicines. World J. Surg. Oncol. 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
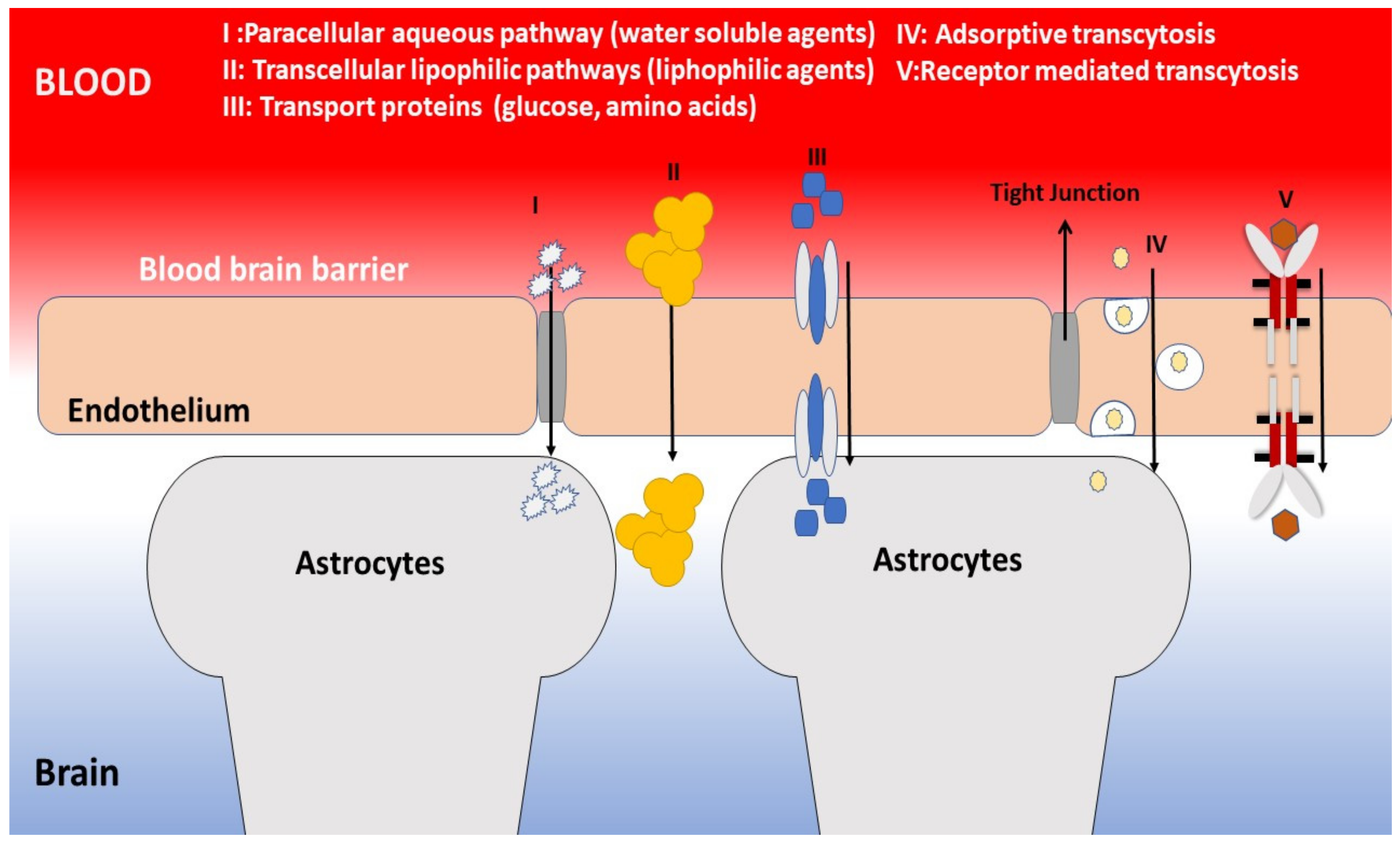
- Hawkins, B.T.; Davis, T.P. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology-Based Strategies for Crossing the Blood-Brain Barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Jiang, Y.; Lv, W.; Xu, J. Liposome-Based Drug Delivery for Brain Tumor Theranostics. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors. Academic Press: Cambridge, MA, USA, 2018; pp. 245–266. ISBN 9780128122181. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Jabir, N.R.; Anwar, K.; Firoz, C.K.; Oves, M.; Kamal, M.A.; Tabrez, S. An Overview on the Current Status of Cancer Nanomedicines. Curr. Med. Res. Opin. 2018, 34, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clin. Transl. Med. 2017. [Google Scholar] [CrossRef] [Green Version]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Kreuter, J.; Alyautdin, R.N.; Kharkevich, D.A.; Ivanov, A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995, 674, 171–174. [Google Scholar] [CrossRef]
- Lalatsa, A.; Leite, D.M.; Figueiredo, M.F.; O’Connor, M. Nanotechnology in Brain Tumor Targeting: Efficacy and Safety of Nanoenabled Carriers Undergoing Clinical Testing. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Zhang, T.T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Shapira, A.; Livney, Y.D.; Broxterman, H.J.; Assaraf, Y.G. Nanomedicine for Targeted Cancer Therapy: Towards the Overcoming of Drug Resistance. Drug Resist. Updat. 2011. [Google Scholar] [CrossRef]
- Urbańska, K.; Pająk, B.; Orzechowski, A.; Sokołowska, J.; Grodzik, M.; Sawosz, E.; Szmidt, M.; Sysa, P. The Effect of Silver Nanoparticles (AgNPs) on Proliferation and Apoptosis of in Ovo Cultured Glioblastoma Multiforme (GBM) Cells. Nanoscale Res. Lett. 2015. [Google Scholar] [CrossRef] [Green Version]
- Eugenio, M.; Campanati, L.; Müller, N.; Romão, L.F.; de Souza, J.; Alves-Leon, S.; de Souza, W.; Sant’Anna, C. Silver/Silver Chloride Nanoparticles Inhibit the Proliferation of Human Glioblastoma Cells. Cytotechnology 2018, 70, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, X.; Liang, X.J. Gold Nanoparticles: Emerging Paradigm for Targeted Drug Delivery System. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Sonali; Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Narendra; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Drappatz, J.; Wen, P.Y. Novel Anti-Angiogenic Therapies for Malignant Gliomas. Lancet Neurol. 2008, 7, 1152–1160. [Google Scholar] [CrossRef]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Bhang, D.; et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. PLoS ONE 2013. [Google Scholar] [CrossRef] [Green Version]
- Hainfeld, J.F.; Smilowitz, H.M.; O’connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold Nanoparticle Imaging and Radiotherapy of Brain Tumors in Mice. Nanomedicine 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, S.Y.; Green, J.J. Therapeutic Nanomedicine for Brain Cancer. Ther. Deliv. 2013, 4, 687–704. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, E.; Hasan, A.; Karunanidhi, P. Solid Lipid Nanoparticles: A Review. Nanosci. Nanotech. Res. 2011, 2, 67–72. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of Solid Lipid Nanoparticles in Brain Targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles-Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indira, T.K.; Lakshmi, P.K. Magnetic Nanoparticles–A Review. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 1035–1042. [Google Scholar]
