The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review
Abstract
:1. Introduction
1.1. Fundamental Characteristics of Npy and Pyy in Teleost
1.2. Expression of Npy and Pyy in Teleost
1.3. Receptors of the Neuropeptide Y Family in Teleost
2. Neuropeptide Y—Its Role as Feed Regulator in Teleost
3. Peptide YY—Its Role as Feed Regulator in Teleost
4. Key Recognized Appetite-Regulating Endocrine Factors in Teleost
The Cause of the Unusual Role of Appetite-Regulating Endocrine Factors in Teleost (Precise Features Habituating Fish Food Intake)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Base, F.; Froese, R.; Pauly, D. (Eds.) World Wide Web Electronic Publication, Version (01/2016). Available online: http://www.fishbase.org (accessed on 21 May 2021).
- Nelson, J.S. Fishes of the World; John Wiley and Sons: New York, NY, USA, 2006. [Google Scholar]
- Volkoff, H.; Unniappan, S.; Kelly, S.P. The endocrine regulation of food intake. Fish Physiol. 2009, 28, 421–465. [Google Scholar]
- Gerking, S.D. Feeding Ecology of Fish; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Volff, J.N. Genome evolution and biodiversity in teleost fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wootton, R.J. Ecology of Teleost Fishes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sobrino Crespo, C.; Perianes Cachero, A.; Puebla Jiménez, L.; Barrios, V.; Arilla Ferreiro, E. Peptides and food intake. Front. Endocrinol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde-Sieira, M.; Soengas, J.L. Nutrient sensing systems in fish: Impact on food intake regulation and energy homeostasis. Front. Neurosci. 2017, 10, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertucci, J.I.; Blanco, A.M.; Sundarrajan, L.; Rajeswari, J.J.; Velasco, C.; Unniappan, S. Nutrient regulation of endocrine factors influencing feeding and growth in fish. Front. Endocrinol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskins, L.J.; Volkoff, H. The comparative endocrinology of feeding in fish: Insights and challenges. Gen. Comp. Endocrinol. 2012, 176, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.H.A.G.; Flik, G.; Huising, M.O. Peptides and proteins regulating food intake. Anim. Biol. 2006, 56, 447–473. [Google Scholar]
- Volkoff, H.; Canosa, L.F.; Unniappan, S.; Cerdá-Reverter, J.M.; Bernier, N.J.; Kelly, S.P.; Peter, R.E. Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 2005, 142, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lenard, N.R.; Berthoud, H.-R. Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity 2008, 16, S11–S22. [Google Scholar] [CrossRef]
- Matsuda, K. Recent Advances in the regulation of feeding behavior by neuropeptides in fish. Ann. N. Y. Acad. Sci. 2009, 1163, 241–250. [Google Scholar] [CrossRef]
- Volkoff, H.; Hoskins, L.J.; Tuziak, S.M. Influence of intrinsic signals and environmental cues on the endocrine control of feeding in fish: Potential application in aquaculture. Gen. Comp. Endocrinol. 2010, 167, 352–359. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Hao, J.; Chen, D.; Liu, J.; Gao, Y.; Zhu, J.; Wu, H.; Lin, F.; Pu, Y.; et al. Molecular cloning, expression analysis, and appetite regulatory effect of peptide YY in Siberian sturgeon (Acipenser baerii). Gene 2015, 563, 172–179. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Hao, J.; Zhu, J.; Tang, N.; Qi, J.; Wang, S.; Wang, H.; Peng, S.; Liu, J.; et al. Intraperitoneal injection urocortin-3 reduces the food intake of Siberian sturgeon (Acipenser baerii). Peptides 2016, 85, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Tang, N.; Qi, J.; Wu, Y.; Hao, J.; Wang, S.; Chen, D.; Li, Z. One evidence of cocaine- and amphetamine-regulated transcript (CART) has the bidirectional effects on appetite in Siberian sturgeon (Acipenser baerii). Fish Physiol. Biochem. 2018, 44, 411–422. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.; Larhammar, D. Neuropeptide Y family of peptides: Structure, anatomical expression, function, and molecular evolution. Biochem. Cell Biol. 2000, 78, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Sundström, G.; Larsson, T.; Xu, B.; Heldin, J.; Larhammar, D. Interactions of zebrafish peptide YYb with the neuropeptide Y-family receptors Y4, Y7, Y8a, and Y8b. Front. Neurosci. 