Biomechanics of Neutrophil Tethers
Abstract
:1. Introduction
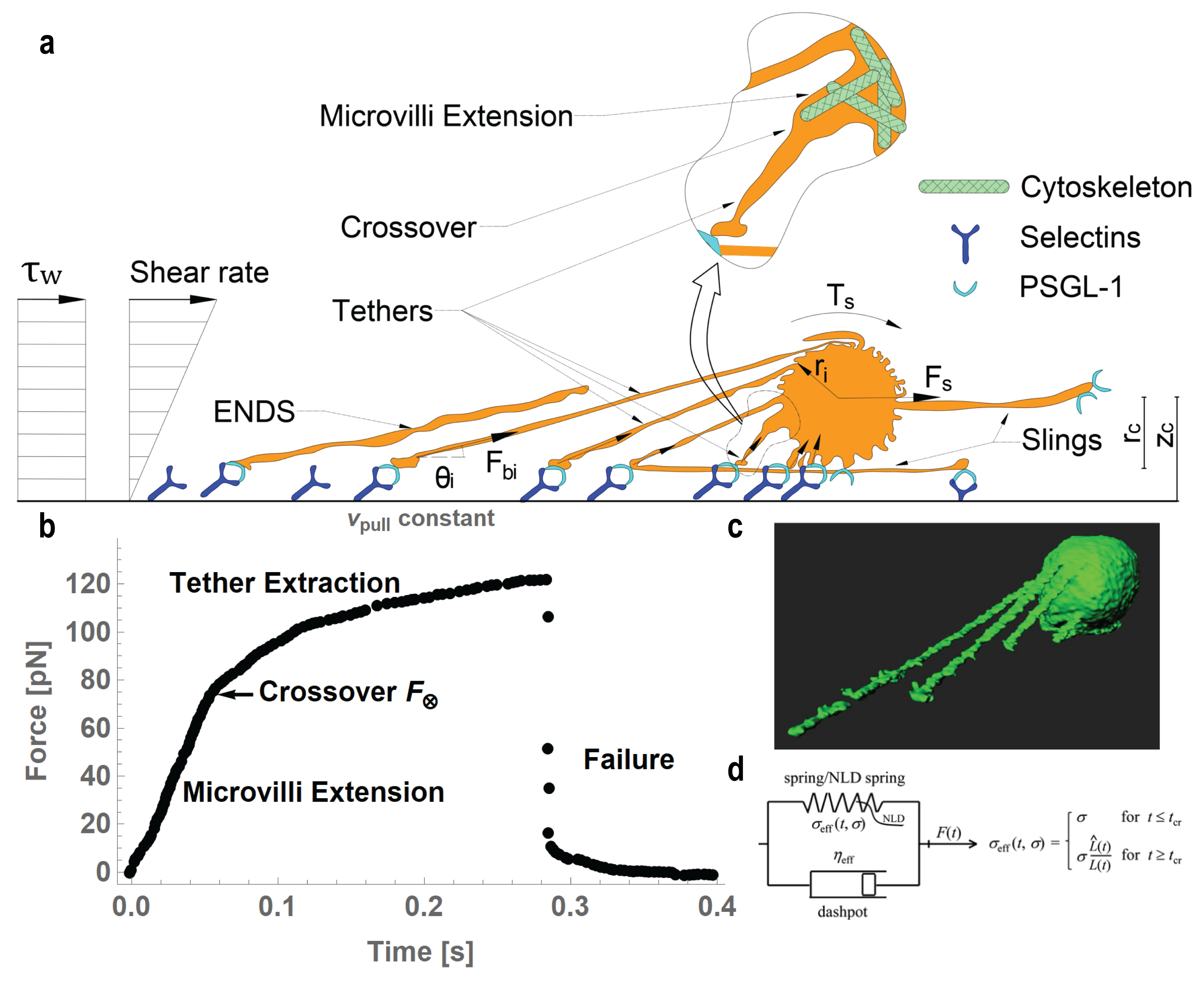

2. Fundamental Physics of Tether Formation
3. Experimental Methods to Pull Tethers
3.1. Tether Extraction with Micropipette Aspiration Technique
3.2. Tether Extraction with Biomembrane Force Probe
3.3. Tether Extraction with Optical Trap
3.4. Tether Extraction with Atomic Force Microscopy
3.5. Flow Chamber and Intravital Microscopy Experiments
4. Insights from Tethers Pulled from Lipid Membranes Vesicles
5. Discussion and Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PMN | Polymorphonuclear leukocytes |
| EC | Endothelial cells |
| PSGL-1 | P-selectin glycoprotein ligand |
| ENDS | Elongated neutrophil-derived structures |
| MAT | Micropipette Aspiration Technique |
| BFP | Biomembrane Force Probe |
| OT | Optical Trap |
| AFM | Atomic force microscopy |
| IM | Intravital microscopy |
| FC | Flow chamber |
| GUVs | Giant unilamellar vesicles |
| qDF | Quantitative dynamic footprinting |
| NLDs | Nonlinearly decaying spring |
| RBC | Red blood cell |
References
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef] [Green Version]
- Reusch, N.; De Domenico, E.; Bonaguro, L.; Schulte-Schrepping, J.; Baßler, K.; Schultze, J.L.; Aschenbrenner, A.C. Neutrophils in COVID-19. Front. Immunol. 2021, 12, 652470. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Marki, A.; Gutierrez, E.; Mikulski, Z.; Groisman, A.; Ley, K. Microfluidics-based side view flow chamber reveals tether-to-sling transition in rolling neutrophils. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundd, P.; Gutierrez, E.; Pospieszalska, M.K.; Zhang, H.; Groisman, A.; Ley, K. Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling. Nat. Methods 2010, 7, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Sundd, P.; Gutierrez, E.; Koltsova, E.K.; Kuwano, Y.; Fukuda, S.; Pospieszalska, M.K.; Groisman, A.; Ley, K. ‘Slings’ enable neutrophil rolling at high shear. Nature 2012, 488, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Schmidtke, D.W.; Diamond, S.L. Direct Observation of Membrane Tethers Formed during Neutrophil Attachment to Platelets or P-Selectin under Physiological Flow. J. Cell Biol. 2000, 149, 719–730. [Google Scholar] [CrossRef]
- Atherton, A.; Born, G.V.R. Relationship between the velocity of rolling granulocytes and that of the blood flow in venules. J. Physiol. 1973, 233, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Chiang, E.Y.; Hidalgo, A.; Chang, J.; Frenette, P.S. Imaging receptor microdomains on leukocyte subsets in live mice. Nat. Methods 2007, 4, 219–222. [Google Scholar] [CrossRef]
- Damiano, E.; Westheider, J.; Tozeren, A.; Ley, K. Variation in the Velocity, Deformation, and Adhesion Energy Density of Leukocytes Rolling Within Venules. Circ. Res. 1996, 79, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Firrell, J.C.; Lipowsky, H.H. Leukocyte margination and deformation in mesenteric venules of rat. Am. J. Physiol. 1989, 256, H1667–H1674. [Google Scholar] [CrossRef] [PubMed]
- Pospieszalska, M.K.; Ley, K. Dynamics of Microvillus Extension and Tether Formation in Rolling Leukocytes. Cell. Mol. Bioeng. 2009, 2, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.; Ley, K. Mice lacking two or all three selectins demonstrate overlapping and distinct functions for each selectin. J. Immunol. 1999, 162, 6755–6762. [Google Scholar] [PubMed]