- Sonali; Agrawal, P.; Singh, R.P.; Rajesh, C.V.; Singh, S.; Vijayakumar, M.R.; Pandey, B.L.; Muthu, M.S. Transferrin Receptor-Targeted Vitamin E TPGS Micelles for Brain Cancer Therapy: Preparation, Characterization and Brain Distribution in Rats. Drug Deliv. 2016. [Google Scholar] [CrossRef] [Green Version]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics-Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660. [Google Scholar] [CrossRef]
- Sonali; Singh, R.P.; Sharma, G.; Kumari, L.; Koch, B.; Singh, S.; Bharti, S.; Rajinikanth, P.S.; Pandey, B.L.; Muthu, M.S. RGD-TPGS Decorated Theranostic Liposomes for Brain Targeted Delivery. Colloids Surf. B Biointerfaces 2016. [Google Scholar] [CrossRef]
- Masood, F. Polymeric Nanoparticles for Targeted Drug Delivery System for Cancer Therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Béduneau, A.; Saulnier, P.; Benoit, J.-P. Active Targeting of Brain Tumors Using Nanocarriers. Biomaterials 2007, 28, 4947–4967. [Google Scholar] [CrossRef] [PubMed]
- Householder, K.T.; Diperna, D.M.; Chung, E.P.; Wohlleb, G.M.; Dhruv, H.D.; Berens, M.E.; Sirianni, R.W. Intravenous Delivery of Camptothecin-Loaded PLGA Nanoparticles for the Treatment of Intracranial Glioma. Int. J. Pharm. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, C.P.; Nishimura, N.; Neeves, K.B.; Schaffer, C.B.; Olbricht, W.L. Real-Time Imaging of Perivascular Transport of Nanoparticles during Convection-Enhanced Delivery in the Rat Cortex. Ann. Biomed. Eng. 2012. [Google Scholar] [CrossRef] [Green Version]
- Alkins, R.D.; Brodersen, P.M.; Sodhi, R.N.S.; Hynynen, K. Enhancing Drug Delivery for Boron Neutron Capture Therapy of Brain Tumors with Focused Ultrasound. Neuro Oncol. 2013. [Google Scholar] [CrossRef]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to Physical Stimulation of Metallic Nanoparticles for Glioblastoma Treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef]
- Crotts, G.; Park, T.G. Protein Delivery from Poly(Lactic-Co-Glycolic Acid) Biodegradable Microspheres: Release Kinetics and Stability Issues. J. Microencapsul. 1998, 15, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Acc. Chem. Res. 2011, 44, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. The First Targeted Delivery of SiRNA in Humans via a Self-Assembling, Cyclodextrin Polymer-Based Nanoparticle: From Concept to Clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacol. Rev. 1999, 51, 691–744. [Google Scholar] [PubMed]
- Schroeder, U.; Sommerfeld, P.; Sabel, B.A. Efficacy of oral dalargin-loaded nanoparticle delivery across the blood-brain barrier. Peptides 1998, 19, 777–780. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate Systems for Brain Delivery of Drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Calvo, P.; Gouritin, B.; Chacun, H.; Desmaile, D.; D’Angelo, J.; Noel, J.P.; Georgin, D.; Fattal, E.; Andreux, J.P.; Couvreur, P. Long-Circulating Pegylated Polycyanoacrylate Nanoparticles as New Drug Carrier for Brain Delivery. Pharm. Res. 2001. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.-B.; Feng, W.; Kang, C.-S.; Pu, P.