Blue Light in Dermatology
Abstract
1. Introduction
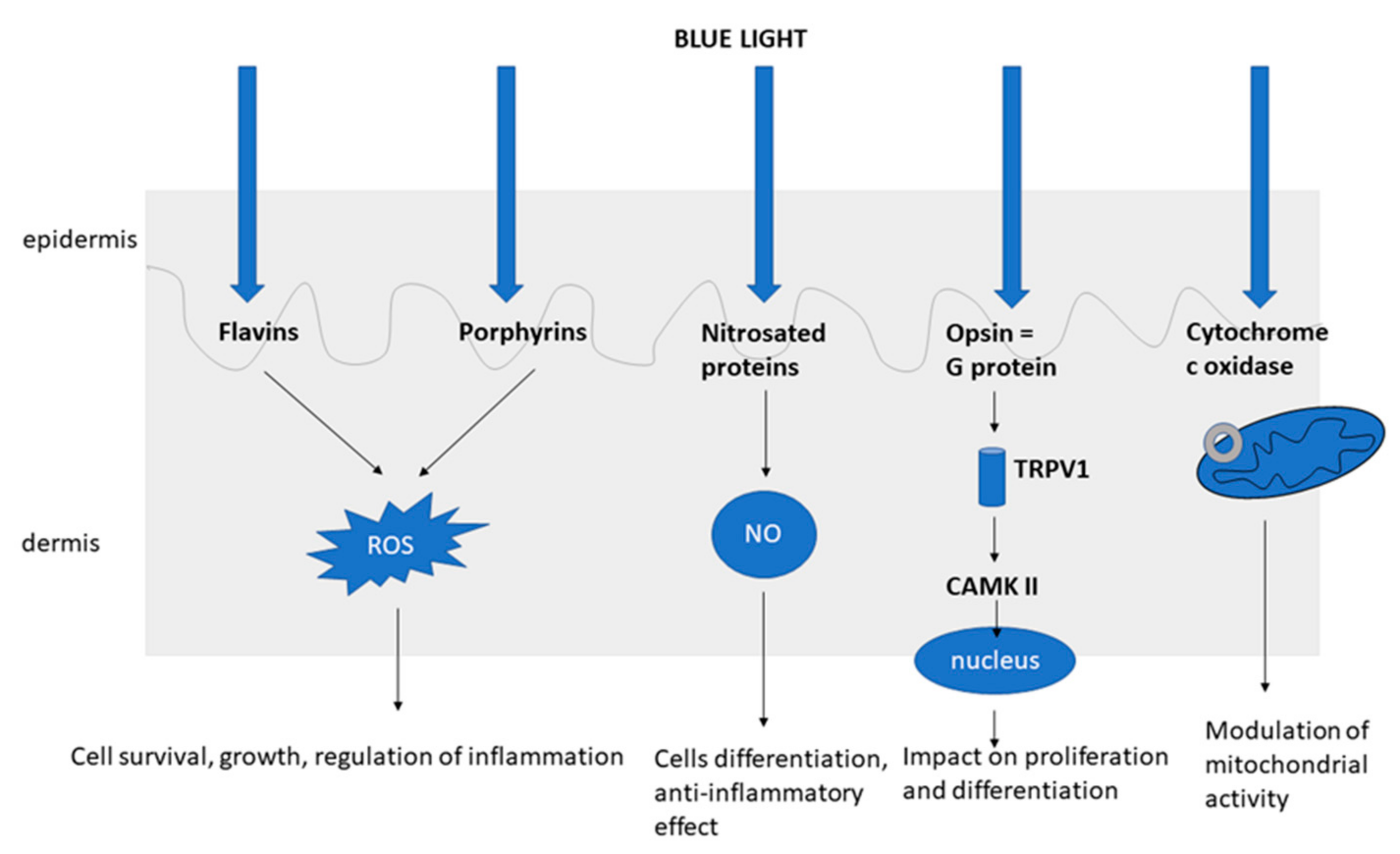
2. The Mechanism of Action of Blue Light
3. Antiproliferative and the Anti-Inflammatory Properties of Blue Light
4. The Negative Aspects of Blue Light
5. Effect on Pigmentation
6. Anticancer Effect
7. The Clinical Use of Blue Light
7.1. Psoriasis
7.2. Atopic Dermatitis and Eczema
7.3. Acne
7.4. Photodynamic Therapy (PDT)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sowa, P.; Rutkowska-Talipska, J.; Rutkowski, K.; Kosztyła-Hojna, B.; Rutkowski, R. Optical Radiation in Modern Medicine. Adv. Dermatol. Allergol. 2013, 4, 246–251. [Google Scholar] [CrossRef]
- Garza, Z.C.F.; Born, M.; Hilbers, P.A.J.; Riel, N.A.W.V.; Liebmann, J. Visible Blue Light Therapy: Molecular Mechanisms and Therapeutic Opportunities. Curr. Med. Chem. 2019, 25, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.; Gelfand, J.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of Care for the Management of Psoriasis and Psoriatic Arthritis. J. Am. Acad. Dermatol. 2010, 62, 114–135. [Google Scholar] [CrossRef]
- Archier, E.; Devaux, S.; Castela, E.; Gallini, A.; Aubin, F.; Le Maître, M.; Aractingi, S.; Bachelez, H.; Cribier, B.; Joly, P.; et al. Carcinogenic Risks of Psoralen UV-A Therapy and Narrowband UV-B Therapy in Chronic Plaque Psoriasis: A Systematic Literature Review. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lindelöf, B.; Sigurgeirsson, B.; Tegner, E.; Larkö, O.; Johannesson, A.; Berne, B.; Ljunggren, B.; Andersson, T.; Molin, L.; Nylander-Lundqvist, E.; et al. PUVA and Cancer Risk: The Swedish Follow-up Study. Br. J. Dermatol. 1999, 141, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Brieva, A.; Guerrero, A.; Pivel, J.P. Incidence and risk factors associated with a second squamous cell carcinoma or basal cell carcinoma in psoralen + ultraviolet light-treated psoriasis patients. J. Investig. Dermatol. 2002, 118, 1038–1043. [Google Scholar] [CrossRef]
- Stern, R.S. The Risk of Squamous Cell and Basal Cell Cancer Associated With Psoralen and Ultraviolet A Therapy: A 30-Year Prospective Study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Hinrichs, R.; Scharffetter-Kochanek, K. Phototherapy and Photochemotherapy. Clin. Dermatol. 2008, 26, 464–476. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers Med Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Dungel, P.; Mittermayr, R.; Haindl, S.; Osipov, A.; Wagner, C.; Redl, H.; Kozlov, A.V. Illumination With Blue Light Reactivates Respiratory Activity of Mitochondria Inhibited by Nitric Oxide, But Not by Glycerol Trinitrate. Arch. Biochem. Biophys. 2008, 471, 109–115. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the Spotlight: Mechanisms of Photobiomodulation Concentrating on Blue and Green Light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Buscone, S.; Mardaryev, A.; Raafs, B.; Bikker, J.W.; Sticht, C.; Gretz, N.; Farjo, N.; Uzunbajakava, N.E.; Botchkareva, N.V. A New Path in Defining Light Parameters for Hair Growth: Discovery and Modulation of Photoreceptors in Human Hair Follicle. Lasers Surg. Med. 2017, 49, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chang, C.-J.; Chen, L.-Y. Blue Light Induced Reactive Oxygen Species from Flavin Mononucleotide and Flavin Adenine Dinucleotide on Lethality of HeLa Cells. J. Photochem. Photobiol. B Biol. 2017, 173, 325–332. [Google Scholar] [CrossRef]
- Buscone, S.; Mardaryev, A.N.; Westgate, G.E.; Uzunbajakava, N.E.; Botchkareva, N.V. Cryptochrome 1 Is Modulated by Blue Light in Human Keratinocytes and Exerts Positive Impact on Human Hair Growth. Exp. Dermatol. 2021, 30, 271–277. [Google Scholar] [CrossRef]
- Maclean, M.; McKenzie, K.; Anderson, J.; Gettinby, G.; MacGregor, S. 405 Nm Light Technology for the Inactivation of Pathogens and Its Potential Role for Environmental Disinfection and Infection Control. J. Hosp. Infect. 2014, 88, 1–11. [Google Scholar] [CrossRef]
- MacLean, M.; MacGregor, S.; Anderson, J.; Woolsey, G. The Role of Oxygen in the Visible-Light Inactivation of Staphylococcus Aureus. J. Photochem. Photobiol. B Biol. 2008, 92, 180–184. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue Light for Infectious Diseases: Propionibacterium Acnes, Helicobacter Pylori, and Beyond? Drug Resist. Updat. 2012, 15, 223–236. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-Light Irradiation Regulates Proliferation and Differentiation in Human Skin Cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Opländer, C.; Deck, A.; Volkmar, C.M.; Kirsch, M.; Liebmann, J.; Born, M.; van Abeelen, F.; van Faassen, E.E.; Kröncke, K.-D.; Windolf, J.; et al. Mechanism and Biological Relevance of Blue-Light (420–453 Nm)-Induced Nonenzymatic Nitric Oxide Generation from Photolabile Nitric Oxide Derivates in Human Skin in Vitro and in Vivo. Free Radic. Biol. Med. 2013, 65, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Choi, M.S.; Bae, I.-H.; Jung, J.-Y.; Son, E.D.; Lee, T.R.; Shin, D.W. Short Wavelength Visible Light Suppresses Innate Immunity-Related Responses by Modulating Protein S-Nitrosylation in Keratinocytes. J. Investig. Dermatol. 2016, 136, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Sticht, C.; Dweep, H.; Van Abeelen, F.A.; Gretz, N.; Oversluizen, G. Impact of Blue LED Irradiation on Proliferation and Gene Expression of Cultured Human Keratinocytes. Mech. Low Light Ther. X 2015, 9309, 930909. [Google Scholar] [CrossRef]
