F4-Neuroprostanes: A Role in Sperm Capacitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
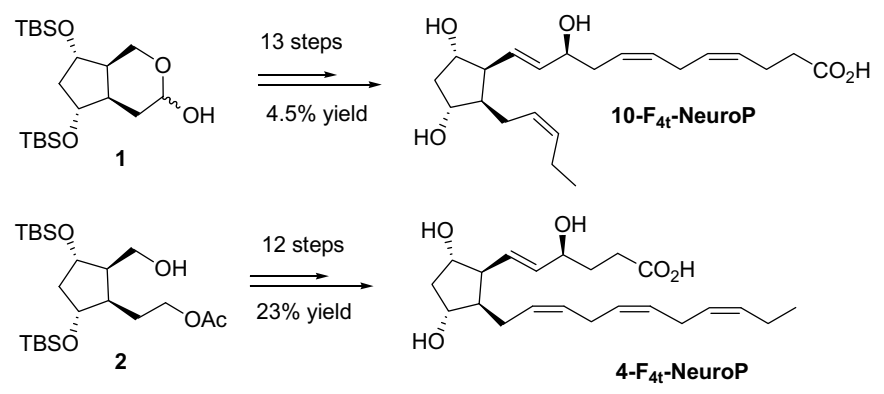
2.2. 4-F4t-NeuroP and 10-F4t-NeuroP Chemical Synthesis
2.3. Neuroprostane Preparation and Determination of Optimal Amount for the In Vitro Study
2.4. Swim-Up
2.5. Computer-Assisted Sperm Analysis
2.6. Sperm Capacitation Patterns in Rabbit Sperm and Swim-Up-Selected Human Sperm
2.7. Immunofluorescence Analysis for the Localization of Phospho-AMPKα (Thr172)
2.8. Statistical Analysis
3. Results
3.1. Determination of Human Sperm Motility after F4-NeuroP Incubation
3.2. Assessment of Capacitation in Rabbit Sperm, Used as Model, and in Swim-Up-Selected Human Sperm
3.3. Localization of Phospho-AMPKα in Swim-Up-Selected Human Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fam, S.S.; Murphey, L.J.; Terry, E.S.; Zackert, W.E.; Chen, Y.; Gao, L.; Pandalai, S.; Milne, G.; Roberts, L.J.; Porter, N.A.; et al. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 2002, 277, 36076–36084. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Milne, G.L.; Yin, H.; Sanchez, S.C.; Porter, N.A.; Morrow, J.D. Formation of highly reactive cyclopentenone isoprostane compounds (A 3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J. Biol. Chem. 2008, 283, 12043–12055. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Corcoran, T.B.; Mas, E.; Durand, T.; Galano, J.M.; Roberts, L.J.; Paech, M.; Muchatuta, N.A.; Phillips, M.; Mori, T.A. Is there a role for isofurans and neuroprostanes in pre-eclampsia and normal pregnancy? Antioxid. Redox Signal. 2012, 16, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Liu, E.H.; Anggård, E.; Halliwell, B. F4-isoprostanes: A novel class of prostanoids formed during peroxidation of docosahexaenoic acid (DHA). Biochem. Biophys. Res. Commun. 1998, 242, 338–344. [Google Scholar] [CrossRef]
- Yin, H.; Musiek, E.S.; Gao, L.; Porter, N.A.; Morrow, J.D. Regiochemistry of neuroprostanes generated from the peroxidation of docosahexaenoic acid in vitro and in vivo. J. Biol. Chem. 2005, 280, 26600–26611. [Google Scholar] [CrossRef]
- Galano, J.M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef]
- Musiek, E.S.; Cha, J.K.; Yin, H.; Zackert, W.E.; Terry, E.S.; Porter, N.A.; Montine, T.J.; Morrow, J.D. Quantification of F-ring isoprostane-like compounds (F4-neuroprostanes) derived from docosahexaenoic acid in vivo in humans by a stable isotope dilution mass spectrometric assay. J. Chromatogr. Analyt. Technol. Biomed. Life Sci. 2004, 799, 95–102. [Google Scholar] [CrossRef]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2014, 2014, 572491. [Google Scholar] [CrossRef]
- Seet, R.C.; Lee, C.Y.; Lim, E.C.; Tan, J.J.; Quek, A.M.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Wang, H.; Chan, Y.H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef]