-Y.; Betbeder, D. Characterization of Endocytosis of Transferrin-Coated PLGA Nanoparticles by the Blood-Brain Barrier. Int. J. Pharm. 2009. [Google Scholar] [CrossRef]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic Brain Tumor Delivery of Synthetic Protein Nanoparticles for Glioblastoma Therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active Targeting of Orthotopic Glioma Using Biomimetic Proteolipid Nanoparticles. ACS Nano 2019. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Wu, Y.; Song, X.; Zhang, S.; Liu, Z. Camouflaging Nanoparticles with Brain Metastatic Tumor Cell Membranes: A New Strategy to Traverse Blood–Brain Barrier for Imaging and Therapy of Brain Tumors. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood–Brain Barrier. Int. J. Nanomed. 2016, 11, 5381. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lee, R.J.; Yu, K.; Bi, Y.; Qi, Y.; Sun, Y.; Li, Y.; Xie, J.; Teng, L. Delivery of SiRNA Using Lipid Nanoparticles Modified with Cell Penetrating Peptide. ACS Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, B.C.; Palei, N.N.; Surendran, V.; Dinda, S.C.; Rajangam, J.; Deb, J.; Sahoo, B.M. Lipid Based Nanoparticles: Current Strategies for Brain Tumor Targeting. Curr. Nanomater. 2019. [Google Scholar] [CrossRef]
- Laschinger, M.; Engelhardt, B. Interaction of Α4-Integrin with VCAM-1 Is Involved in Adhesion of Encephalitogenic T Cell Blasts to Brain Endothelium but Not in Their Transendothelial Migration in Vitro. J. Neuroimmunol. 2000. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier Drug Targeting: The Future of Brain Drug Development. Mol. Interv. 2003, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Lippens, R.J.J. Liposomal Daunorubicin (DaunoXome) in Children with Recurrent or Progressive Brain Tumors. Pediatr. Hematol. Oncol. 1999. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.; Urban, C. Liposomal Cytarabine for Leukemic and Lymphomatous Meningitis: Recent Developments. Expert Opin. Pharmacother. 2008. [Google Scholar] [CrossRef] [PubMed]
- Ananda, S.; Nowak, A.K.; Cher, L.; Dowling, A.; Brown, C.; Simes, J.; Rosenthal, M.A. Phase 2 Trial of Temozolomide and Pegylated Liposomal Doxorubicin in the Treatment of Patients with Glioblastoma Multiforme Following Concurrent Radiotherapy and Chemotherapy. J. Clin. Neurosci. 2011. [Google Scholar] [CrossRef]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated Liposomal Doxorubicine and Prolonged Temozolomide in Addition to Radiotherapy in Newly Diagnosed Glioblastoma-A Phase II Study. BMC Cancer 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C.; et al. Pegylated Liposomal Doxorubicin-Efficacy in Patients with Recurrent High-Grade Glioma. Cancer 2004. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Rosenthal, M.A.; Wong, S.S.; Ashley, D.M.; Woods, A.M.; Dowling, A.; Cher, L.M. Phase 2 Study of Temozolomide and Caelyx in Patients with Recurrent Glioblastoma Multiforme. Neuro Oncol. 2004. [Google Scholar] [CrossRef]
- Wagner, S.; Peters, O.; Fels, C.; Janssen, G.; Liebeskind, A.K.; Sauerbrey, A.; Suttorp, M.; Hau, P.; Wolff, J.E.A. Pegylated-Liposomal Doxorubicin and Oral Topotecan in Eight Children with Relapsed High-Grade Malignant Brain Tumors. J. Neurooncol. 2008. [Google Scholar] [CrossRef]