- Mittermayr, R.; Osipov, A.; Piskernik, C.; Haindl, S.; Dungel, P.; Weber, C.; Vladimirov, Y.A.; Redl, H.; Kozlov, A.V. Blue Laser Light Increases Perfusion of a Skin Flap Via Release of Nitric Oxide from Hemoglobin. Mol. Med. 2007, 13, 22–29. [Google Scholar] [CrossRef]
- Yoo, J.A.; Yu, E.; Park, S.-H.; Oh, S.W.; Kwon, K.; Park, S.J.; Kim, H.; Yang, S.; Park, J.Y.; Cho, J.Y.; et al. Blue Light Irradiation Induces Human Keratinocyte Cell Damage via Transient Receptor Potential Vanilloid 1 (TRPV1) Regulation. Oxidative Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Trotter, L.A.; Patel, D.; Dubin, S.; Guerra, C.; McCloud, V.; Lockwood, P.; Messer, R.; Wataha, J.C.; Lewis, J.B. Violet/Blue Light Activates Nrf2 Signaling and Modulates the Inflammatory Response of THP-1 Monocytes. Photochem. Photobiol. Sci. 2017, 16, 883–889. [Google Scholar] [CrossRef]
- Patel, A.D.; Rotenberg, S.; Messer, R.L.W.; Wataha, J.C.; Ogbureke, K.; McCloud, V.V.; Lockwood, P.; Hsu, S.; Lewis, J.B. Blue Light Activates Phase 2 Response Proteins and Slows Growth of a431 Epidermoid Carcinoma Xenografts. Anticancer Res. 2014, 34, 6305–6313. [Google Scholar]
- Fischer, M.R.; Abel, M.; Kostka, S.L.; Rudolph, B.; Becker, D.; Von Stebut, E. Blue Light Irradiation Suppresses Dendritic Cells Activationin Vitro. Exp. Dermatol. 2013, 22, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Monfrecola, G.; Lembo, S.; Cantelli, M.; Ciaglia, E.; Scarpato, L.; Fabbrocini, G.; Balato, A. The Effect of Visible Blue Light on the Differentiation of Dendritic Cells in Vitro. Biochimie 2014, 101, 252–255. [Google Scholar] [CrossRef]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of Blue Light Irradiation on Human Dermal Fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Goyarts, E.C.; Pelle, E.; Trivero, J.; Pernodet, N. Blue Light Disrupts the Circadian Rhythm and Create Damage in Skin Cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue Light-Induced Oxidative Stress in Live Skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-Violet Light Irradiation Dose Dependently Decreases Carotenoids in Human Skin, Which Indicates the Generation of Free Radicals. Oxidative Med. Cell. Longev. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Klapczynski, A.; Kuch, N.; Arpino, F.; Simon-Keller, K.; De La Torre, C.; Sticht, C.; Van Abeelen, F.A.; Oversluizen, G.; Gretz, N. Gene Expression Profiling Reveals Aryl Hydrocarbon Receptor As a Possible Target for Photobiomodulation When Using Blue Light. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Ristow, M. Unraveling the Truth About Antioxidants: Mitohormesis Explains ROS-Induced Health Benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef]