- Zerbinati, C.; Caponecchia, L.; Rago, R.; Leoncini, E.; Bottaccioli, A.G.; Ciacciarelli, M.; Pacelli, A.; Salacone, P.; Sebastianelli, A.; Pastore, A.; et al. Fatty acids profiling reveals potential candidate markers of semen quality. Andrology 2016, 4, 1094–1101. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Noto, D.; Iacoponi, F.; Signorini, C. Fatty Acid Profile and Metabolism Are Related to Human Sperm Parameters and Are Relevant in Idiopathic Infertility and Varicocele. Mediat. Inflamm. 2020, 2020, 3640450. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Collodel, G. Role of isoprostanes in human male infertility. Syst. Biol. Reprod. Med. 2020, 66, 291–299. [Google Scholar] [CrossRef]
- Longini, M.; Moretti, E.; Signorini, C.; Noto, D.; Iacoponi, F.; Collodel, G. Relevance of seminal F2-dihomo-IsoPs, F2-IsoPs and F4-NeuroPs in idiopathic infertility and varicocele. Prostagland. Lipid Mediat. 2020, 149, 106448. [Google Scholar] [CrossRef]
- Collodel, G.; Castellini, C.; Lee, J.C.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell. Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary n-3 Source on Rabbit Male Reproduction. Oxid. Med. Cell. Longev. 2019, 2019, 3279670. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Galano, J.M.; Leung, H.H.; Balas, L.; Oger, C.; Durand, T.; Lee, J.C. Nonenzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F4t -neuroprostane, acts as a bioactive lipid molecule in neuronal cells. FEBS Lett. 2020, 594, 1797–1808. [Google Scholar] [CrossRef]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef]
- Crescente, M.; Armstrong, P.C.; Kirkby, N.S.; Edin, M.L.; Chan, M.V.; Lih, F.B.; Jiao, J.; Maffucci, T.; Allan, H.E.; Mein, C.A.; et al. Profiling the eicosanoid networks that underlie the anti- and pro-thrombotic effects of aspirin. FASEB J. 2020, 34, 10027–10040. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 26, 107879. [Google Scholar] [CrossRef]
- Roy, J.; Oger, C.; Thireau, J.; Roussel, J.; Mercier-Touzet, O.; Delinger, F.; Pinot, E.; Farah, C.; Taber, D.F.; Cristol, J.P.; et al. Nonenzymatic lipid mediators, neuroprostanes, exert the antiarrhythmic properties of docosahexaenoic acid. Free Radic. Biol. Med. 2015, 86, 269–278. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Boiti, C.; Castellini, C.; Theau Clement, M.; Besenfelder, U.; Liguori, L.; Renieri, T.; Pizzi, F. Guidelines for the handing of rabbit bucks and semen. World Rabbit Sci. 2005, 13, 71–91. [Google Scholar]
- Grillo, E.; Lo Rizzo, C.; Bianciardi, L.; Bizzarri, V.; Baldassarri, M.; Spiga, O.; Furini, S.; De Felice, C.; Signorini, C.; Leoncini, S.; et al. Revealing the complexity of a monogenic disease: Rett syndrome exome sequencing. PLoS ONE 2013, 8, e56599. [Google Scholar] [CrossRef] [PubMed]
- Oger, C.; Brinkmann, Y.; Bouazzaoui, S.; Durand, T.; Galano, J.M. Stereocontrolled access to isoprostanes via a bicyclo[3.3.0]octene framework. Org. Lett. 2008, 10, 5087–5090. [Google Scholar] [CrossRef] [PubMed]