- Di Legge, A.; Trivellizzi, I.N.; Moruzzi, M.C.; Pesce, A.; Scambia, G.; Lorusso, D. Phase 2 Trial of Nonpegylated Doxorubicin (Myocet) as Second-Line Treatment in Advanced or Recurrent Endometrial Cancer. Int. J. Gynecol. Cancer 2011. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Advances in the Use of Nanocarriers for Cancer Diagnosis and Treatment. Einstein 2016, 14, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Prokai-Tatrai, K.; Prokai, L. Prodrugs of Thyrotropin-Releasing Hormone and Related Peptides as Central Nervous System Agents. Molecules 2009, 14, 633–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Liang, M.; Wang, Y.; Cui, L.; Gao, C.; Chu, X.; Liu, Q.; Feng, Y.; Gong, W.; Yang, M.; et al. Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Guo, X.Y.; Yang, T.; Yu, M.Z.; Chen, D.W.; Wang, J.C. Brain Tumor-Targeted Therapy by Systemic Delivery of SiRNA with Transferrin Receptor-Mediated Core-Shell Nanoparticles. Int. J. Pharm. 2016. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood-Brain Barrier. Nature 2010. [Google Scholar] [CrossRef] [Green Version]
- Kesari, S. Understanding Glioblastoma Tumor Biology: The Potential to Improve Current Diagnosis and Treatments. Semin. Oncol. 2011, 38, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Badie, B.; Chen, M.; Liu, A.; Blanchard, S.; Synold, T.W. The Neuropharmacokinetics of Temozolomide in Patients with Resectable Brain Tumors: Potential Implications for the Current Approach to Chemoradiation. Clin. Cancer Res. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. Alzheimer’s Disease Drug Development and the Problem of the Blood-Brain Barrier. Alzheimer’s Dement. 2009, 5, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, Y.; Dong, S.; Lee, R.J.; Yang, D.; Zhang, H.; Teng, L. Cell-Penetrating Peptide and Transferrin Co-Modified Liposomes for Targeted Therapy of Glioma. Molecules 2019, 24, 3540. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, C.; Bai, E.; Yang, K.; Xu, R. Transferrin and Cell-Penetrating Peptide Dual-Functioned Liposome for Targeted Drug Delivery to Glioma. Int. J. Clin. Exp. Med. 2015, 8, 1658. [Google Scholar]
- Mäe, M.; Langel, Ü. Cell-Penetrating Peptides as Vectors for Peptide, Protein and Oligonucleotide Delivery. Curr. Opin. Pharmacol. 2006, 6, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F. Cell-Penetrating Peptides: Classes, Origin, and Current Landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel Loaded Liposomes Decorated with a Multifunctional Tandem Peptide for Glioma Targeting. Biomaterials 2014. [Google Scholar] [CrossRef]
- Qin, Y.; Fan, W.; Chen, H.; Yao, N.; Tang, W.; Tang, J.; Yuan, W.; Kuai, R.; Zhang, Z.; Wu, Y.; et al. In Vitro and in Vivo Investigation of Glucose-Mediated Brain-Targeting Liposomes. J. Drug Target. 2010. [Google Scholar] [CrossRef]
- Park, S.H.; Yoon, Y.; Moon, H.; Lee, G.H.; Lee, B.H.; Yoon, T.J.; Lee, H.J. Development of a Novel Microbubble-Liposome Complex Conjugated with Peptide Ligands Targeting IL4R on Brain Tumor Cells. Oncol. Rep. 2016. [Google Scholar] [CrossRef] [Green Version]
- Arcella, A.; Palchetti, S.; Digiacomo, L.; Pozzi, D.; Capriotti, A.L.; Frati, L.; Oliva, M.A.; Tsaouli, G.; Rota, R.; Screpanti, I.; et al. Brain Targeting by Liposome-Biomolecular Corona Boosts Anticancer Efficacy of Temozolomide in Glioblastoma Cells. ACS Chem. Neurosci. 2018. [Google Scholar] [CrossRef]