- Wataha, J.; Lewis, J.; Lockwood, P.; Hsu, S.; Messer, R.; Rueggeberg, F.; Bouillaguet, S. Blue Light Differentially Modulates Cell Survival and Growth. J. Dent. Res. 2004, 83, 104–108. [Google Scholar] [CrossRef]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.-P.; Passeron, T. Differences in Visible Light-Induced Pigmentation According to Wavelengths: A Clinical and Histological Study in Comparison With UVB Exposure. Pigment. Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef]
- Falcone, D.; Uzunbajakava, N.E.; van Abeelen, F.; Oversluizen, G.; Peppelman, M.; van Erp, P.E.J.; van de Kerkhof, P.C.M. Effects of blue light on inflammation and skin barrier recovery following acute perturbation. Pilot study results in healthy human subjects. Photodermatol. Photoimmunol. Photomed. 2018, 34, 184–193. [Google Scholar] [CrossRef]
- Weinstabl, A.; Hoff-Lesch, S.; Merk, H.F.; Von Felbert, V. Prospective Randomized Study on the Efficacy of Blue Light in the Treatment of Psoriasis Vulgaris. Dermatology 2011, 223, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kleinpenning, M.; Otero, M.; van Erp, P.; Gerritsen, M.; Van De Kerkhof, P. Efficacy of Blue Light Vs. Red Light in the Treatment of Psoriasis: A Double-Blind, Randomized Comparative Study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 219–225. [Google Scholar] [CrossRef]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; Van Erp, P.E.J.; Van De Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and Histological Effects of Blue Light on Normal Skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Ohara, M.; Kawashima, Y.; Katoh, O.; Watanabe, H. Blue Light Inhibits the Growth of B16 Melanoma Cells. Jpn. J. Cancer Res. 2002, 93, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Fujikura, T.; Fujiwara, H. Augmentation of the Inhibitory Effect of Blue Light on the Growth of B16 Melanoma Cells by Riboflavin. Int. J. Oncol. 2003, 22, 1291–1295. [Google Scholar] [CrossRef]
- Sparsa, A.; Faucher, K.; Sol, V.; Durox, H.; Boulinguez, S.; Doffoel-Hantz, V.; Calliste, C.-A.; Cook-Moreau, J.; Krausz, P.; Sturtz, F.G.; et al. Blue Light Is Phototoxic for B16F10 Murine Melanoma and Bovine Endothelial Cell Lines by Direct Cytocidal Effect. Anticancer. Res. 2010, 30, 143–148. [Google Scholar] [PubMed]
- Chen, Z.; Li, W.; Hu, X.; Liu, M. Irradiance Plays a Significant Role in Photobiomodulation of B16F10 Melanoma Cells by Increasing Reactive Oxygen Species and Inhibiting Mitochondrial Function. Biomed. Opt. Express 2019, 11, 27–39. [Google Scholar] [CrossRef]