2013, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlestedt, C.; Reis, D.J. Neuropeptide Y-related peptides and their receptors—Are the receptors potential therapeutic drug targets? Annu. Rev. Pharmacol. Toxicol. 1993, 33, 309–352. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, L.J.; Volkoff, H. Daily patterns of mRNA expression of two core circadian regulatory proteins, Clock2 and Per1, and two appetite-regulating peptides, OX and NPY, in goldfish (Carassius auratus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, M.; Kojima, K.; Maruyama, K.; Konno, N.; Motohashi, E.; Ikegami, T.; Uchiyama, M.; Shioda, S.; Ando, H.; Matsuda, K. Neuropeptide Y in Tiger Puffer (Takifugu rubripes): Distribution, cloning, characterization, and mRNA expression responses to prandial condition. Zoological Sci. 2011, 28, 882–890. [Google Scholar] [CrossRef]
- Yokobori, E.; Azuma, M.; Nishiguchi, R.; Kang, K.S.; Kamijo, M.; Uchiyama, M.; Matsuda, K. Neuropeptide Y stimulates food intake in the zebrafish, Danio rerio. J. Neuroendocrinol. 2012, 24, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Aldegunde, M.; Mancebo, M. Effects of neuropeptide Y on food intake and brain biogenic amines in the rainbow trout (Oncorhynchus mykiss). Peptides 2006, 27, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Brothers, S.P.; Wahlestedt, C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol. Med. 2010, 2, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, Y.; Pandit, N.P.; Fu, J.; Li, D.; Li, J. Molecular cloning, expression analysis, and potential food intake attenuation effect of peptide YY in grass carp (Ctenopharyngodon idellus). Gen. Comp. Endocrinol. 2013, 187, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pandit, N.P.; Fu, J.; Li, D.; Li, J. Identification, characterization and feeding response of peptide YYb (PYYb) gene in grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2014, 40, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Unniappan, S. Molecular characterization, appetite regulatory effects and feeding-related changes of peptide YY in goldfish. Gen. Comp. Endocrinol. 2010, 166, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, N.; Furutani, T.; Takahashi, N.; Masumoto, T.; Fukada, H. Yellowtail neuropeptide Y: Molecular cloning, tissue distribution, and response to fasting. Fish. Sci. 2014, 80, 483–492. [Google Scholar] [CrossRef]
- Pereira, R.T.; Costa, L.S.; Oliveira, I.R.C.; da Araújo, J.C.; Aerts, M.; Vigliano, F.A.; Rosa, P.V. Relative distribution of gastrin-, CCK-8-, NPY-and CGRP-immunoreactive cells in the digestive tract of dorado (Salminus brasiliensis). Tissue Cell 2015, 47, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkoff, H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Front. Neurosci. 2016, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 1982, 296, 659–660. [Google Scholar] [CrossRef]
- Blomqvist, A.G.; Söderberg, C.; Lundell, I.; Milner, R.J.; Larhammar, D. Strong evolutionary conservation of neuropeptide Y: Sequences of chicken, goldfish, and Torpedo marmorata DNA clones. Proc. Natl. Acad. Sci. USA 1992, 89, 2350–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurokawa, T.; Suzuki, T. Development of Neuropeptide Y-related peptides in the digestive organs during the larval stage of Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2002, 126, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kouhei, M.; Azuma, M.; Kang, K.S. Orexin system in teleost fish. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2012; pp. 341–361. [Google Scholar]
- Lopez-Patino, M.A.; Guijarro, A.I.; Isorna, E.; Delgado, M.J.; Alonso-Bedate, M.; de Pedro, N. Neuropeptide Y Has a Stimulatory Action on Feeding Behavior in Goldfish (Carassius Auratus). Eur. J. Pharmacol. 1999, 377, 147–153. [Google Scholar] [CrossRef]
- Tang, Z.; Sun, C.; Yan, A.; Wu, S.; Qin, C.; Zhang, Y.; Li, W. Genes involved in fatty acid metabolism: Molecular characterization and hypothalamic mRNA response to energy status and neuropeptide Y treatment in the orange-spotted grouper Epinephelus coioides. Mol. Cell. Endocrinol. 2013, 376, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, D.R. Multiple receptors for the pancreatic polypeptide (PP-Fold) family: Physiological implications. Proc. Soc. Exp. Biol. Med. 1998, 218, 7–22. [Google Scholar] [CrossRef]
