Therapeutic Nanoparticles for the Different Phases of Ischemic Stroke
Abstract
1. Introduction
2. Stroke: Physiopathology and Treatment Limitations
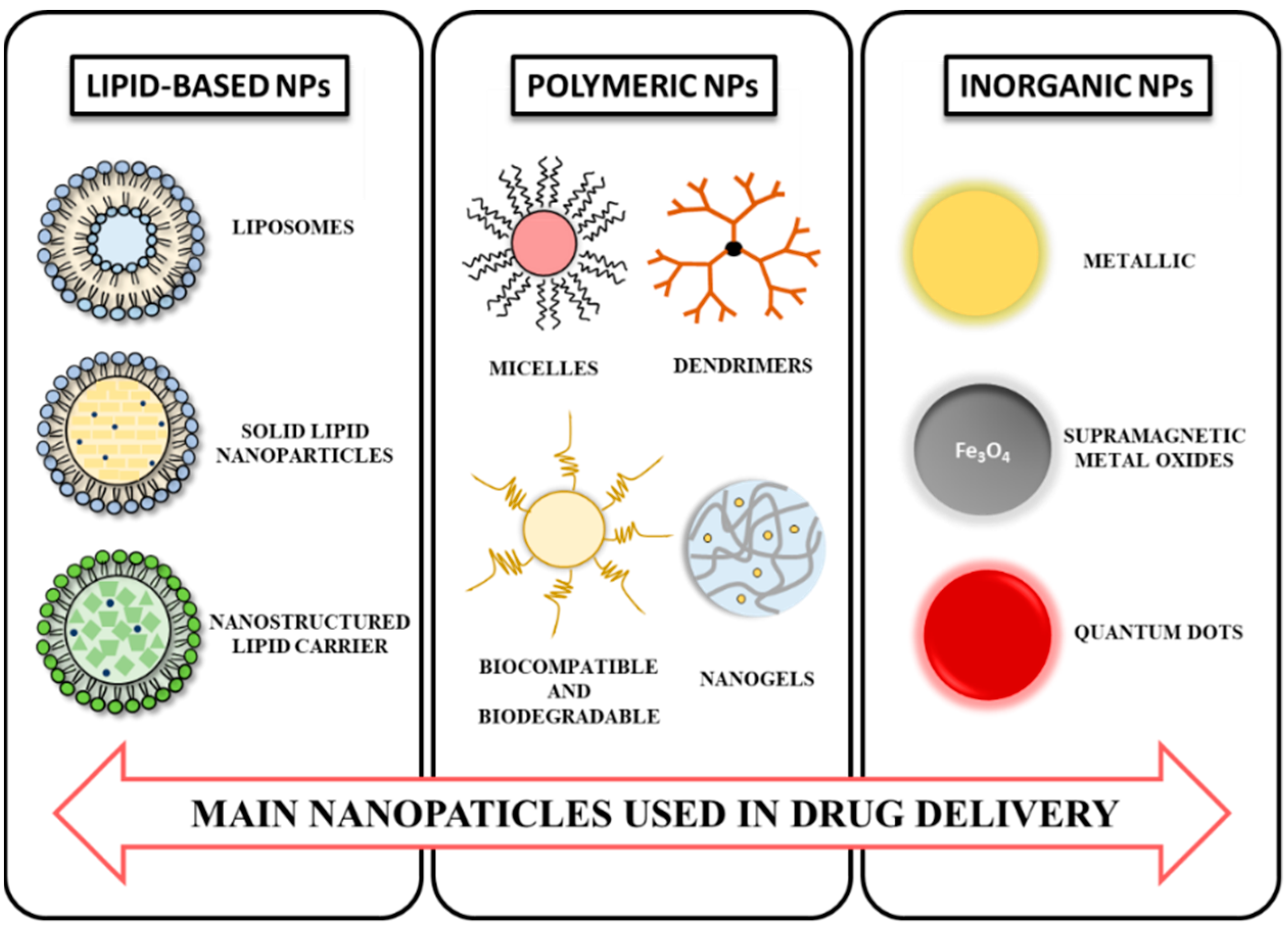
3. NPs: Composition and Properties
4. NPs in Stroke Diagnosis
5. NPs for Stroke Treatment
5.1. NPs in the Hyperacute Phase of Stroke
5.2. NPs and the Acute Phase of the Stroke: Tackling Neuroinflammation
5.3. NPs in the Subacute Phase: Targeting Angiogenesis
5.4. NPs in the Chronic Phase of Stroke: Promoting Neurorepair and Functional Recovery
6. Potential Harmful Effects of NPS and Nanotoxicity
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Papanagiotou, P.; Ntaios, G. Endovascular Thrombectomy in Acute Ischemic Stroke. Circ. Cardiovasc. Interv. 2018, 11, e005362. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 1605. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Donato, H.; Ferreira, L.; Sargento-Freitas, J. Permeability of the blood-brain barrier through the phases of ischaemic stroke and relation with clinical outcome: Protocol for a systematic review. BMJ Open 2020, 10, e039280. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Su, Y.; Wang, Z. Nanomedicine for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7600. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Sharma, C.P. Inorganic nanoparticles for targeted drug delivery. In Biointegration of Medical Implant Materials: Science and Design; Sharma, C.P., Ed.; Woodhead Publishing Limited: London, UK, 2010; pp. 204–235. [Google Scholar]
- Bonnard, T.; Gauberti, M.; de Lizarrondo, S.M.; Campos, F.; Vivien, D. Recent Advances in Nanomedicine for Ischemic and Hemorrhagic Stroke. Stroke 2019, 50, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.; Sandercock, P.; Dennis, M.; Warlow, C.; Burn, J. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991, 337, 1521–1526. [Google Scholar] [CrossRef]
- Shiber, J.R.; Fontane, E.; Adewale, A. Stroke registry: Hemorrhagic vs ischemic strokes. Am. J. Emerg. Med. 2010, 28, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Liu, R.; Pan, M.-X.; Tang, J.-C.; Zhang, Y.; Liao, H.-B.; Zhuang, Y.; Zhao, D.; Wan, Q. Role of neuroinflammation in ischemic stroke. Neuroimmunol. Neuroinflamm. 2017, 4, 158. [Google Scholar] [CrossRef]
- Carmichael, S.T. The 3 Rs of Stroke Biology: Radial, Relayed, and Regenerative. Neurotherapeutics 2016, 13, 348–359. [Google Scholar] [CrossRef]