- Oger, C.; Bultel-Poncé, V.; Guy, A.; Balas, L.; Rossi, J.C.; Durand, T.; Galano, J.M. The handy use of Brown’s catalyst for a skipped diyne deuteration: Application to the synthesis of a d4-labeled-F4t-neuroprostane. Chem. Eur. J. 2010, 16, 13976–13980. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Ruggeri, S.; Collodel, G. What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil. Steril. 2011, 96, 24–27. [Google Scholar] [CrossRef]
- Kaul, G.; Sharma, G.S.; Singh, B.; Gandhi, K.K. Capacitation and acrosome reaction in buffalo bull spermatozoa assessed by chlortetracycline and pisum sativum agglutinin fluorescence assay. Theriogenology 2001, 55, 1457–1468. [Google Scholar] [CrossRef]
- Signorini, C.; De Felice, C.; Durand, T.; Galano, J.M.; Oger, C.; Leoncini, S.; Ciccoli, L.; Carone, M.; Ulivelli, M.; Manna, C.; et al. Relevance of 4-F4t-neuroprostane and 10-F4t-neuroprostane to neurological diseases. Free Radic. Biol. Med. 2018, 115, 278–287. [Google Scholar] [CrossRef]
- Yen, H.C.; Wei, H.J.; Lin, C.L. Unresolved issues in the analysis of F2-isoprostanes, F4-neuroprostanes, isofurans, neurofurans, and F2-dihomo-isoprostanes in body fluids and tissue using gas chromatography/negative-ion chemical-ionization mass spectrometry. Free Radic. Res. 2015, 49, 861–880. [Google Scholar] [CrossRef]
- Signorini, C.; De Felice, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Della Ragione, F.; Rossi, M.; Pecorelli, A.; Valacchi, G.; et al. F₄-neuroprostanes mediate neurological severity in Rett syndrome. Clin. Chim. Acta 2011, 412, 1399–1406. [Google Scholar] [CrossRef]
- Medina, S.; Miguel-Elízaga, I.D.; Oger, C.; Galano, J.M.; Durand, T.; Martínez-Villanueva, M.; Castillo, M.L.; Villegas-Martínez, I.; Ferreres, F.; Martínez-Hernández, P.; et al. Dihomo-isoprostanes-nonenzymatic metabolites of AdA-are higher in epileptic patients compared to healthy individuals by a new ultrahigh pressure liquid chromatography-triple quadrupole-tandem mass spectrometry method. Free Radic. Biol. Med. 2015, 79, 154–163. [Google Scholar] [CrossRef]
- Galano, J.M.; Mas, E.; Barden, A.; Mori, T.A.; Signorini, C.; De Felice, C.; Barrett, A.; Opere, C.; Pinot, E.; Schwedhelm, E.; et al. Isoprostanes and neuroprostanes: Total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostagland. Lipid Mediat. 2013, 107, 95–102. [Google Scholar] [CrossRef]
- Roy, J.; Fauconnier, J.; Oger, C.; Farah, C.; Angebault-Prouteau, C.; Thireau, J.; Bideaux, P.; Scheuermann, V.; Bultel-Poncé, V.; Demion, M.; et al. Non-enzymatic oxidized metabolite of DHA, 4(RS)-4-F4t-neuroprostane protects the heart against reperfusion injury. Free Radic. Biol. Med. 2017, 102, 229–239. [Google Scholar] [CrossRef]
- Galano, J.M.; Roy, J.; Durand, T.; Lee, J.C.; Le Guennec, J.Y.; Oger, C.; Demion, M. Biological activities of non-enzymatic oxygenated metabolites of polyunsaturated fatty acids (NEO-PUFAs) derived from EPA and DHA: New anti-arrhythmic compounds? Mol. Asp. Med. 2018, 64, 161–168. [Google Scholar] [CrossRef]