- Kim, M.B.; Sarelius, I.H. Role of shear forces and adhesion molecule distribution on P-selectin-mediated leukocyte rolling in postcapillary venules. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2705–H2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, K.; Bullard, D.C.; Arbonés, M.L.; Bosse, R.; Vestweber, D.; Tedder, T.F.; Beaudet, A.L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995, 181, 669–675. [Google Scholar] [CrossRef]
- Lawrence, M.B.; Springer, T.A. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell 1991, 65, 859–873. [Google Scholar] [CrossRef]
- Lawrence, M.B.; Springer, T.A. Neutrophils roll on E-selectin. J. Immunol. 1993, 151, 6338–6346. [Google Scholar] [PubMed]
- McEver, R.P.; Zhu, C. Rolling Cell Adhesion. Annu. Rev. Cell Dev. Biol. 2010, 26, 363–396. [Google Scholar] [CrossRef]
- Walcheck, B.; Moore, K.L.; McEver, R.P.; Kishimoto, T.K. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J. Clin. Investig. 1996, 98, 1081–1087. [Google Scholar] [CrossRef]
- Sundd, P.; Pospieszalska, M.K.; Cheung, L.S.L.; Konstantopoulos, K.; Ley, K. Biomechanics of leukocyte rolling. Biorheology 2011, 48, 1–35. [Google Scholar] [CrossRef]
- Kunkel, E.J.; Dunne, J.L.; Ley, K. Leukocyte Arrest During Cytokine-Dependent Inflammation In Vivo. J. Immunol. 2000, 164, 3301–3308. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, E.J.; Chomas, J.E.; Ley, K. Role of Primary and Secondary Capture for Leukocyte Accumulation In Vivo. Circ. Res. 1998, 82, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Müller, H.; Kuwano, Y.; Ley, K. PSGL-1-dependent myeloid leukocyte activation. J. Leukoc. Biol. 2009, 86, 1119–1124. [Google Scholar] [CrossRef]
- Hidalgo, A.; Peired, A.J.; Wild, M.K.; Vestweber, D.; Frenette, P.S. Complete Identification of E-Selectin Ligands on Neutrophils Reveals Distinct Functions of PSGL-1, ESL-1, and CD44. Immunity 2007, 26, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Adrover, J.M.; del Fresno, C.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossío, I.; et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 2019, 50, 390–402.e10. [Google Scholar] [CrossRef] [Green Version]
- Wild, M.K.; Lühn, K.; Marquardt, T.; Vestweber, D. Leukocyte Adhesion Deficiency II: Therapy and Genetic Defect. Cells Tissues Organs 2002, 172, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.Y. Chapter 2 Biomechanics of Leukocyte and Endothelial Cell Surface. In Current Topics in Membranes, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 64, pp. 25–45. [Google Scholar] [CrossRef]
- Finger, E.B.; Bruehl, R.E.; Bainton, D.F.; Springer, T.A. A differential role for cell shape in neutrophil tethering and rolling on endothelial selectins under flow. J. Immunol. 1996, 157, 5085–5096. [Google Scholar]
- Chen, Y.; Yao, D.K.; Shao, J.Y. The Constitutive Equation for Membrane Tether Extraction. Ann. Biomed. Eng. 2010, 38, 3756–3765. [Google Scholar] [CrossRef] [Green Version]