- Sargento-Freitas, J.; Aday, S.; Nunes, C.; Cordeiro, M.; Gouveia, A.; Silva, F.; Machado, C.; Rodrigues, B.; Santo, G.C.; Ferreira, C.D.C.; et al. Endothelial progenitor cells enhance blood–brain barrier permeability in subacute stroke. Neurology 2017, 90, e127–e134. [Google Scholar] [CrossRef]
- Petty, K.; Lemkuil, B.P.; Gierl, B. Acute Ischemic Stroke. Anesthesiol. Clin. 2021, 39, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.B.; Kaltenbach, L.; Goldstein, L.B.; Olson, D.M.; Smith, E.E.; Peterson, E.D.; Schwamm, L.; Lichtman, J.H.; Furie, K.L. Regional Variation in Recommended Treatments for Ischemic Stroke and TIA. Stroke 2012, 43, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Gupta, R.; Guerrero, W.R.; Rose, D.Z.; Burgin, W.S.; Sivakanthan, S. ASPECTS decay during inter-facility transfer in patients with large vessel occlusion strokes. J. NeuroInterventional Surg. 2017, 9, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Ornello, R.; Degan, D.; Tiseo, C.; di Carmine, C.; Perciballi, L.; Pistoia, F.; Carolei, A.; Sacco, S. Distribution and Temporal Trends From 1993 to 2015 of Ischemic Stroke Subtypes. Stroke 2018, 49, 814–819. [Google Scholar] [CrossRef]
- Cramer, S.C. Recovery After Stroke. Contin. Lifelong Learn. Neurol. 2020, 26, 415–434. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Q.; Hu, Z.; Tang, X. Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front. Neurosci. 2019, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Strbian, D.; Sairanen, T.; Meretoja, A.; Pitkäniemi, J.; Putaala, J.; Salonen, O.; Silvennoinen, H.; Kaste, M.; Tatlisumak, T. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011, 77, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Fiorelli, M.; Steiner, T.; Schäbitz, W.-R.; Bozzao, L.; Bluhmki, E.; Hacke, W.; von Kummer, R. Hemorrhagic Transformation of Ischemic Brain Tissue. Stroke 2001, 32, 1330–1335. [Google Scholar] [CrossRef]
- Neuberger, U.; Kickingereder, P.V.; Schönenberger, S.; Schieber, S.; Ringleb, P.A.; Bendszus, M.; Pfaff, J.; Möhlenbruch, M.A. Risk factors of intracranial hemorrhage after mechanical thrombectomy of anterior circulation ischemic stroke. Neuroradiology 2019, 61, 461–469. [Google Scholar] [CrossRef]
- European Commission. Definition—Nanomaterials. Available online: https://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm (accessed on 1 March 2021).