- Patel, B.K.; Parikh, R.H. Formulation Development and Evaluation of Temozolomide Loaded Hydrogenated Soya Phosphatidylcholine Liposomes for the Treatment of Brain Cancer. Asian J. Pharm. Clin. Res. 2016, 9, 340–343. [Google Scholar]
- Temsamani, J.; Rees, A.R.; Scherrmann, J.M. Vector-Mediated Drug Delivery to the Brain. Expert Opin. Biol. Ther. 2001. [Google Scholar] [CrossRef]
- Soni, V.; Kohli, D.V.; Jain, S.K. Transferrin-Conjugated Liposomal System for Improved Delivery of 5-Fluorouracil to Brain. J. Drug Target. 2008. [Google Scholar] [CrossRef] [PubMed]
- Doi, A.; Kawabata, S.; Iida, K.; Yokoyama, K.; Kajimoto, Y.; Kuroiwa, T.; Shirakawa, T.; Kirihata, M.; Kasaoka, S.; Maruyama, K.; et al. Tumor-Specific Targeting of Sodium Borocaptate (BSH) to Malignant Glioma by Transferrin-PEG Liposomes: A Modality for Boron Neutron Capture Therapy. J. Neurooncol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wen, H.; Lu, W.L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.J.; Yang, T.Y.; et al. Dual-Targeting Daunorubicin Liposomes Improve the Therapeutic Efficacy of Brain Glioma in Animals. J. Control. Release 2010. [Google Scholar] [CrossRef]
- Gupta, B.; Levchenko, T.S.; Torchilin, V.P. TAT Peptide-Modified Liposomes Provide Enhanced Gene Delivery to Intracranial Human Brain Tumor Xenografts in Nude Mice. Oncol. Res. 2007. [Google Scholar] [CrossRef]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017, 8, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, M. Clinical Applications of Polymeric Micelle Carrier Systems in Chemotherapy and Image Diagnosis of Solid Tumors. J. Exp. Clin. Med. 2011, 3, 151–158. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Kunjachan, S.; Wu, Z.; Gremse, F.; Moeckel, D.; Van Zandvoort, M.; Kiessling, F.; Storm, G.; Van Nostrum, C.F.; Hennink, W.E.; et al. Fluorophore Labeling of Core-Crosslinked Polymeric Micelles for Multimodal in Vivo and Ex Vivo Optical Imaging. Nanomedicine 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, C.; Gu, B.; Xie, C.; Li, J.; Liu, Y.; Lu, W. Cyclic RGD Conjugated Poly(Ethylene Glycol)-Co-Poly(Lactic Acid) Micelle Enhances Paclitaxel Anti-Glioblastoma Effect. J. Control. Release 2010, 143, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as Nanocarrier for Drug Delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Mishra, V.; Jain, N.K. Acetazolamide Encapsulated Dendritic Nano-Architectures for Effective Glaucoma Management in Rabbits. Int. J. Pharm. 2014. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future. Cambridge University Press: Cambridge, UK, 2012; ISBN 9780521515801. [Google Scholar]
- Mishra, V.; Kesharwani, P. Dendrimer Technologies for Brain Tumor. Drug Discov. Today 2016, 21, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, B.; Liang, J.; Bi, J.; Dai, S.; Qiao, S.Z. Developing Functionalized Dendrimer-like Silica Nanoparticles with Hierarchical Pores as Advanced Delivery Nanocarriers. Adv. Mater. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, C.; Pang, Z. Dendrimer-Based Drug Delivery Systems for Brain Targeting. Biomolecules 2019, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Poldrack, R.A.; Farah, M.J. Progress and Challenges in Probing the Human Brain. Nature 2015, 526, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Moscariello, P.; Ng, D.Y.W.; Jansen, M.; Weil, T.; Luhmann, H.J.; Hedrich, J. Brain Delivery of Multifunctional Dendrimer Protein Bioconjugates. Adv. Sci. 2018. [Google Scholar] [CrossRef]