- Mentlein, R.; Dahms, P.; Grandt, D.; Krüger, R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993, 49, 133–144. [Google Scholar] [CrossRef]
- Medeiros, M.D.; Turner, A.J. Processing and metabolism of peptide-YY: Pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology 1994, 134, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.H.; Smedh, U.; Kinzig, K.P.; Scott, K.A.; Knipp, S.; Ladenheim, E.E. Peptide YY (3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R384–R388. [Google Scholar] [CrossRef]
- Volkoff, H. Appetite regulating peptides in red-bellied piranha, Pygocentrus nattereri: Cloning, tissue distribution and effect of fasting on mRNA expression levels. Peptides 2014, 56, 116–124. [Google Scholar] [CrossRef]
- Wall, A.; Volkoff, H. Effects of fasting and feeding on the brain mRNA expressions of orexin, tyrosine hydroxylase (TH), PYY and CCK in the Mexican blind cavefish (Astyanax fasciatus mexicanus). Gen. Comp. Endocrinol. 2003, 183, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Murashita, K.; Fukada, H.; Hosokawa, H.; Masumoto, T. Cholecystokinin and peptide Y in yellowtail (Seriola quinqueradiata): Molecular cloning, real-time quantitative RT-PCR, and response to feeding and fasting. Gen. Comp. Endocrinol. 2006, 145, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H. Cloning and tissue distribution of appetite-regulating peptides in pirapitinga (Piaractus brachypomus). J. Anim. Physiol. Anim. Nutr. 2015, 99, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Michael Conlon, J. The origin and evolution of peptide YY (PYY) and pancreatic polypeptide (PP). Peptides 2002, 23, 269–278. [Google Scholar] [CrossRef]
- Nässel, D.R.; Larhammar, D. Neuropeptides and peptide hormones. In Neurosciences-From Molecule to Behavior: A University Textbook; Springer: Berlin/Heidelberg, Germany, 2013; pp. 213–237. [Google Scholar]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A. Gut hormone PYY 3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Murashita, K.; Kurokawa, T.; Nilsen, T.O.; Rønnestad, I. Ghrelin, cholecystokinin, and peptide YY in Atlantic salmon (Salmo salar): Molecular cloning and tissue expression. Gen. Comp. Endocrinol. 2009, 160, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Narnaware, Y.K.; Peyon, P.P.; Lin, X.; Peter, R.E. Regulation of food intake by neuropeptide Y in goldfish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.-F.; Li, G.-Z.; Yao, W.; Cheong, L.-W.; Liao, W.-Q. Molecular characterization of neuropeptide Y gene in Chinese perch, an acanthomorph fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.S.; Jordal, A.-E.O.; Olsen, K.; Harboe, T.; Power, D.M.; Rønnestad, I. Neuroendocrine control of appetite in Atlantic halibut (Hippoglossus hippoglossus): Changes during metamorphosis and effects of feeding. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 183, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Murashita, K.; Kurokawa, T.; Ebbesson, L.O.E.; Stefansson, S.O.; Rønnestad, I. Characterization, tissue distribution, and regulation of agouti-related protein (AgRP), cocaine- and amphetamine-regulated transcript (CART) and neuropeptide Y (NPY) in Atlantic salmon (Salmo salar). Gen. Comp. Endocrinol. 2009, 162, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Iinuma, N.; Unuma, T.; Tanaka, H.; Kagawa, H.; Ohta, H.; Suzuki, T. Development of endocrine system regulating exocrine pancreas and estimation of feeding and digestive ability in Japanese eel larvae. Aquaculture 2004, 234, 513–525. [Google Scholar] [CrossRef]
- Amores, A.; Force, A.; Yan, Y.L.; Joly, L.; Amemiya, C.; Fritz, A.; Ho, R.K.; Langeland, J.; Prince, V.; Wang, Y.L.; et al. Zebrafish hox clusters and vertebrate genome evolution. Science 1998, 282, 1711–1714. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Zhou, C.; Yuan, D.; Wang, T.; Lin, F.; Chen, H.; Wu, H.; Xin, Z.; Yang, S.; Wang, Y.; et al. Characterization, tissue distribution and regulation of neuropeptide Y in Schizothorax prenanti. J. Fish Biol. 2014, 85, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Sjödin, P.; Larson, E.T.; Conlon, J.M.; Larhammar, D. Cloning and characterization of a zebrafish Y2 receptor. Regul. Pept. 2006, 133, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Larson, E.T.; Yan, Y.-L.; Postlethwait, J.-H.; Larhammar, D. Novel Neuropeptide Y Y2-Like receptor subtype in zebrafish and frogs supports early vertebrate chromosome duplications. J. Mol. Evol. 2004, 58, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lundell, I.; Berglund, M.M.; Starback, P.; Salaneck, E.; Gehlert, D.R.; Larhammar, D. Cloning and characterization of a novel neuropeptide Y receptor subtype in the zebrafish. DNA Cell Biol. 1997, 16, 1357. [Google Scholar] [CrossRef] [PubMed]