- De Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Alkaff, S.A.; Radhakrishnan, K.; Nedumaran, A.M.; Liao, P.; Czarny, B. Nanocarriers for Stroke Therapy: Advances and Obstacles in Translating Animal Studies. Int. J. Nanomed. 2020, 15, 445–464. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Daglar, B.; Ozgur, E.; Çorman, M.E.; Uzun, L.; Demirel, G.B. Polymeric nanocarriers for expected nanomedicine: Current challenges and future prospects. RSC Adv. 2014, 4, 48639–48659. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.; Chen, Q.; Liu, P.; Guo, Q.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhou, W.; Liang, D.; et al. Microthrombus-Targeting Micelles for Neurovascular Remodeling and Enhanced Microcirculatory Perfusion in Acute Ischemic Stroke. Adv. Mater. 2019, 31, 1808361. [Google Scholar] [CrossRef]
- Santos, S.D.; Xavier, M.; Leite, D.M.; Moreira, D.A.; Custódio, B.; Torrado, M.; Castro, R.; Leiro, V.; Rodrigues, J.; Tomás, H.; et al. PAMAM dendrimers: Blood-brain barrier transport and neuronal uptake after focal brain ischemia. J. Control. Release 2018, 291, 65–79. [Google Scholar] [CrossRef]
- Cui, W.; Liu, R.; Jin, H.; Lv, P.; Sun, Y.; Men, X.; Yang, S.; Qu, X.; Yang, Z.; Huang, Y. pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J. Control. Release 2016, 225, 53–63. [Google Scholar] [CrossRef]
- Yemisci, M.; Caban, S.; Gursoy-Ozdemir, Y.; Lule, S.; Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E.; Andrieux, K.; Couvreur, P.; Capan, Y.; et al. Systemically Administered Brain-Targeted Nanoparticles Transport Peptides across the Blood—Brain Barrier and Provide Neuroprotection. J. Cereb. Blood Flow Metab. 2015, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Praça, C.; Rai, A.; Santos, T.; Cristóvão, A.C.; Pinho, S.L.; Cecchelli, R.; Dehouck, M.-P.; Bernardino, L.; Ferreira, L.S. A nanoformulation for the preferential accumulation in adult neurogenic niches. J. Control. Release 2018, 284, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci. 2017, 4, 1600279. [Google Scholar] [CrossRef] [PubMed]
- Sivaji, K.; Kannan, R.R. Polysorbate 80 Coated Gold Nanoparticle as a Drug Carrier for Brain Targeting in Zebrafish Model. J. Clust. Sci. 2019, 30, 897–906. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, D.-S. Fabrication, characterization, and biological evaluation of anti-HER2 indocyanine green-doxorubicin-encapsulated PEG-b-PLGA copolymeric nanoparticles for targeted photochemotherapy of breast cancer cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Hu, K.; Shi, Y.; Jiang, W.; Han, J.; Huang, S.; Jiang, X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease. Int. J. Pharm. 2011, 415, 273–283. [Google Scholar] [CrossRef]
- Landowski, L.M.; Niego, B.; Sutherland, B.A.; Hagemeyer, C.E.; Howells, D.W. Applications of Nanotechnology in the Diagnosis and Therapy of Stroke. Semin. Thromb. Hemost. 2020, 46, 592–605. [Google Scholar] [CrossRef]
- Sarmah, D.; Banerjee, M.; Datta, A.; Kalia, K.; Dhar, S.; Yavagal, D.R.; Bhattacharya, P. Nanotechnology in the diagnosis and treatment of stroke. Drug Discov. Today 2020, 26, 585–592. [Google Scholar] [CrossRef]
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Gao, X.; Wang, Y.-C.; Peng, X.-G.; Chang, D.; Zheng, S.; Li, C.; Ju, S. Image-guided Pro-angiogenic Therapy in Diabetic Stroke Mouse Models Using a Multi-modal Nanoprobe. Theranostics 2014, 4, 787–797. [Google Scholar] [CrossRef]
- Saleh, A.; Schroeter, M.; Ringelstein, A.; Hartung, H.-P.; Siebler, M.; Mödder, U.; Jander, S. Iron Oxide Particle-Enhanced MRI Suggests Variability of Brain Inflammation at Early Stages After Ischemic Stroke. Stroke 2007, 38, 2733–2737. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Ryu, J.H.; Schellingerhout, D.; Sun, I.-C.; Lee, S.-K.; Jeon, S.; Kim, J.; Kwon, I.C.; Nahrendorf, M.; Ahn, C.-H.; et al. Direct Imaging of Cerebral Thromboemboli Using Computed Tomography and Fibrin-targeted Gold Nanoparticles. Theranostics 2015, 5, 1098–1114. [Google Scholar] [CrossRef] [PubMed]
- Wicha, P.; Tocharus, J.; Janyou, A.; Jittiwat, J.; Changtam, C.; Suksamrarn, A.; Tocharus, C. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS ONE 2017, 12, e0189211. [Google Scholar] [CrossRef]