- Aulenta, F.; Hayes, W.; Rannard, S. Dendrimers: A New Class of Nanoscopic Containers and Delivery Devices. Eur. Polym. J. 2003, 39, 1741–1771. [Google Scholar] [CrossRef]
- Rhyner, M.N.; Smith, A.M.; Goo, X.; Mao, H.; Yang, L.; Nie, S. Quantum Dots and Multifunctional Nanoparticles: New Contrast Agents for Tumor Imaging. Nanomedicine 2006. [Google Scholar] [CrossRef] [Green Version]
- Bilan, R.; Nabiev, I.; Sukhanova, A. Quantum Dot-Based Nanotools for Bioimaging, Diagnostics, and Drug Delivery. ChemBioChem 2016, 17, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Dey, R.; Mitra, M.K.; Mukherjee, S.; Das, G.C. Review: Biofunctionalized Quantum Dots in Biology and Medicine. J. Nanomater. 2009, 2009, 815734. [Google Scholar] [CrossRef] [Green Version]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Silicon Quantum Dots for Biological Applications. Adv. Healthc. Mater. 2014, 3, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008, 17, 89–101. [Google Scholar] [CrossRef]
- Miller, M.A.; Arlauckas, S.; Weissleder, R. Prediction of Anti-Cancer Nanotherapy Efficacy by Imaging. Nanotheranostics 2017, 1, 296. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20. [Google Scholar] [CrossRef]
- Kawasaki, E.S.; Player, A. Nanotechnology, Nanomedicine, and the Development of New, Effective Therapies for Cancer. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, M.S.; Van Dort, M.; Rehemtulla, A.; Ross, B.D. Targeted Imaging and Therapy of Brain Cancer Using Theranostic Nanoparticles. Mol. Pharm. 2010. [Google Scholar] [CrossRef] [Green Version]
- Muthu, M.S.; Mei, L.; Feng, S.S. Nanotheranostics: Advanced Nanomedicine for the Integration of Diagnosis and Therapy. Nanomedicine 2014, 9, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Zhang, M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery across Biological Barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakaş, C.Y.; Tekarslan Şahin, H.; İnan, B.; Özçimen, D.; Erginer, Y. In Vitro Cytotoxic Activity of Microalgal Extracts Loaded Nano–Micro Particles Produced via Electrospraying and Microemulsion Methods. Biotechnol. Prog. 2019. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Mason, R.P.; Sarkaria, J.N.; Zhao, D. Convertible MRI Contrast: Sensing the Delivery and Release of Anti-Glioma Nano-Drugs. Sci. Rep. 2015, 5, 9874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, A.; Yang, B.; Bienemann, A.S.; Barua, N.U.; Wyatt, M.J.; Woolley, M.; Johnson, D.E.; Edler, K.J.; Gill, S.S. Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Mu, Q.; Lin, G.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Gemcitabine and Chlorotoxin Conjugated Iron Oxide Nanoparticles for Glioblastoma Therapy. J. Mater. Chem. B 2016. [Google Scholar] [CrossRef] [Green Version]
- Mirgani, M.T.; Isacchi, B.; Sadeghizadeh, M.; Marra, F.; Bilia, A.R.; Mowla, S.J.; Najafi, F.; Babaei, E. Dendrosomal Curcumin Nanoformulation Downregulates Pluripotency Genes via MiR-145 Activation in U87MG Glioblastoma Cells. Int. J. Nanomed. 2014. [Google Scholar] [CrossRef] [Green Version]