- Larhammar, D. Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 1996, 62, 1–11. [Google Scholar] [CrossRef]
- Fällmar, H.; Åkerberg, H.; Gutiérrez-de-Terán, H.; Lundell, I.; Mohell, N.; Larhammar, D. Identification of positions in the human neuropeptide Y/peptide YY receptor Y2 that contribute to pharmacological differences between receptor subtypes. Neuropeptides 2011, 45, 293–300. [Google Scholar] [CrossRef]
- Larhammar, D.; Salaneck, E. Molecular evolution of NPY receptor subtypes. Neuropeptides 2004, 38, 141–151. [Google Scholar] [CrossRef]
- Yi, M.; Li, H.; Wu, Z.; Yan, J.; Liu, Q.; Ou, C.; Chen, M. A promising therapeutic target for metabolic diseases: Neuropeptide Y receptors in humans. Cell. Physiol. Biochem. 2018, 45, 88–107. [Google Scholar] [CrossRef]
- Mercer, R.; Chee, M.; Colmers, W. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011, 32, 398–415. [Google Scholar] [CrossRef]
- Thorsell, A. Brain neuropeptide Y and corticotropin-releasing hormone in mediating stress and anxiety. Exp. Biol. Med. (Maywood). 2010, 235, 1163–1167. [Google Scholar] [CrossRef]
- Dumont, Y.; Fournier, A.; St-Pierre, S.; Quirion, R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31, Pro34] peptide YY and [125I] peptide YY3-36 as selective Y1 and Y2 radioligands. J. Pharmacol. Exp. Ther. 1995, 272, 673–680. [Google Scholar]
- Nguyen, A.; Mitchell, N.; Lin, S.; Macia, L.; Yulyaningsih, E.; Baldock, P.; Enriquez, R.; Zhang, L.; Shi, Y.; Zolotukhin, S.; et al. Y1 and Y5 receptors are both required for the regulation of food intake and energy homeostasis in mice. PLoS ONE 2012, 7, e40191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaneck, E.; Larsson, T.A.; Larson, E.T.; Larhammar, D. Birth and death of neuropeptide Y receptor genes in relation to the teleost fish tetraploidization. Gene 2008, 409, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Day, D.E.; Keen-Rhinehart, E.; Bartness, T.J. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R29–R36. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kang, K.; Sakashita, A.; Yahashi, S.; Vaudry, H. Behavioral effect of neuropeptides related to feeding regulation in fish. Ann. N. Y. Acad. Sci. 2011, 1220, 117–126. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakashita, A.; Yokobori, E.; Azuma, M. Neuroendocrine control of feeding behavior and psychomotor activity by neuropeptide Y in fish. Neuropeptides 2012, 46, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y. Neuropeptide Y family. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2016; pp. 211–225. [Google Scholar]
- Clark, J.T.; Kalra, P.S.; Crowley, W.R.; Kalra, S.P. Neuropeptide y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 1984, 115, 427–429. [Google Scholar] [CrossRef]
- Levine, A.S.; Morley, J.E. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides 1984, 5, 1025–1029. [Google Scholar] [CrossRef]
- Stanley, B.G.; Leibowitz, S.F. Neuropeptide Y injected in the paraventricular hypothalamus: A powerful stimulant of feeding behavior. Proc. Natl. Acad. Sci. USA 1985, 82, 3940–3943. [Google Scholar] [CrossRef] [Green Version]
- Allen, Y.S.; Adrian, T.E.; Allen, J.M.; Tatemoto, K.; Crow, T.J.; Bloom, S.R.; Polak, J.M. Neuropeptide Y distribution in the rat brain. Science 1983, 221, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Transgenic approach to the study of body weight regulation. Pharmacol. Rev. 2000, 52, 35–61. [Google Scholar] [PubMed]
- Dailey, M.J.; Bartness, T.J. Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R877–R892. [Google Scholar] [CrossRef] [Green Version]
- Campos, V.F.; Robaldo, R.B.; Deschamps, J.C.; Seixas, F.K.; McBride, A.J.A.; Marins, L.F.; Okamoto, M.; Sampaio, L.A.; Collares, T. Neuropeptide Y gene expression around mealtime in the Brazilian flounder, Paralichthys orbignyanus. J. Biosci. 2012, 37, 227–232. [Google Scholar] [CrossRef]