- Holt, W.; Harrison, R. Bicarbonate stimulation of boar sperm motility via a protein kinase A-dependent pathway: Between cell and between-ejaculated differences are not due deficies in protein kinase a activation. J. Androl. 2002, 23, 557–565. [Google Scholar]
- García Herreros, M.; Aparicio, I.M.; Núñez, I.; García-Marín, L.J.; Gil, M.C.; Peña Vega, F.J. Boar sperm velocity and motility patterns under capacitating and non-capacitating incubation conditions. Theriogenology 2005, 63, 795–805. [Google Scholar] [CrossRef]
- Soriano-Úbeda, C.; Romero-Aguirregomezcorta, J.; Matás, C.; Visconti, P.E.; García-Vázquez, F.A. Manipulation of bicarbonate concentration in sperm capacitation media improves in vitro fertilisation output in porcine species. J. Anim. Sci. Biotechnol. 2019, 10, 19. [Google Scholar] [CrossRef]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E.; et al. Astaxanthin Improves Human Sperm Capacitation by Inducing Lyn Displacement and Activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef]
- Fraser, L.R.; Osiguwa, O.O. Human sperm responses to calcitonin, angiotensin II and fertilization-promoting peptide in prepared semen samples from normal donors and infertility patients. Hum. Reprod. 2004, 19, 596–606. [Google Scholar] [CrossRef][Green Version]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef]
- de Lamirande, E.; Leclerc, P.; Gagnon, C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krähling, M.; Müller, A.; Kaupp, U.B.; Strünker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Vaquer, C.C.; Suhaiman, L.; Pavarotti, M.A.; De Blas, G.A.; Belmonte, S.A. Ceramide induces a multicomponent intracellular calcium increase triggering the acrosome secretion in human sperm. Biochim. Biophys Acta Mol. Cell Res. 2020, 1867, 118704. [Google Scholar] [CrossRef]
- Signorelli, J.R.; Díaz, E.S.; Fara, K.; Barón, L.; Morales, P. Protein phosphatases decrease their activity during capacitation: A new requirement for this event. PLoS ONE 2013, 8, e81286. [Google Scholar] [CrossRef]
- Martínez-León, E.; Osycka-Salut, C.; Signorelli, J.; Pozo, P.; Pérez, B.; Kong, M.; Morales, P.; Pérez-Martínez, S.; Díaz, E.S. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum. Reprod. 2015, 30, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: Modulation and protein kinase A dependency. Mol. Hum. Reprod. 2004, 10, 355–363. [Google Scholar] [CrossRef]
- Bedu-Addo, K.; Lefièvre, L.; Moseley, F.L.; Barratt, C.L.; Publicover, S.J. Bicarbonate and bovine serum albumin reversibly ’switch’ capacitation-induced events in human spermatozoa. Mol. Hum. Reprod. 2005, 11, 683–691. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Alves, S.; Grasseau, I.; Métayer-Coustard, S.; Praud, C.; Froment, P.; Blesbois, E. Central role of 5’-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014, 91, 121. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Froment, P.; Combarnous, Y.; Blesbois, É. L’AMPK, régulateur de l’énergie et des fonctions des spermatozoïdes [AMPK, regulator of sperm energy and functions]. Med. Sci. (Paris) 2016, 32, 491–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, T.Y.; Lv, D.L.; Zhang, X.; Du, Y.Q.; Yuan, Y.T.; Chen, M.J.; Xi, H.M.; Li, Y.; Han, N.; Hu, J.H. Rosmarinic acid improves boar sperm quality, antioxidant capacity and energy metabolism at 17 °C via AMPK activation. Reprod. Domest. Anim. 2020, 55, 1714–1724. [Google Scholar] [CrossRef] [PubMed]