- Lu, X.; Dong, J.; Zheng, D.; Li, X.; Ding, D.; Xu, H. Reperfusion combined with intraarterial administration of resveratrol-loaded nanoparticles improved cerebral ischemia–reperfusion injury in rats. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102208. [Google Scholar] [CrossRef]
- Amani, H.; Habibey, R.; Shokri, F.; Hajmiresmail, S.J.; Akhavan, O.; Mashaghi, A.; Pazoki-Toroudi, H. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci. Rep. 2019, 9, 6044. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Cano-Sarabia, M.; Simats, A.; Guillamon, M.M.H.; Rosell, A.; Maspoch, D.; Campos-Martorell, M. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int. J. Nanomed. 2016, 11, 3035–3048. [Google Scholar] [CrossRef]
- Fukuta, T.; Asai, T.; Yanagida, Y.; Namba, M.; Koide, H.; Shimizu, K.; Oku, N. Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J. 2017, 31, 1879–1890. [Google Scholar] [CrossRef]
- Petro, M.; Jaffer, H.; Yang, J.; Kabu, S.; Morris, V.B.; Labhasetwar, V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 2016, 81, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Mei, H.; Shi, W.; Pang, Z.-Q.; Zhang, B.; Guo, T.; Wang, H.-F.; Jiang, X.-G.; Hu, Y. Recombinant Tissue Plasminogen Activator-conjugated Nanoparticles Effectively Targets Thrombolysis in a Rat Model of Middle Cerebral Artery Occlusion. Curr. Med. Sci. 2018, 38, 427–435. [Google Scholar] [CrossRef]
- Absar, S.; Nahar, K.; Kwon, Y.M.; Ahsan, F. Thrombus-Targeted Nanocarrier Attenuates Bleeding Complications Associated with Conventional Thrombolytic Therapy. Pharm. Res. 2013, 30, 1663–1676. [Google Scholar] [CrossRef]
- Juenet, M.; Aid, R.; Li, B.; Berger, A.; Aerts, J.; Ollivier, V.; Nicoletti, A.; Letourneur, D.; Chauvierre, C. Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials 2018, 156, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Yin, H.; Cao, X.; Hu, Q.; Lv, W.; Xu, Q.; Gu, Z.; Xin, H. Sequentially Site-Specific Delivery of Thrombolytics and Neuroprotectant for Enhanced Treatment of Ischemic Stroke. ACS Nano 2019, 13, 8577–8588. [Google Scholar] [CrossRef]
- Bonoiu, A.; Mahajan, S.D.; Ye, L.; Kumar, R.; Ding, H.; Yong, K.-T.; Roy, I.; Aalinkeel, R.; Nair, B.; Reynolds, J.L.; et al. MMP-9 gene silencing by a quantum dot–siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res. 2009, 1282, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Islam, Y.; Khalid, A.; Pluchino, S.; Sivakumaran, M.; Teixidò, M.; Leach, A.; Fatokun, A.A.; Downing, J.; Coxon, C.; Ehtezazi, T. Development of Brain Targeting Peptide Based MMP-9 Inhibiting Nanoparticles for the Treatment of Brain Diseases with Elevated MMP-9 Activity. J. Pharm. Sci. 2020, 109, 3134–3144. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.; Molino, Y.; Bojja, S.; Khrestchatisky, M.; Kaczmarek, L. Tissue inhibitor of matrix metalloproteinases-1 loaded poly(lactic-co-glycolic acid) nanoparticles for delivery across the blood–brain barrier. Int. J. Nanomed. 2014, 9, 575–588. [Google Scholar] [CrossRef]
- Liu, S.; Jin, R.; Wang, M.; Li, G. Nanoparticle Delivery of CD147 Antagonistic Peptide-9 Protects against Acute Ischemic Brain Injury and tPA-Induced Intracerebral Hemorrhage in Mice. ACS Appl. Bio Mater. 2020, 3, 1976–1985. [Google Scholar] [CrossRef]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780–792. [Google Scholar]
- Machado-Pereira, M.; Santos, T.; Ferreira, L.; Bernardino, L.; Ferreira, R. Anti-Inflammatory Strategy for M2 Microglial Polarization Using Retinoic Acid-Loaded Nanoparticles. Mediat. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.-H.; Wang, H.-C.; Kuan, C.-H.; Chen, M.-H.; Wu, H.-C.; Sun, J.-S.; Wang, T.-W. Glycosaminoglycan-based hybrid hydrogel encapsulated with polyelectrolyte complex nanoparticles for endogenous stem cell regulation in central nervous system regeneration. Biomaterials 2018, 174, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Seo, Y.K.; Thambi, T.; Moon, G.J.; Son, J.P.; Li, G.; Park, J.H.; Lee, J.H.; Kim, H.H.; Lee, D.S.; et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer. Biomaterials 2015, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, F.; Wu, Y.; Luo, J.; Mao, X.; Long, L.; Gou, M.; Yang, L.; Deng, D.Y.B. RGD-Modified Nanocarrier-Mediated Targeted Delivery of HIF-1α-AA Plasmid DNA to Cerebrovascular Endothelial Cells for Ischemic Stroke Treatment. ACS Biomater. Sci. Eng. 2018, 5, 6254–6264. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 2019, 17, 29. [Google Scholar] [CrossRef]