- Girdhar, G.; Shao, J.Y. Simultaneous Tether Extraction from Endothelial Cells and Leukocytes: Observation, Mechanics, and Significance. Biophys. J. 2007, 93, 4041–4052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marki, A.; Buscher, K.; Lorenzini, C.; Meyer, M.; Saigusa, R.; Fan, Z.; Yeh, Y.T.; Hartmann, N.; Dan, J.M.; Kiosses, W.B.; et al. Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Park, E.Y.; Smith, M.J.; Stropp, E.S.; Snapp, K.R.; DiVietro, J.A.; Walker, W.F.; Schmidtke, D.W.; Diamond, S.L.; Lawrence, M.B. Comparison of PSGL-1 Microbead and Neutrophil Rolling: Microvillus Elongation Stabilizes P-Selectin Bond Clusters. Biophys. J. 2002, 82, 1835–1847. [Google Scholar] [CrossRef] [Green Version]
- Galkina, S.I.; Fedorova, N.V.; Golenkina, E.A.; Stadnichuk, V.I.; Sud’ina, G.F. Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes. Int. J. Mol. Sci. 2020, 21, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkina, S.I.; Sud’ina, G.F.; Ullrich, V. Inhibition of Neutrophil Spreading during Adhesion to Fibronectin Reveals Formation of Long Tubulovesicular Cell Extensions (Cytonemes). Exp. Cell Res. 2001, 266, 222–228. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Romanova, J.M.; Golyshev, S.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F.; Klein, T. Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Molotkovsky, J.G.; Ullrich, V.; Sud’ina, G.F. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide. Exp. Cell Res. 2005, 304, 620–629. [Google Scholar] [CrossRef]
- Galkina, S.I.; Romanova, J.M.; Stadnichuk, V.I.; Molotkovsky, J.G.; Sud’ina, G.F.; Klein, T. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol. Med. Microbiol. 2009, 56, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’ina, G.F. Inhibition of the GTPase dynamin or actin depolymerisation initiates outward plasma membrane tubulation/vesiculation (cytoneme formation) in neutrophils. Biol. Cell 2015, 107, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Corriden, R.; Self, T.; Akong-Moore, K.; Nizet, V.; Kellam, B.; Briddon, S.J.; Hill, S.J. Adenosine-A 3 receptors in neutrophil microdomains promote the formation of bacteria-tethering cytonemes. EMBO Rep. 2013, 14, 726–732. [Google Scholar] [CrossRef]
- Shao, J.; Hochmuth, R. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys. J. 1996, 71, 2892–2901. [Google Scholar] [CrossRef]
- Ramachandran, V.; Williams, M.; Yago, T.; Schmidtke, D.W.; McEver, R.P. Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. Proc. Natl. Acad. Sci. USA 2004, 101, 13519–13524. [Google Scholar] [CrossRef] [Green Version]
- Marki, A.; Buscher, K.; Mikulski, Z.; Pries, A.; Ley, K. Rolling neutrophils form tethers and slings under physiologic conditions in vivo. J. Leukoc. Biol. 2017, 103, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.Y.; Ting-Beall, H.P.; Hochmuth, R.M. Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. USA 1998, 95, 6797–6802. [Google Scholar] [CrossRef] [Green Version]
- Sundd, P.; Pospieszalska, M.K.; Ley, K. Neutrophil rolling at high shear: Flattening, catch bond behavior, tethers and slings. Mol. Immunol. 2013, 55, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, E.; Heinrich, V.; Leung, A.; Kinoshita, K. Nano- to Microscale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. I. Membrane Separation from the Cytoskeleton. Biophys. J. 2005, 88, 2288–2298. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.Y.; Xu, J. A Modified Micropipette Aspiration Technique and Its Application to Tether Formation From Human Neutrophils. J. Biomech. Eng. 2002, 124, 388–396. [Google Scholar] [CrossRef]