- Xu, M.; Volkoff, H. Molecular characterization of prepro-orexin in Atlantic cod (Gadus morhua): Cloning, localization, developmental profile and role in food intake regulation. Mol. Cell. Endocrinol. 2007, 271, 28–37. [Google Scholar] [CrossRef]
- Silverstein, J.T.; Breininger, J.; Baskin, D.G.; Plisetskaya, E.M. Neuropeptide Y-like gene expression in the salmon brain increases with fasting. Gen. Comp. Endocrinol. 1998, 110, 157–165. [Google Scholar] [CrossRef]
- Perboni, S.; Vignoni, M.; Inui, A. NPY. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1143–1148. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, X.; Du, S.; Sun, W.; You, F.; Zhang, P. Characterization, tissue distribution, and expression of neuropeptide Y in olive flounder Paralichthys olivaceus. Chin. J. Oceanol. Limnol. 2015, 33, 553–558. [Google Scholar] [CrossRef]
- Marshall, W.J.; Lapsley, M.; Day, A.P.; Ayling, R.M. Chapter 11–Nutritional disorders and their management. In Churchill Livingstone; London, United Kingdom. In Clinical Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; pp. 200–213. [Google Scholar] [CrossRef]
- Schartl, M.; Kneitz, S.; Volkoff, H.; Adolfi, M.; Schmidt, C.; Fischer, P.; Minx, P.; Tomlinson, C.; Meyer, A.; Warren, W.; et al. The piranha draft genome provides molecular insight associated to its unique feeding behavior. Genome Biol. Evol. 2019, 11, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Sabioni, R.E.; Cyrino, J.E.P. Appetite regulating factors in dourado, Salminus brasiliensis: cDNA cloning and effects of fasting and feeding on gene expression. Gen. Comp. Endocrinol. 2016, 237, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Wei, R.; Wang, T.; Wu, Y.; Lin, F.; Chen, H.; Liu, J.; Gao, Y.; Zhou, C.; Chen, D.; et al. Appetite regulation in Schizothorax prenanti by three CART genes. Gen. Comp. Endocrinol. 2015, 224, 194–204. [Google Scholar] [CrossRef]
- Hatef, A.; Shajan, S.; Unniappan, S. Nutrient status modulates the expression of nesfatin-1 encoding nucleobindin 2A and 2B mRNAs in zebrafish gut, liver and brain. Gen. Comp. Endocrinol. 2015, 215, 51–60. [Google Scholar] [CrossRef]
- Patel, H.R.; Qi, Y.; Hawkins, E.J.; Hileman, S.M.; Elmquist, J.K.; Imai, Y.; Ahima, R.S. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes 2006, 55, 3091–3098. [Google Scholar] [CrossRef] [Green Version]
- Narnaware, Y.K.; Peter, R.E. Neuropeptide Y stimulates food consumption through multiple receptors in goldfish. Physiol. Behav. 2001, 74, 185–190. [Google Scholar] [CrossRef]
- Ji, W.; Ping, H.-C.; Wei, K.-J.; Zhang, G.-R.; Shi, Z.-C.; Yang, R.-B.; Zou, G.-W.; Wang, W.-M. Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. Gen. Comp. Endocrinol. 2015, 223, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, A.S.; Volkoff, H. Cloning and characterization of neuropeptide Y (NPY) and cocaine and amphetamine-regulated transcript (CART) in Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 451–461. [Google Scholar] [CrossRef]
- Muller, E.E.; Locatelli, V.; Cocchi, D. Neuroendocrine control of growth hormone secretion. Physiol. Rev. 1999, 79, 511–607. [Google Scholar] [CrossRef]
- Peng, C.; Peter, R.E. Neuroendocrine regulation of growth hormone secretion and growth in fish. Zool. Stud. 1997, 36, 79–89. [Google Scholar]
- Kiris, G.A.; Kumlu, M.; Dikel, S. Stimulatory effects of neuropeptide Y on food intake and growth of Oreochromis niloticus. Aquaculture 2007, 264, 383–389. [Google Scholar] [CrossRef]
- Carpio, Y.; León, K.; Acosta, J.; Morales, R.; Estrada, M.P. Recombinant tilapia Neuropeptide Y promotes growth and antioxidant defenses in African catfish (Clarias gariepinus) fry. Aquaculture 2007, 272, 649–655. [Google Scholar] [CrossRef]
- Wu, S.; Li, B.; Lin, H.; Li, W. Stimulatory effects of neuropeptide Y on the growth of orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2012, 179, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, M.; Lal, B.; Sakharkar, A.J.; Deshmukh, M.; Singru, P.S.; Subhedar, N. Involvement of neuropeptide Y Y1 receptors in the regulation of LH and GH cells in the pituitary of the catfish, Clarias batrachus: An immunocytochemical study. Gen. Comp. Endocrinol. 2006, 149, 190–196. [Google Scholar] [CrossRef]