- Wang, C.; Lin, G.; Luan, Y.; Ding, J.; Li, P.-C.; Zhao, Z.; Qian, C.; Liu, G.; Ju, S.; Teng, G.-J. HIF-prolyl hydroxylase 2 silencing using siRNA delivered by MRI-visible nanoparticles improves therapy efficacy of transplanted EPCs for ischemic stroke. Biomaterials 2018, 197, 229–243. [Google Scholar] [CrossRef]
- Ferreira, R.; Fonseca, M.C.; Santos, T.; Sargento-Freitas, J.; Tjeng, R.; Paiva, F.; Castelo-Branco, M.; Ferreira, L.S.; Bernardino, L. Retinoic acid-loaded polymeric nanoparticles enhance vascular regulation of neural stem cell survival and differentiation after ischaemia. Nanoscale 2016, 8, 8126–8137. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Wang, Y.-S.; Hsu, P.-Y.; Chen, C.-Y.; Liao, Y.-C.; Juo, S.-H.H. miR-195 Has a Potential to Treat Ischemic and Hemorrhagic Stroke through Neurovascular Protection and Neurogenesis. Mol. Ther. Methods Clin. Dev. 2019, 13, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cooke, M.J.; Sachewsky, N.; Morshead, C.M.; Shoichet, M.S. Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J. Control. Release 2013, 172, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Spagnoli, F.; Burris, M.; Rolland, W.B.; Fajilan, A.; Dou, H.; Tang, J.; Zhang, J.H. Nanoerythropoietin Is 10-Times More Effective Than Regular Erythropoietin in Neuroprotection in a Neonatal Rat Model of Hypoxia and Ischemia. Stroke 2012, 43, 884–887. [Google Scholar] [CrossRef]
- Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. Circumventing the blood–brain barrier: Local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J. Control. Release 2015, 215, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.; Wen, Y.; Gou, R.; Wang, Y.; Xu, Q. The Experimental Therapy on Brain Ischemia by Improvement of Local Angiogenesis with Tissue Engineering in the Mouse. Cell Transplant. 2014, 23, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Talhada, D.; Rai, A.; Ferreira, R.; Ferreira, L.; Bernardino, L.; Ruscher, K. MicroRNA-124-loaded nanoparticles increase survival and neuronal differentiation of neural stem cells in vitro but do not contribute to stroke outcome in vivo. PLoS ONE 2018, 13, e0193609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Nih, L.; Carmichael, S.T.; Lu, Y.; Segura, T. Enzyme-Responsive Delivery of Multiple Proteins with Spatiotemporal Control. Adv. Mater. 2015, 27, 3620–3625. [Google Scholar] [CrossRef]
- Zhao, H.; Bao, X.-J.; Wang, R.-Z.; Li, G.-L.; Gao, J.; Ma, S.-H.; Wei, J.-J.; Feng, M.; Zhao, Y.-J.; Ma, W.-B.; et al. Postacute Ischemia Vascular Endothelial Growth Factor Transfer by Transferrin-Targeted Liposomes Attenuates Ischemic Brain Injury After Experimental Stroke in Rats. Hum. Gene Ther. 2011, 22, 207–215. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Lv, W.; Wang, Z.; Lv, L.; Wang, B.; Liu, X.; Liu, Y.; Hu, Q.; Sun, W.; et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J. Control. Release 2016, 233, 64–71. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; De Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Steam Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef]
- Xin, H.; Wang, F.; Li, Y.; Lu, Q.-E.; Cheung, W.L.; Zhang, Y.; Zhang, Z.G.; Chopp, M. Secondary Release of Exosomes from Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery after Stroke in Rats Treated with Exosomes Harvested from MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transplant. 2017, 26, 243–257. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Su, J.; Wang, X.; Li, X. Rapid Enkephalin Delivery Using Exosomes to Promote Neurons Recovery in Ischemic Stroke by Inhibiting Neuronal p53/Caspase-3. BioMed. Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, G.; Xia, Y.; Zhu, Q.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Yang, Y.; Wang, Y.; et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell. Mol. Med. 2020, 24, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, B.R.; Treadwell, S.; Robinson, T. Haemorrhagic transformation in acute ischaemic stroke following thrombolysis therapy: Classification, pathogenesis and risk factors. Postgrad. Med. J. 2008, 84, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic Transformation after Ischemic Stroke in Animals and Humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef]