- Girdhar, G.; Shao, J.Y. Membrane Tether Extraction from Human Umbilical Vein Endothelial Cells and Its Implication in Leukocyte Rolling. Biophys. J. 2004, 87, 3561–3568. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, V.; Leung, A.; Evans, E. Nano- to Microscale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. II. Tether Flow Terminated by P-Selectin Dissociation from PSGL-1. Biophys. J. 2005, 88, 2299–2308. [Google Scholar] [CrossRef] [Green Version]
- Merkel, R.; Nassoy, P.; Leung, A.; Ritchie, K.; Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 1999, 397, 50–53. [Google Scholar] [CrossRef]
- King, M.R.; Heinrich, V.; Evans, E.; Hammer, D.A. Nano-to-Micro Scale Dynamics of P-Selectin Detachment from Leukocyte Interfaces. III. Numerical Simulation of Tethering under Flow. Biophys. J. 2005, 88, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Anvari, B.; Takashima, M.; Brecht, P.; Torres, J.H.; Brownell, W.E. Membrane Tether Formation from Outer Hair Cells with Optical Tweezers. Biophys. J. 2002, 82, 1386–1395. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Shao, J.Y. Human neutrophil surface protrusion under a point load: location independence and viscoelasticity. Am. J. Physiol. Cell Physiol. 2008, 295, C1434–C1444. [Google Scholar] [CrossRef] [Green Version]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Parekh, S.H.; Lam, W.A.; Fletcher, D.A. Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells. Nat. Methods 2009, 6, 383–387. [Google Scholar] [CrossRef]
- Pontes, B.; Monzo, P.; Gauthier, N.C. Membrane tension: A challenging but universal physical parameter in cell biology. Semin. Cell Dev. Biol. 2017, 71, 30–41. [Google Scholar] [CrossRef]
- Diz-Muñoz, A.; Thurley, K.; Chintamen, S.; Altschuler, S.J.; Wu, L.F.; Fletcher, D.A.; Weiner, O.D. Membrane Tension Acts Through PLD2 and mTORC2 to Limit Actin Network Assembly During Neutrophil Migration. PLoS Biol. 2016, 14, e1002474. [Google Scholar] [CrossRef] [Green Version]
- Cuvelier, D.; Derényi, I.; Bassereau, P.; Nassoy, P. Coalescence of Membrane Tethers: Experiments, Theory, and Applications. Biophys. J. 2005, 88, 2714–2726. [Google Scholar] [CrossRef] [Green Version]
- Lemière, J.; Valentino, F.; Campillo, C.; Sykes, C. How cellular membrane properties are affected by the actin cytoskeleton. Biochimie 2016, 130, 33–40. [Google Scholar] [CrossRef]
- Guevorkian, K.; Manzi, J.; Pontani, L.L.; Brochard-Wyart, F.; Sykes, C. Mechanics of Biomimetic Liposomes Encapsulating an Actin Shell. Biophys. J. 2015, 109, 2471–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, A.; Bouzid, M.; Betz, T.; Simon, C.; Abou-Ghali, M.; Lemière, J.; Valentino, F.; Manzi, J.; Brochard-Wyart, F.; Guevorkian, K.; et al. Actin modulates shape and mechanics of tubular membranes. Sci. Adv. 2020, 6, eaaz3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prévost, C.; Tsai, F.C.; Bassereau, P.; Simunovic, M. Pulling Membrane Nanotubes from Giant Unilamellar Vesicles. J. Vis. Exp. 2017, 2017, 56086. [Google Scholar] [CrossRef] [PubMed]