- Karra, E.; Chandarana, K.; Batterham, R.L. The role of peptide YY in appetite regulation and obesity. J. Physiol. 2009, 587, 19–25. [Google Scholar] [CrossRef]
- Zhang, L.; Nguyen, A.D.; Lee, I.; Yulyaningsih, E.; Riepler, S.J.; Stehrer, B.; Enriquez, R.F.; Lin, S.; Shi, Y.; Baldock, P.A. NPY modulates PYY function in the regulation of energy balance and glucose homeostasis. Diabetes Obes. Metab. 2012, 14, 727–736. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, C.; Wang, T.; Lin, F.; Chen, H.; Wu, H.; Wei, R.; Xin, Z.; Liu, J.; Gao, Y. Molecular characterization and tissue expression of peptide YY in Schizothorax prenanti: Effects of preprandial changes and fasting on expression in the hypothalamus. Regul. Pept. 2014, 190, 32–38. [Google Scholar] [CrossRef]
- Zhou, C.; Lei, L.; Yuan, D.; Deng, X.; Ye, H.; Luo, H.; Fang, J.; Yang, M.; Li, Y.; Zhang, C. Structural and functional characterization of peptide YY on feeding in Schizothorax davidi. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Valen, R.; Jordal, A.-E.; Murashita, K.; Rønnestad, I. Postprandial effects on appetite-related neuropeptide expression in the brain of Atlantic salmon, Salmo salar. Gen. Comp. Endocrinol. 2011, 171, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Humphries, S.; Peter, R.E.; Rivier, J.E.; Blomqvist, A.G.; Larhammar, D. Actions of goldfish neuropeptide Y on the secretion of growth hormone and gonadotropin-II in female goldfish. Gen. Comp. Endocrinol. 1993, 90, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Peter, R.E. Characterization of two forms of cocaine-and amphetamine-regulated transcript (CART) peptide precursors in goldfish: Molecular cloning and distribution, modulation of expression by nutritional status, and interactions with leptin. Endocrinology 2001, 142, 5076–5088. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Cooper, K.A.; Dickhoff, W.W.; Beckman, B.R. Postprandial changes in plasma growth hormone, insulin, insulin-like growth factor (IGF)-I, and IGF-binding proteins in coho salmon fasted for varying periods. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R352–R361. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.L.; Shimizu, M.; Beckman, B.R.; Baker, D.M.; Dickhoff, W.W. Time course of the GH/IGF axis response to fasting and increased ration in chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 2005, 140, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Miura, T.; Kaiya, H.; Maruyama, K.; Shimakura, S.-I.; Uchiyama, M.; Kangawa, K.; Shioda, S. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides 2006, 27, 2321–2325. [Google Scholar] [CrossRef]
- Johansson, V.; Winberg, S.; Björnsson, B.T. Growth hormone-induced stimulation of swimming and feeding behavior of rainbow trout is abolished by the D1 dopamine antagonist SCH23390. Gen. Comp. Endocrinol. 2005, 141, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, E.; Johnsson, J.I.; Björnsson, B.T. Growth hormone increases predation exposure of rainbow trout. Proc. Royal Soc. London Ser. B Biol. Sci. 1996, 263, 647–651. [Google Scholar]
- Martínez-Álvarez, R.M.; Volkoff, H.; Muñoz-Cueto, J.A.; Delgado, M.J. Effect of calcitonin gene-related peptide (CGRP), adrenomedullin and adrenomedullin-2/intermedin on food intake in goldfish (Carassius auratus). Peptides 2009, 30, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Bowen, A.J.; Schwartz, M.W.; Palmiter, R.D. Parabrachial CGRP neurons control meal termination. Cell Metab. 2016, 23, 811–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, S.P.; Peter, R.E. Prolactin-releasing peptide, food intake, and hydromineral balance in goldfish. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R1474–R1481. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, A.B.; Nisembaum, L.G.; Isorna, E.; Delgado, M.J.; de Pedro, N. Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides 2012, 34, 329–335. [Google Scholar] [CrossRef]
- Hoskins, L.J.; Xu, M.; Volkoff, H. Interactions between gonadotropin-releasing hormone (GnRH) and orexin in the regulation of feeding and reproduction in goldfish (Carassius auratus). Horm. Behav. 2008, 54, 379–385. [Google Scholar] [CrossRef]
- Cerda-Reverter, J.M.; Peter, R.E. Endogenous melanocortin antagonist in fish: Structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology 2003, 144, 4552–4561. [Google Scholar] [CrossRef] [Green Version]