- Zenych, A.; Fournier, L.; Chauvierre, C. Nanomedicine progress in thrombolytic therapy. Biomaterials 2020, 258, 120297. [Google Scholar] [CrossRef]
- Hsu, H.-L.; Chen, J.-P. Preparation of thermosensitive magnetic liposome encapsulated recombinant tissue plasminogen activator for targeted thrombolysis. J. Magn. Magn. Mater. 2017, 427, 188–194. [Google Scholar] [CrossRef]
- Voros, E.; Cho, M.; Ramirez, M.; Palange, A.L.; de Rosa, E.; Key, J.; Garami, Z.; Lumsden, A.B.; Decuzzi, P. TPA Immobilization on Iron Oxide Nanocubes and Localized Magnetic Hyperthermia Accelerate Blood Clot Lysis. Adv. Funct. Mater. 2015, 25, 1709–1718. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Wu, J.-M.; Su, T.; Zhang, S.-Y.; Lin, X.-J. Fasudil, a Rho-Kinase Inhibitor, Exerts Cardioprotective Function in Animal Models of Myocardial Ischemia/Reperfusion Injury: A Meta-Analysis and Review of Preclinical Evidence and Possible Mechanisms. Front. Pharmacol. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Bauer, A.T.; Bürgers, H.F.; Rabie, T.; Marti, H.H. Matrix Metalloproteinase-9 Mediates Hypoxia-Induced Vascular Leakage in the Brain via Tight Junction Rearrangement. J. Cereb. Blood Flow Metab. 2009, 30, 837–848. [Google Scholar] [CrossRef]
- Famakin, B.M. The Immune Response to Acute Focal Cerebral Ischemia and Associated Post-stroke Immunodepression: A Focused Review. Aging Dis. 2014, 5, 307–326. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Werner, Y.; Mass, E.; Kumar, P.A.; Ulas, T.; Händlers, K.; Horne, A.; Klee, K.; Lupp, A.; Schütz, D.; Saaber, F.; et al. Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nat. Neurosci. 2020, 23, 351–362. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Lénárt, N.; Fekete, R.; László, Z.I.; Lele, Z.; Orsolits, B.; Molnár, G.; Heindl, S.; Schwarcz, A.D.; et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 2020, 367, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Bonds, J.A.; Minshall, R.D.; Pelligrino, D.A.; Testai, F.D.; Lazarov, O. Neurogenesis and Inflammation after Ischemic Stroke: What is Known and Where We Go from Here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Zhang, F.; Nance, E.; Alnasser, Y.; Kannan, R.; Kannan, S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J. Neuroinflamm. 2016, 13, 65. [Google Scholar] [CrossRef]
- Pinkernelle, J.; Calatayud, P.; Goya, G.F.; Fansa, H.; Keilhoff, G. Magnetic nanoparticles in primary neural cell cultures are mainly taken up by microglia. BMC Neurosci. 2012, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, L.; Tsang, W.; Soltanian-Zadeh, H.; Morris, D.C.; Zhang, R.; Goussev, A.; Powers, C.; Yeich, T.; Chopp, M. Correlation of VEGF and Angiopoietin Expression with Disruption of Blood–Brain Barrier and Angiogenesis after Focal Cerebral Ischemia. Br. J. Pharmacol. 2002, 22, 379–392. [Google Scholar] [CrossRef]
- Moon, S.-K.; Alaverdashvili, M.; Cross, A.; Whishaw, I.Q. Both compensation and recovery of skilled reaching following small photothrombotic stroke to motor cortex in the rat. Exp. Neurol. 2009, 218, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, L.; An, C.; Wang, R.; Yang, L.; Yu, W.; Li, P.; Gao, Y. The blood brain barrier in cerebral ischemic injury—Disruption and repair. Hemorrhagic Stroke 2020, 1, 34–53. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, Y.; Yang, S.; Li, M.; Wen, J.; Li, L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Spellicy, S.E.; Kaiser, E.E.; Bowler, M.M.; Jurgielewicz, B.J.; Webb, R.L.; West, F.D.; Stice, S.L. Neural Stem Cell Extracellular Vesicles Disrupt Midline Shift Predictive Outcomes in Porcine Ischemic Stroke Model. Transl. Stroke Res. 2020, 11, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Cambre, M.; Lee, H.-J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of Nanoparticles on Brain Health: An Up to Date Overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Simak, J.; De Paoli, S. The effects of nanomaterials on blood coagulation in hemostasis and thrombosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1448. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Gao, J.-Q. Potential neurotoxicity of nanoparticles. Int. J. Pharm. 2010, 394, 115–121. [Google Scholar] [CrossRef]