- Sorre, B.; Callan-Jones, A.; Manzi, J.; Goud, B.; Prost, J.; Bassereau, P.; Roux, A. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc. Natl. Acad. Sci. USA 2012, 109, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, V.; Waugh, R.E. A piconewton force transducer and its application to measurement of the bending stiffness of phospholipid membranes. Ann. Biomed. Eng. 1996, 24, 595–605. [Google Scholar] [CrossRef]
- Simunovic, M.; Lee, K.Y.C.; Bassereau, P. Celebrating Soft Matter’s 10th anniversary: Screening of the calcium-induced spontaneous curvature of lipid membranes. Soft Matter 2015, 11, 5030–5036. [Google Scholar] [CrossRef]
- Nussenzveig, H.M. Cell membrane biophysics with optical tweezers. Eur. Biophys. J. 2018, 47, 499–514. [Google Scholar] [CrossRef]
- Pontes, B.; Viana, N.; Salgado, L.; Farina, M.; Neto, V.M.; Nussenzveig, H. Cell Cytoskeleton and Tether Extraction. Biophys. J. 2011, 101, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Pontes, B.; Ayala, Y.; Fonseca, A.C.C.; Romão, L.F.; Amaral, R.F.; Salgado, L.T.; Lima, F.R.; Farina, M.; Viana, N.B.; Moura-Neto, V.; et al. Membrane Elastic Properties and Cell Function. PLoS ONE 2013, 8, e67708. [Google Scholar] [CrossRef] [Green Version]
- Alimohamadi, H.; Vasan, R.; Hassinger, J.; Stachowiak, J.; Rangamani, P. The role of traction in membrane curvature generation. Mol. Biol. Cell 2018, 29, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Fraldi, M.; Cugno, A.; Deseri, L.; Dayal, K.; Pugno, N. A frequency-based hypothesis for mechanically targeting and selectively attacking cancer cells. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef] [Green Version]
- Fraldi, M.; Cugno, A.; Carotenuto, A.R.; Cutolo, A.; Pugno, N.M.; Deseri, L. Small-on-Large Fractional Derivative–Based Single-Cell Model Incorporating Cytoskeleton Prestretch. J. Eng. Mech. 2017, 143, D4016001. [Google Scholar] [CrossRef] [Green Version]
- Deseri, L.; Zingales, M.; Pollaci, P. The state of fractional hereditary materials (FHM). Discret. Contin. Dyn. Syst. B 2014, 19, 2065–2089. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1993; p. 568. [Google Scholar]
- Pospieszalska, M.K.; Lasiecka, I.; Ley, K. Cell Protrusions and Tethers: A Unified Approach. Biophys. J. 2011, 100, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Pickard, J.E.; Ley, K. Micro-PTV measurement of the fluid shear stress acting on adherent leukocytes in vivo. Biophys. J. 2009, 96, 4249–4259. [Google Scholar] [CrossRef] [Green Version]
- Goldman, A.; Cox, R.; Brenner, H. Slow viscous motion of a sphere parallel to a plane wall—II Couette flow. Chem. Eng. Sci. 1967, 22, 653–660. [Google Scholar] [CrossRef]