- Steiniger, S.C.J.; Kreuter, J.; Khalansky, A.S.; Skidan, I.N.; Bobruskin, A.I.; Smirnova, Z.S.; Severin, S.E.; Uhl, R.; Kock, M.; Geiger, K.D.; et al. Chemotherapy of Glioblastoma in Rats Using Doxorubicin-Loaded Nanoparticles. Int. J. Cancer 2004. [Google Scholar] [CrossRef]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection Enhanced Delivery of Cisplatin-Loaded Brain Penetrating Nanoparticles Cures Malignant Glioma in Rats. J. Control. Release 2017. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, M.; Grześkowiak, B.F.; Szutkowski, K.; Wawrzyniak, D.; Głodowicz, P.; Barciszewski, J.; Jurga, S.; Rolle, K.; Mrówczyński, R. Nano-Mediated Delivery of Double-Stranded RNA for Gene Therapy of Glioblastoma Multiforme. PLoS ONE 2019. [Google Scholar] [CrossRef]
- Shatsberg, Z.; Zhang, X.; Ofek, P.; Malhotra, S.; Krivitsky, A.; Scomparin, A.; Tiram, G.; Calderón, M.; Haag, R.; Satchi-Fainaro, R. Functionalized Nanogels Carrying an Anticancer MicroRNA for Glioblastoma Therapy. J. Control. Release 2016. [Google Scholar] [CrossRef]
- Huang, J.L.; Jiang, G.; Song, Q.X.; Gu, X.; Hu, M.; Wang, X.L.; Song, H.H.; Chen, L.P.; Lin, Y.Y.; Jiang, D.; et al. Lipoprotein-Biomimetic Nanostructure Enables Efficient Targeting Delivery of SiRNA to Ras-Activated Glioblastoma Cells via Macropinocytosis. Nat. Commun. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Zhang, L.; Wang, Z.; Cheng, Y.; Zhang, P.; Wang, X.; Wen, W.; Yang, H.; Liu, H.; Jin, W.; et al. MicroRNA-101 Inhibits Proliferation, Migration and Invasion of Human Glioblastoma by Targeting SOX9. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Yoon, J.W.; Jiang, W.; Rutka, J.T.; Huang, Y.; Kim, B.Y.S. Perspectives of Nanotechnology in the Management of Gliomas. Prog. Neurol. Surg. 2018. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Gioacchino, M.; Petrarca, C.; Lazzarin, F.; Di Giampaolo, L.; Sabbioni, E.; Boscolo, P.; Mariani-Costantini, R.; Bernardini, G. Immunotoxicity of nanoparticles. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. S1), 65S–71S. [Google Scholar] [PubMed]
- Del Grosso, A.; Galliani, M.; Angella, L.; Santi, M.; Tonazzini, I.; Parlanti, G.; Signore, G.; Cecchini, M. Brain-targeted enzyme-loaded nanoparticles: A breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Sci. Adv. 2019, 5, eaax7462. [Google Scholar] [CrossRef] [Green Version]
- Rigon, L.; Salvalaio, M.; Pederzoli, F.; Legnini, E.; Duskey, J.T.; D’Avanzo, F.; De Filippis, C.; Ruozi, B.; Marin, O.; Vandelli, M.A.; et al. Targeting Brain Disease in MPSII: Preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2014. [Google Scholar] [CrossRef] [Green Version]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef]
- Stocki, P.; Szary, J.; Rasmussen, C.L.M.; Demydchuk, M.; Northall, L.; Logan, D.B.; Gauhar, A.; Thei, L.; Moos, T.; Walsh, F.S.; et al. Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. FASEB J. 2021, 35, e21172. [Google Scholar] [CrossRef] [PubMed]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Magnetic Nanoparticles: An Emerging Technology for Malignant Brain Tumor Imaging and Therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yong, W.H.; Sun, Y.; Vernier, P.T.; Koeffler, H.P.; Gundersen, M.A.; Marcu, L. Receptor-Targeted Quantum Dots: Fluorescent Probes for Brain Tumor Diagnosis. J. Biomed. Opt. 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medicine USlo. Available online: Https://Clinicaltrials.Gov/ (accessed on 2 January 2021).