- Hagan, J.J.; Leslie, R.A.; Patel, S.; Evans, M.L.; Wattam, T.A.; Holmes, S.; Benham, C.D.; Taylor, S.G.; Routledge, C.; Hemmati, P. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. USA 1999, 96, 10911–10916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurokawa, T.; Suzuki, T.; Hashimoto, H. Identification of gastrin and multiple cholecystokinin genes in teleost. Peptides 2003, 24, 227–235. [Google Scholar] [CrossRef]
- de Pedro, N.; Céspedes, M.V.; Delgado, M.J.; Alonso-Bedate, M. The galanin-induced feeding stimulation is mediated via α2-adrenergic receptors in goldfish. Regul. Pept. 1995, 57, 77–84. [Google Scholar] [CrossRef]
- Himick, B.A.; Peter, R.E. CCK/gastrin-like immunoreactivity in brain and gut, and CCK suppression of feeding in goldfish. Am. J. Physiol. Integr. Comp. Physiol. 1994, 267, R841–R851. [Google Scholar] [CrossRef]
- Yeung, C.-M.; Mojsov, S.; Mok, P.-Y.; Chow, B.K.C. Isolation and structure-function studies of a glucagon-like peptide 1 receptor from goldfish Carassius auratus: Identification of three charged residues in extracellular domains critical for receptor function. Endocrinology 2002, 143, 4646–4654. [Google Scholar] [CrossRef] [Green Version]
- Plisetskaya, E.M.; Mommsen, T.P. Glucagon and glucagon-like peptides in fishes. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 187–257. [Google Scholar]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-controlling endocrine systems in teleosts. Front. Endocrinol. (Lausanne) 2007, 8, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.M.; Martel, G.; Martin, D.W. Comparisons of the feeding activity and diets of male and female cunners Tautogolabrus adspersus (Pisces: Labridae). Mar. Biol. 1984, 84, 7–11. [Google Scholar] [CrossRef]
- Parhar, I.S.; Sato, H.; Sakuma, Y. Ghrelin gene in cichlid fish is modulated by sex and development. Biochem. Biophys. Res. Commun. 2003, 305, 169–175. [Google Scholar] [CrossRef]
- Sakata, I.; Mori, T.; Kaiya, H.; Yamazaki, M.; Kangawa, K.; Inoue, K.; Sakai, T. Localization of ghrelin-producing cells in the stomach of the rainbow trout (Oncorhynchus mykiss). Zoological Sci. 2004, 21, 757–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ginneken, V.J.T.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Miller, K.M.; Schulze, A.D.; Ginther, N.; Li, S.; Patterson, D.A.; Farrell, A.P.; Hinch, S.G. Salmon Spawning Migration: Metabolic Shifts and Environmental Triggers. Comp. Biochem. Physiol. Part D Genomics Proteomics. 2009, 4, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Fordham, B.S.E.; Trippel, E.A. Feeding Behaviour of Cod (Gadus Morhua) in Relation to Spawning. J. Appl. Ichthyol. 1999, 15, 1–9. [Google Scholar] [CrossRef]
- Skjæraasen, J.E.; Salvanes, A.G.V.; Karlsen, Ø.; Dahle, R.; Nilsen, T.; Norberg, B. The effect of photoperiod on sexual maturation, appetite and growth in wild Atlantic cod (Gadus morhua L.). Fish Physiol. Biochem. 2004, 30, 163–174. [Google Scholar] [CrossRef]
- Mann, D.A.; Sancho, G. Feeding ecology of the domino damselfish, Dascyllus albisella. Copeia 2007, 2007, 566–576. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.F.; Collares, T.V.; Seixas, F.K.; Deschamps, J.C.; Marins, L.F.F.; Okamoto, M.H.; Sampaio, L.A.N.; Robaldo, R.B. NPY and sbGnRH gene expression in juvenile and adult male Brazilian flounder Paralichthys orbignyanus. Ciencia Rural 2011, 41, 1927–1930. [Google Scholar] [CrossRef] [Green Version]
- Leal, E.; Sánchez, E.; Muriach, B.; Cerdá-Reverter, J.M. Sex steroid-induced inhibition of food intake in sea bass (Dicentrarchus labrax). J. Comp. Physiol. B 2009, 179, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazumdar, M.; Sakharkar, A.J.; Singru, P.S.; Subhedar, N. Reproduction phase-related variations in neuropeptide Y immunoreactivity in the olfactory system, forebrain, and pituitary of the female catfish, Clarias batrachus (Linn.). J. Comp. Neurol. 2007, 504, 450–469. [Google Scholar] [CrossRef]
- van de Pol, I.; Flik, G.; Gorissen, M. Comparative physiology of energy metabolism: Fishing for endocrine signals in the early vertebrate pool. Front. Endocrinol. 2017, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Boujard, T. Daily feeding rhythms and fish physiology. Vie Milieu 2001, 51, 237–245. [Google Scholar]