- Fan, Z.; McArdle, S.; Marki, A.; Mikulski, Z.; Gutierrez, E.; Engelhardt, B.; Deutsch, U.; Ginsberg, M.; Groisman, A.; Ley, K. Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis. Nat. Commun. 2016, 7, 12658. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Springer, T.A. An Automatic Braking System That Stabilizes Leukocyte Rolling by an Increase in Selectin Bond Number with Shear. J. Cell Biol. 1999, 144, 185–200. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shao, J.Y. Simultaneous tether extraction contributes to neutrophil rolling stabilization: A model study. Biophys. J. 2007, 92, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, A.; De Leon, D.; Li, H.; Noiri, E.; Moy, V.T.; Goligorsky, M.S. Atomic force microscopy measurement of leukocyte-endothelial interaction. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H359–H367. [Google Scholar] [CrossRef]
- Sun, M.; Graham, J.S.; Hegedüs, B.; Marga, F.; Zhang, Y.; Forgacs, G.; Grandbois, M. Multiple Membrane Tethers Probed by Atomic Force Microscopy. Biophys. J. 2005, 89, 4320–4329. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.Y.; Yu, Y.; Oswald, S.J. From Surface Protrusion to Tether Extraction: A Mechanistic Model. ACS Biomater. Sci. Eng. 2017, 3, 3036–3042. [Google Scholar] [CrossRef]
- Borghi, N.; Brochard-Wyart, F. Tether Extrusion from Red Blood Cells: Integral Proteins Unbinding from Cytoskeleton. Biophys. J. 2007, 93, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Waugh, R.; Hochmuth, R. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys. J. 1987, 52, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Hochmuth, F.; Shao, J.; Dai, J.; Sheetz, M. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J. 1996, 70, 358–369. [Google Scholar] [CrossRef] [Green Version]
- Brochard-Wyart, F.; Borghi, N.; Cuvelier, D.; Nassoy, P. Hydrodynamic narrowing of tubes extruded from cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7660–7663. [Google Scholar] [CrossRef] [Green Version]
- Campillo, C.; Sens, P.; Köster, D.; Pontani, L.L.; Lévy, D.; Bassereau, P.; Nassoy, P.; Sykes, C. Unexpected Membrane Dynamics Unveiled by Membrane Nanotube Extrusion. Biophys. J. 2013, 104, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitarska, E.; Diz-Muñoz, A. Pay attention to membrane tension: Mechanobiology of the cell surface. Curr. Opin. Cell Biol. 2020, 66, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Diz-Muñoz, A.; Weiner, O.D.; Fletcher, D.A. In pursuit of the mechanics that shape cell surfaces. Nat. Phys. 2018, 14, 648–652. [Google Scholar] [CrossRef]
- Bergert, M.; Lembo, S.; Sharma, S.; Russo, L.; Milovanović, D.; Gretarsson, K.H.; Börmel, M.; Neveu, P.A.; Hackett, J.A.; Petsalaki, E.; et al. Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation. Cell Stem Cell 2021, 28, 209–216.4. [Google Scholar] [CrossRef] [PubMed]
- Raucher, D.; Stauffer, T.; Chen, W.; Shen, K.; Guo, S.; York, J.D.; Sheetz, M.P.; Meyer, T. Phosphatidylinositol 4,5-Bisphosphate Functions as a Second Messenger that Regulates Cytoskeleton–Plasma Membrane Adhesion. Cell 2000, 100, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Steinkühler, J.; Zhao, Z.; Lipowsky, R.; Dimova, R. Mechanical Tension of Biomembranes Can Be Measured by Super Resolution (STED) Microscopy of Force-Induced Nanotubes. Nano Lett. 2020, 20, 3185–3191. [Google Scholar] [CrossRef] [PubMed]
- Raucher, D.; Sheetz, M.P. Characteristics of a Membrane Reservoir Buffering Membrane Tension. Biophys. J. 1999, 77, 1992–2002. [Google Scholar] [CrossRef] [Green Version]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Liu, B.; Shao, J.Y. Tangential Tether Extraction and Spontaneous Tether Retraction of Human Neutrophils. Biophys. J. 2012, 103, 2257–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, W.D.; Hochmuth, R.M. Experimental Studies of Membrane Tethers Formed from Human Neutrophils. Ann. Biomed. Eng. 2002, 30, 1273–1280. [Google Scholar] [CrossRef]