- Song, G.; Zhao, M.; Chen, H.; Lenahan, C.; Zhou, X.; Ou, Y.; He, Y. The Role of Nanomaterials in Stroke Treatment: Targeting Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Kyle, S.; Saha, S. Nanotechnology for the Detection and Therapy of Stroke. Adv. Heal. Mater. 2014, 3, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. The toxicity of nanoparticles to human endothelial cells. In Advances in Experimental Medicine and Biology; Saquib, Q., Faisal, M., Al-Khedhairy, A., Alatar, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 1048, pp. 59–69. ISBN 9783319720418. [Google Scholar]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Fernández-Bertólez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Bessa, M.J.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef] [PubMed]

| Phase | Target | NPs | Payload | Outcome | Model | Ref. |
|---|---|---|---|---|---|---|
| HYPERACUTE | Rho-kinase | Liposomes | Fausidil | Protection against tPA harmful effects | SD MCAO rat | [62] |
| ROS | Polymeric | Resveratrol | Protection against EVT harmful effects | SD tMCAO rat | [58] | |
| Biodegradable PLGA | CAT and SOD | Protection against tPA harmful effects | SD thrombo rat | [63] | ||
| Fibrin | PEG-PCL | rtPA | Improved and no harmful reperfusion | SD MCAO rat | [64] | |
| GPIIb/IIIa of platelets | Liposomes with FGG C-terminal peptide | tPA | Improved reperfusion with no harmful effect | SD IVC trhombosis rat | [65] | |
| P-selectin of platelets | Polysaccharide-poly-IBCA + Fucoidan | rtPA | Improved reperfusion without harmful effect | Rat venous thrombosis | [66] | |
| TfR/GLUT receptor | Liposome dual-target nanocarrier | ZL006 | Efficient trhombolysis and reduced cell apoptosis and ischemia | SD MCAO rat/ ICR mice | [67] | |
| MMP-9 | Quantum dot nanoplexes | MMP-9 siRNA | ECM proteins upregulation and BBBP decrease | Human BMVEC/NHAs | [68] | |
| Amphibilic peptide | MMP-9-inhibiting peptide | MMP-9 inhibition | BBB model: hCMEC/D3 cell line | [69] | ||
| Ps80-coated PLGA | TIMP-1 | Early inhibition of MMP-9 | In vitro: RBE4 / RBCEC+ astrocytes; In vivo: mice | [70] | ||
| Polymeric NPs | CD147-antagonist peptide-9 | Reduced brain infarct size and HT appearance | C57BL/6 tMCAO mice | [71] | ||
| ACUTE | Microglia activation | Adipose-derived stem cells exosomes | miR-126 | Inhibition of microglial activation and inflammatory factors expression | MCAO rats | [72] |
| Retinoic acid NPs | Retinoic acid | Reduction in microglia activation | N9 microglia cells; Organotypic hippocampal slices culture | [73] | ||
| Transferrin receptor | PEGylated Selenium NPs | siRNA STAT3 | Suppression of excessive inflammation and oxidative metabolism | MCAO rats | [59] | |
| SUBACUTE | Stroke cavity | RGD-HA hydrogel | VEGF | Better angioenesis/establish axonal nets | Mouse MCAO | [74] |
| PCN-NPs | SDF-1a, bFGF | Enhanced neurogenesis and angiogenesis | PTI | [75] | ||
| Ischemic area | SDF-1-loaded micelles | SDF-1α | Enhanced neurogenesis and angiogenesis | Rat MCAO | [76] | |
| Integrin receptor | cRGD-dendrimer | N/A | Improved angiogenesis | PTI | [54] | |
| DMAPA-NPs | HIF-1α-AA plasmid | Enhanced angiogenesis, reduced infarct volume, and improved neurological function | Zebrafish AIS/Rat MCAO | [77] | ||
| RGD-EVs | miR-210 | Improved angiogenesis | MCAO mouse | [78] | ||
| Neurons | RVG-EVs | miR-124 | Enhanced cortical neurogenesis | PTI | [60] | |
| CHRONIC | siRNA delivery/EPCs | Alkyl-PEI/SPIO | PHD2 siRNA | MRI/BLI tracking, Increased functional recovery, vascularization, neurogenesis, and Cxcr4 expression inducing cell mobilization and migration. Decreased infarct volume | In vitro: umbellical cord UCB EPCs In vivo: BALB/c nude mice | [79] |
| Angio/neurogenesis | PEI | retinoic acid | NSC proliferation and differentiation, protection of ECs ischemic death | hEPC from stroke patients | [80] | |
| Neurovascular protection | PEI | miR-195 | Improved neurogenesis, neuroprotection EC function/ less inflammation | In vitro: SH-sy5 In vivo: tMCAO/MCAO rat | [81] | |