- Kulczykowska, E.; Sánchez Vázquez, F.J. Neurohormonal regulation of feed intake and response to nutrients in fish: Aspects of feeding rhythm and stress. Aquac. Res. 2010, 41, 654–667. [Google Scholar] [CrossRef]
- Cowan, M.; Azpeleta, C.; López-Olmeda, J.F. Rhythms in the endocrine system of fish: A review. J. Comp. Physiol. B 2017, 187, 1057–1089. [Google Scholar] [CrossRef]
- Isorna, E.; de Pedro, N.; Valenciano, A.I.; Alonso-Gómez, Á.L.; Delgado, M.J. Interplay between the endocrine and circadian systems in fishes. J. Endocrinol. 2017, 232, R141–R159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehoe, A.S.; Volkoff, H. The effects of temperature on feeding and expression of two appetite-related factors, neuropeptide Y and cocaine-and amphetamine-regulated transcript, in Atlantic cod, Gadus morhua. J. World Aquac. Soc. 2008, 39, 790–796. [Google Scholar] [CrossRef]
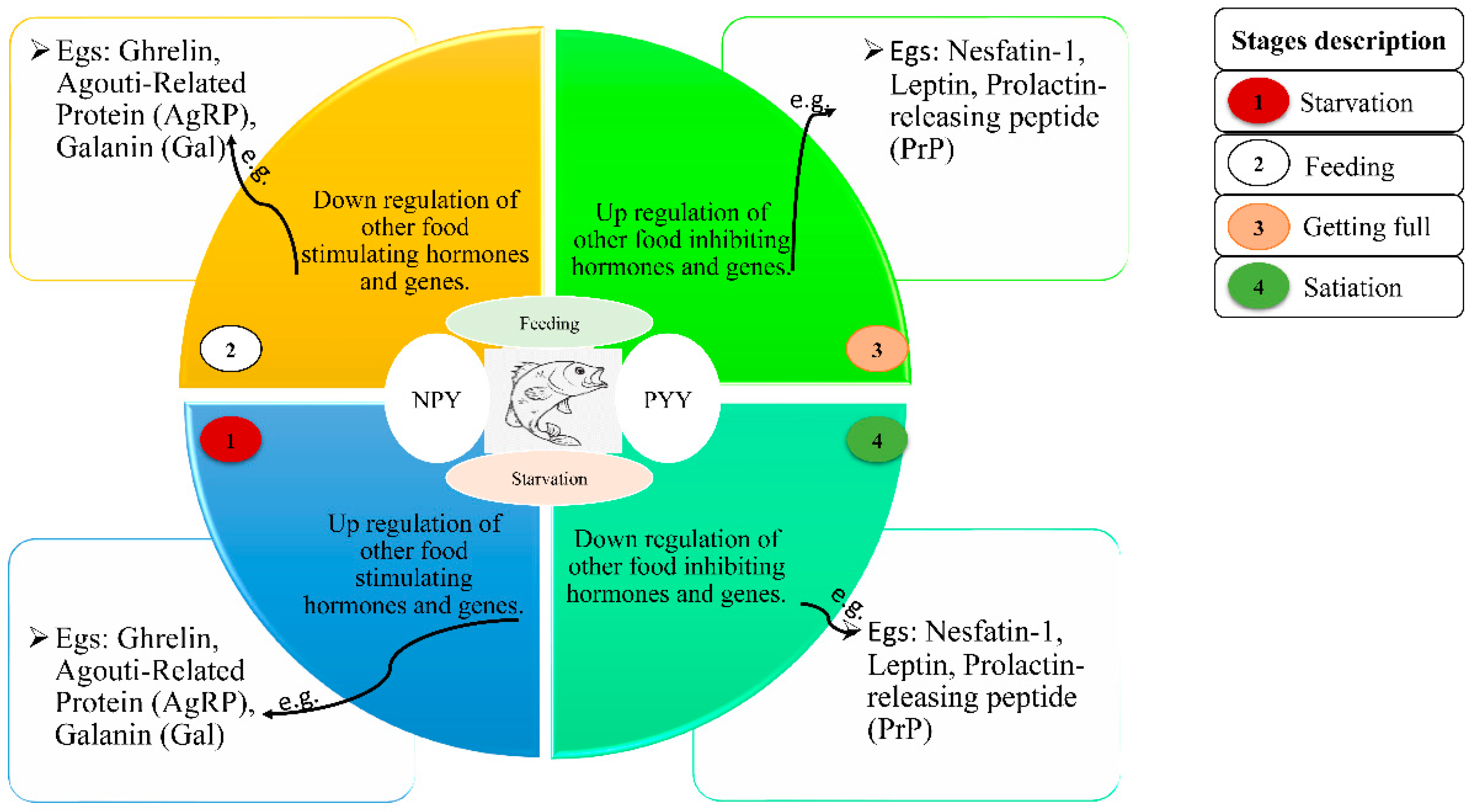
| Appetite-Regulating Hormones | Treatment | Duration | Gene Regulation | Fish | Reference |
|---|---|---|---|---|---|
| Neuropeptide Y(Npy) | Starvation | 3 weeks | Upregulation | Chinook salmon (Oncorhynchus tshawytscha) | [83] |
| Coho salmon (Oncorhynchus kisutch) | |||||
| 3 days to 1 week | Goldfish (Carassius auratus) | [52] | |||
| 1 week | Zebrafish (Danio rerio) | [24] | |||
| 2 weeks | Ya-fish (Schizothorax prenanti) | [58] | |||
| 1 day | Blunt snout bream (Megalobrama amblycephala) | [93] | |||
| 1 week | No effect of Npy expression | Atlantic cod (Gadus morhua) | [94] | ||
| 1–2 days | Downregulation | Olive flounder (Paralichthys olivaceus) | [85] | ||
| Peptide YY(Pyy) | Feeding and or refeeding | 9 days | Upregulation | Schizothorax davidi | [104] |
| Refeeding after fasting | 3 days | Yellowtail (Seriola quinqueradiata) | [46] | ||
| 1 week | Upregulation, as compared to fish fed for the whole week | Goldfish (Carassius auratus) | [29] | ||
| Starvation | 6 days | No effect of Pyy expression | Atlantic salmon (Salmo salar) | [51] | |
| 10 days | No effect of Pyy expression or no significant difference between fed and fasted fish | Mexican blind cavefish (Astyanax fasciatus mexicanus) | [45] | ||
| 9 days | Downregulation; increased after refeeding | Ya-fish (Schizothorax prenanti) | [103] | ||
| 1 week | Downregulation | Red-bellied piranha (Pygocentrus nattereri) | [44] |
| Appetite-Regulating Hormone | Orexigenic/Anorexigenic Actions | Tissues | Reference |
|---|---|---|---|
| Ghrelin | Orexigenic | Gastrointestinal tract | [110] |
| Growth hormone (GH) | Orexigenic | Pituitary | [111,112] |
| Calcitonin gene-related peptide | Orexigenic | Brain | [113,114] |
| Prolactin-releasing peptide (PrP) | Anorexigenic | Brain | [115] |
| Leptin | Anorexigenic | Liver | [116] |
| Gonadotropin-releasing hormone (GrH) | Anorexigenic | Brain | [117] |
| Agouti-related protein (AgRP) | Orexigenic | Brain | [88,118] |
| Proopiomelanocortin (POMC) | Anorexigenic | Pituitary | [88] |
| Cocaine- and amphetamine-regulated transcript (CART) | Anorexigenic | Brain, pituitary | [107] |
| Orexins | Orexigenic | Hypothalamus | [88,119] |
| Nesfatin-1 | Anorexigenic | Hypothalamus | [120] |
| Galanin (Gal) | Orexigenic | Gastrointestinal tract and the brain | [121] |
| Cholecystokinin (CCK) | Anorexigenic | Gastrointestinal tract and the brain | [120,122] |
| Glucagon-like peptide-1 | Anorexigenic | Brain and intestine | [9,123,124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assan, D.; Mustapha, U.F.; Chen, H.; Li, Z.; Peng, Y.; Li, G. The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review. Life 2021, 11, 547. https://doi.org/10.3390/life11060547
Assan D, Mustapha UF, Chen H, Li Z, Peng Y, Li G. The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review. Life. 2021; 11(6):547. https://doi.org/10.3390/life11060547
Chicago/Turabian StyleAssan, Daniel, Umar Farouk Mustapha, Huapu Chen, Zhiyuan Li, Yuhao Peng, and Guangli Li. 2021. "The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review" Life 11, no. 6: 547. https://doi.org/10.3390/life11060547
APA StyleAssan, D., Mustapha, U. F., Chen, H., Li, Z., Peng, Y., & Li, G. (2021). The Roles of Neuropeptide Y (Npy) and Peptide YY (Pyy) in Teleost Food Intake: A Mini Review. Life, 11(6), 547. https://doi.org/10.3390/life11060547