- Xu, G.; Shao, J.Y. Double Tether Extraction from Human Neutrophils and Its Comparison with CD4+ T-Lymphocytes. Biophys. J. 2005, 88, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, B.; Xu, G.; Shao, J.Y. Validation, In-Depth Analysis, and Modification of the Micropipette Aspiration Technique. Cell. Mol. Bioeng. 2009, 2, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Datar, A.; Bornschlögl, T.; Bassereau, P.; Prost, J.; Pullarkat, P.A. Dynamics of Membrane Tethers Reveal Novel Aspects of Cytoskeleton-Membrane Interactions in Axons. Biophys. J. 2015, 108, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Gómez, F.; Silva, L.S.; Araújo, G.R.d.S.; Frases, S.; Pinheiro, A.A.S.; Agero, U.; Pontes, B.; Viana, N.B. Effect of cell geometry in the evaluation of erythrocyte viscoelastic properties. Phys. Rev. E 2020, 101, 062403. [Google Scholar] [CrossRef]
- Nambiar, R.; McConnell, R.E.; Tyska, M.J. Control of cell membrane tension by myosin-I. Proc. Natl. Acad. Sci. USA 2009, 106, 11972–11977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fygenson, D.K.; Marko, J.F.; Libchaber, A. Mechanics of Microtubule-Based Membrane Extension. Phys. Rev. Lett. 1997, 79, 4497–4500. [Google Scholar] [CrossRef] [Green Version]
- Sorre, B.; Callan-Jones, A.; Manneville, J.B.; Nassoy, P.; Joanny, J.F.; Prost, J.; Goud, B.; Bassereau, P. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 5622–5626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, R.S.; Viana, N.B.; Maia Neto, P.A.; Nussenzveig, H.M. Absolute calibration of forces in optical tweezers. Phys. Rev. A 2014, 90, 013825. [Google Scholar] [CrossRef]
- Zlatanova, J.; Lindsay, S.M.; Leuba, S.H. Single molecule force spectroscopy in biology using the atomic force microscope. Prog. Biophys. Mol. Biol. 2000, 74, 37–61. [Google Scholar] [CrossRef]
- Marshall, B.T.; Long, M.; Piper, J.W.; Yago, T.; McEver, R.P.; Zhu, C. Direct observation of catch bonds involving cell-adhesion molecules. Nature 2003, 423, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, Y.; Yuan, Y.; Huang, B. Applications of atomic force microscopy in immunology. Front. Med. 2021, 15, 43–52. [Google Scholar] [CrossRef]
- Chu, C.; Celik, E.; Rico, F.; Moy, V.T. Elongated Membrane Tethers, Individually Anchored by High Affinity α4β1/VCAM-1 Complexes, Are the Quantal Units of Monocyte Arrests. PLoS ONE 2013, 8, e64187. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; García, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009, 185, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Marshall, B.T.; Sarangapani, K.K.; Wu, J.; Lawrence, M.B.; McEver, R.P.; Zhu, C. Measuring Molecular Elasticity by Atomic Force Microscope Cantilever Fluctuations. Biophys. J. 2006, 90, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Houk, A.R.; Jilkine, A.; Mejean, C.O.; Boltyanskiy, R.; Dufresne, E.R.; Angenent, S.B.; Altschuler, S.J.; Wu, L.F.; Weiner, O.D. Membrane Tension Maintains Cell Polarity by Confining Signals to the Leading Edge during Neutrophil Migration. Cell 2012, 148, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Ingber, D.E.; Wang, N.; Stamenović, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef] [Green Version]