| Sequential growth factor release | PLGA and PLGA/ poly(sebacic acid) NPs on HAMC hydrogel | EGF-PEG and erythropoietin | Controlled release of growth factor to the brain circumvents the BBB, neurogenesis | C57BL/6 murine stroke | [82] | |
| EPO dose reduction | PLGA | Erythropoietin | Effects of the EPO-NPs equivalent to 10 times the amount of free EPO | Unilateral AIS neonatal rat | [83] | |
| Increase efficiency of drug delivery | PLGA NPs in HAMC hydrogel | Cyclosporin A | Higher levels of CsA delivered with local injection, NSC survival, proliferation, and migration | Long-Evans endothelin-1 stroke rats | [84] | |
| Neural restoration via angiogenesis | PLGA NPs in a HA scaffold + anti-NOGO receptor antibody | VEGF and Ang-1 | Behavioral improvement, vascularization, axonal growth | In vitro: HUAECs/ primary NSCs; in vivo: C57BL/6J MCAO rats | [85] | |
| Identification of new stroke therapeutics | PLGA | miR-124 | SVZ neurogenesis, increased survival and neuronal differentiation of NSCs in vitro but no effects in vivo | In vitro: primary NSCs/ In vivo: C57BL/6 J PTI mice | [86] | |
| BBB crossing | Chitosan NPs + anti-tfR antibody | bFGF | Accumulation of NPs in brain parenchyma, neuroprotection | MCAO swiss albino mice | [43] | |
| Biomolecules delivery | Enantiomeric protein nanocapsules in HA hydrogel + RGD motif | VEGF and PDGF | Controlled release thanks to MMP-sensitive crosslinker, improved vascularization | C57BL/6 MCAO mice | [87] | |
| Increased brain delivery of VEGF | Liposomes functionalized with transferrin | VEGF | Neurogenesis, increased mRNA and protein VEGF, decreased infarct volume, functional recovery | SD MCAO rats | [88] | |
| Design of stroke dual-targeted lipososmes | liposomes conjugated with T7 peptide and stroke homing peptide (SHp) | neuroprotectant ZL006 | BBB crossing, targeting of the ischemic area, improved neurological deficit, protection against apoptosis | In vitro: BCEC cells and PC-12 cells In vivo: SD MCAO rat and mice | [89] | |
| Stroke therapy with EVs | EVs from MSCs | N/A | Increased axonal density, functional recovery, neurogenesis, angiogenesis | MCAO Wistar rats | [90] | |
| MSC and MSC-EVs comparison | EVs from BMSCs | N/A | Improved motor coordination, neurogenesis, neuroprotection, angiogenesis | MCAO Mice C57BL6 | [91] | |
| EVs’ study as therapeutics | Evs from MSCs | miR-133b | Motor recovery, neurite remodeling | In vitro: Primary neurons In vivo: MCAO rats | [92] | |
| Neurogenesis | EVs from BMSCs modified with transferrin | Enkephalin | Increased neuronal density, decreased p53 and caspase-3 levels | In vitro: primary neurons In vivo: MCAO rats | [93] | |
| Therapeutic effect of EVs from ADSC | EVs from adipose-derived stem cells (ADSC) | miR-126 | Neurogenesis, angiogenesis, functional recovery | MCAO rats | [72] | |
| Effect of urine EVs on neurogenesis | Evs from urine | miR-26a | Proliferation and differentiation of NSC | MCAO rats | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo-Castro, S.; Albino, I.; Barrera-Sandoval, Á.M.; Tomatis, F.; Sousa, J.A.; Martins, E.; Simões, S.; Lino, M.M.; Ferreira, L.; Sargento-Freitas, J. Therapeutic Nanoparticles for the Different Phases of Ischemic Stroke. Life 2021, 11, 482. https://doi.org/10.3390/life11060482
Bernardo-Castro S, Albino I, Barrera-Sandoval ÁM, Tomatis F, Sousa JA, Martins E, Simões S, Lino MM, Ferreira L, Sargento-Freitas J. Therapeutic Nanoparticles for the Different Phases of Ischemic Stroke. Life. 2021; 11(6):482. https://doi.org/10.3390/life11060482
Chicago/Turabian StyleBernardo-Castro, Sara, Inês Albino, Ángela María Barrera-Sandoval, Francesca Tomatis, João André Sousa, Emanuel Martins, Susana Simões, Miguel M. Lino, Lino Ferreira, and João Sargento-Freitas. 2021. "Therapeutic Nanoparticles for the Different Phases of Ischemic Stroke" Life 11, no. 6: 482. https://doi.org/10.3390/life11060482
APA StyleBernardo-Castro, S., Albino, I., Barrera-Sandoval, Á. M., Tomatis, F., Sousa, J. A., Martins, E., Simões, S., Lino, M. M., Ferreira, L., & Sargento-Freitas, J. (2021). Therapeutic Nanoparticles for the Different Phases of Ischemic Stroke. Life, 11(6), 482. https://doi.org/10.3390/life11060482