| Nanomaterials | Activity | Advantages | References |
|---|---|---|---|
| Silver Nanoparticles |
|
| [72] |
| Gold Nanoparticles |
|
| [24,77,78,195] |
| Magnetic nanoparticles |
|
| [12,196] |
| Quantum dots |
|
| [33,197] |
| Liposomes |
|
| [62,126,129,133] |
| Dendrimers |
|
| [153,155] |
| Polymeric nanomaterials |
|
| [34,84,88,89] |
| Nanoparticle Type (Study Number) | Phase | Formulation | Disease Condition | References |
|---|---|---|---|---|
| Polymer-gadolinium chelates (NCT02820454) | II | AGuIX (polysiloxane gadolinium-chelates-based nanoparticles) concurrently with whole brain radiation | Brain metastases | [7,198] |
| Gold (NCT03020017) | Early phase-I | NU-0129 | Glio sarcoma, recurrent glioblastoma | |
| Cationic liposomes (NCT02340156) | II | Liposomes encapsulated p53 cDNA in combination with oral temozolomide | Recurrent glioblastoma | |
| Silica (NCT01266096) | Micro dosing | 124I-cRGDY-PEG-dots for PET scan | Recurrent metastatic melanoma, malignant brain tumor | |
| Liposome (NCT00734682) | I | CPT-11 | CPT-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moin, A.; Rizvi, S.M.D.; Hussain, T.; Gowda, D.V.; Subaiea, G.M.; Elsayed, M.M.A.; Ansari, M.; Alanazi, A.S.; Yadav, H. Current Status of Brain Tumor in the Kingdom of Saudi Arabia and Application of Nanobiotechnology for Its Treatment: A Comprehensive Review. Life 2021, 11, 421. https://doi.org/10.3390/life11050421
Moin A, Rizvi SMD, Hussain T, Gowda DV, Subaiea GM, Elsayed MMA, Ansari M, Alanazi AS, Yadav H. Current Status of Brain Tumor in the Kingdom of Saudi Arabia and Application of Nanobiotechnology for Its Treatment: A Comprehensive Review. Life. 2021; 11(5):421. https://doi.org/10.3390/life11050421
Chicago/Turabian StyleMoin, Afrasim, Syed Mohd Danish Rizvi, Talib Hussain, D. V. Gowda, Gehad M. Subaiea, Mustafa M. A. Elsayed, Mukhtar Ansari, Abulrahman Sattam Alanazi, and Hemant Yadav. 2021. "Current Status of Brain Tumor in the Kingdom of Saudi Arabia and Application of Nanobiotechnology for Its Treatment: A Comprehensive Review" Life 11, no. 5: 421. https://doi.org/10.3390/life11050421
APA StyleMoin, A., Rizvi, S. M. D., Hussain, T., Gowda, D. V., Subaiea, G. M., Elsayed, M. M. A., Ansari, M., Alanazi, A. S., & Yadav, H. (2021). Current Status of Brain Tumor in the Kingdom of Saudi Arabia and Application of Nanobiotechnology for Its Treatment: A Comprehensive Review. Life, 11(5), 421. https://doi.org/10.3390/life11050421