- Usami, S.; Chen, H.H.; Zhao, Y.; Chien, S.; Skalak, R. Design and construction of a linear shear stress flow chamber. Ann. Biomed. Eng. 1993, 21, 77–83. [Google Scholar] [CrossRef]
- Van Kooten, T.G.; Schakenraad, J.M.; Van der Mei, H.C.; Busscher, H.J. Development and use of a parallel-plate flow chamber for studying cellular adhesion to solid surfaces. J. Biomed. Mater. Res. 1992, 26, 725–738. [Google Scholar] [CrossRef]
- Munn, L.; Melder, R.; Jain, R. Analysis of cell flux in the parallel plate flow chamber: Implications for cell capture studies. Biophys. J. 1994, 67, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Kucik, D.F.; Szalai, A.J.; Edberg, J.C. Human Neutrophil Flow Chamber Adhesion Assay. J. Vis. Exp. 2014, 51410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnutt, B.C.; Smith, D.F.; Raffler, N.A.; Smith, M.L.; White, E.J.; Ley, K. Induction of LFA-1-dependent neutrophil rolling on ICAM-1 by engagement of E-selectin. Microcirculation 2006, 13, 99–109. [Google Scholar] [CrossRef]
- Bhatia, T.; Husen, P.; Brewer, J.; Bagatolli, L.A.; Hansen, P.L.; Ipsen, J.H.; Mouritsen, O.G. Preparing giant unilamellar vesicles (GUVs) of complex lipid mixtures on demand: Mixing small unilamellar vesicles of compositionally heterogeneous mixtures. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 3175–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimova, R.; Marques, C. The Giant Vesicle Book, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Callan-Jones, A.; Sorre, B.; Bassereau, P. Curvature-Driven Lipid Sorting in Biomembranes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004648. [Google Scholar] [CrossRef] [Green Version]
- Tsai, F.C.; Bertin, A.; Bousquet, H.; Manzi, J.; Senju, Y.; Tsai, M.C.; Picas, L.; Miserey-Lenkei, S.; Lappalainen, P.; Lemichez, E.; et al. Ezrin enrichment on curved membranes requires a specific conformation or interaction with a curvature-sensitive partner. eLife 2018, 7, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Derényi, I.; Jülicher, F.; Prost, J. Formation and Interaction of Membrane Tubes. Phys. Rev. Lett. 2002, 88, 238101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraschiv, A.; Lagny, T.J.; Campos, C.V.; Coudrier, E.; Bassereau, P.; Šarić, A. Influence of membrane-cortex linkers on the extrusion of membrane tubes. Biophys. J. 2021, 120, 598–606. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cugno, A.; Marki, A.; Ley, K. Biomechanics of Neutrophil Tethers. Life 2021, 11, 515. https://doi.org/10.3390/life11060515
Cugno A, Marki A, Ley K. Biomechanics of Neutrophil Tethers. Life. 2021; 11(6):515. https://doi.org/10.3390/life11060515
Chicago/Turabian StyleCugno, Andrea, Alex Marki, and Klaus Ley. 2021. "Biomechanics of Neutrophil Tethers" Life 11, no. 6: 515. https://doi.org/10.3390/life11060515
APA StyleCugno, A., Marki, A., & Ley, K. (2021). Biomechanics of Neutrophil Tethers. Life, 11(6), 515. https://doi.org/10.3390/life11060515