| Control | F4- | F4 | F4+ | Statistics | |
|---|---|---|---|---|---|
| Total progressive motility % | 29.4 ± 12 | 27.4 ± 11.3 | 34.4 ± 12.1 | 13.6 ± 5.9 | Control vs. F4 p < 0.05 Control vs. F4+ p < 0.01 |
| Rapid progressive motility % | 10.4 ± 4.0 | 9.8 ± 3.8 | 16.6 ± 4.2 | 2.4 ± 1.1 | Control vs. F4 p < 0.01 Control vs. F4+ p < 0.01 |
| Samples (n°5) | Time 0 | Time 2 h | Time 4 h | Statistics | |
|---|---|---|---|---|---|
| Non-capacitated sperm % | Control | 95.0 ± 1.4 | 59.4 ± 1.8 | 34.5 ± 3.2 | |
| Calcium ionophore | 95.6 ± 0.5 | 22.1 ± 1.3 | 20.7 ± 0.6 | ||
| F4-NeuroPs | 95.9 ± 1.2 | 31.6 ± 2.1 | 12.6 ± 0.9 | ||
| Capacitated sperm % | Control | 4.9 ± 1.4 | 29.3 ± 2.5 | 53.8 ± 1.9 | Control vs. calcium ionophore 2 h Control vs. F4-NeuroPs 2 h |
| Calcium ionophore | 4.4 ± 0.5 | 71.7 ± 0.9 | 58.0 ± 0.3 | ||
| F4-NeuroPs | 4.1 ± 1.2 | 57.8 ± 1.9 | 61.7 ± 1.5 | ||
| Acrosome-reacted sperm % | Control | 0 | 11.3 ± 4.2 | 11.6 ± 1.3 | Control vs. calcium ionophore 4 h Control vs. F4-NeuroPs 4 h |
| Calcium ionophore | 0 | 6.3 ± 0.6 | 21.3 ± 0.9 | ||
| F4-NeuroPs | 0 | 10.9 ± 1.2 | 25.7 ± 0.8 |
| Control (2 h) | F4-NeuroPs (2 h) | Control (4 h) | F4-NeuroPs (4 h) | |
|---|---|---|---|---|
| Mid-piece labeling % | 66 ± 1.8 * | 13.7 ± 1.7 ** | 17.5 ± 1.7 | 3.2 ± 1.2 |
| Dotted tail labeling % | 0.5 ± 0.5 | 64.7 ± 1 *** | 51 ± 2.6 | 15 ± 0.8 |
| Acrosomal labeling % | 1.2 ± 0.5 | 18.7 ± 2.2 | 17.5 ± 2.1 | 57.2 ± 1.7 ** |
| Any signal % | 33.5 ± 1.3 | 3.7 ± 1.2 | 14 ± 1.6 | 24.5 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorini, C.; Moretti, E.; Noto, D.; Mattioli, S.; Castellini, C.; Pascarelli, N.A.; Durand, T.; Oger, C.; Galano, J.-M.; De Felice, C.; et al. F4-Neuroprostanes: A Role in Sperm Capacitation. Life 2021, 11, 655. https://doi.org/10.3390/life11070655
Signorini C, Moretti E, Noto D, Mattioli S, Castellini C, Pascarelli NA, Durand T, Oger C, Galano J-M, De Felice C, et al. F4-Neuroprostanes: A Role in Sperm Capacitation. Life. 2021; 11(7):655. https://doi.org/10.3390/life11070655
Chicago/Turabian StyleSignorini, Cinzia, Elena Moretti, Daria Noto, Simona Mattioli, Cesare Castellini, Nicola Antonio Pascarelli, Thierry Durand, Camille Oger, Jean-Marie Galano, Claudio De Felice, and et al. 2021. "F4-Neuroprostanes: A Role in Sperm Capacitation" Life 11, no. 7: 655. https://doi.org/10.3390/life11070655
APA StyleSignorini, C., Moretti, E., Noto, D., Mattioli, S., Castellini, C., Pascarelli, N. A., Durand, T., Oger, C., Galano, J.-M., De Felice, C., Lee, J. C.-Y., & Collodel, G. (2021). F4-Neuroprostanes: A Role in Sperm Capacitation. Life, 11(7), 655. https://doi.org/10.3390/life11070655