- Maari, C.; Viau, G.; Bissonnette, R. Repeated Exposure to Blue Light Does Not Improve Psoriasis. J. Am. Acad. Dermatol. 2003, 49, 55–58. [Google Scholar] [CrossRef]
- Pfaff, S.; Liebmann, J.; Born, M.; Merk, H.F.; Von Felbert, V. Prospective Randomized Long-Term Study on the Efficacy and Safety of UV-Free Blue Light for Treating Mild Psoriasis Vulgaris. Dermatology 2015, 231, 24–34. [Google Scholar] [CrossRef]
- Krings, L.; Liebmann, J.; Born, M.; Leverkus, M.; Von Felbert, V. A randomized study comparing the efficacy and safety of blue light and topical vitamin D treatments for mild Psoriasis vulgaris. Trends Photochem. Photobiol. 2019, 18, 1–11. [Google Scholar]
- Lesiak, A.; Bednarski, I.A.; Narbutt, J. Prospective 3-month study on the efficacy of UV-free blue light in mild psoriasis vulgaris treatment. Adv. Dermatol. Allergol. 2021, 38. [Google Scholar]
- Krutmann, J.; Medve-Koenigs, K.; Ruzicka, T.; Ranft, U.; Wilkens, J.H. Ultraviolet-free phototherapy. Photodermatol. Photoimmunol. Photomed. 2005, 21, 59–61. [Google Scholar] [CrossRef]
- Becker, D.; Langer, E.; Seemann, M.; Seemann, G.; Fell, I.; Saloga, J.; Grabbe, S.; Von Stebut, E. Clinical Efficacy of Blue Light Full Body Irradiation As Treatment Option for Severe Atopic Dermatitis. PLoS ONE 2011, 6, e20566. [Google Scholar] [CrossRef]
- Keemss, K.; Pfaff, S.C.; Born, M.; Liebmann, J.; Merk, H.F.; Von Felbert, V. Prospective, Randomized Study on the Efficacy and Safety of Local UV-Free Blue Light Treatment of Eczema. Dermatology 2016, 232, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Harrison, A.; Drew, S.; Whittall, R. A Randomized Controlled Study for the Treatment of Acne Vulgaris Using High-Intensity 414 Nm Solid State Diode Arrays. J. Cosmet. Laser Ther. 2015, 17, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Pan, C.; Li, K.; Tan, Y.; Wei, X. Phototherapy for Mild to Moderate Acne Vulgaris with Portable Blue and Red Led. J. Innov. Opt. Heal. Sci. 2011, 4, 45–52. [Google Scholar] [CrossRef]
- Kawada, A.; Aragane, Y.; Kameyama, H.; Sangen, Y. An open study and in vitro investigation of acne phototherapy with a high-intensity, enhanced, narrowband, blue light source. J. Am. Acad. Derm. 2005, 52, P14. [Google Scholar] [CrossRef]
- Noborio, R.; Nishida, E.; Kurokawa, M.; Morita, A. A New Targeted Blue Light Phototherapy for the Treatment of Acne. Photodermatol. Photoimmunol. Photomed. 2007, 23, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Ammad, S.; Gonzales, M.; Edwards, C.; Finlay, A.Y.; Mills, C. An Assessment of the Efficacy of Blue Light Phototherapy in the Treatment of Acne Vulgaris. J. Cosmet. Dermatol. 2008, 7, 180–188. [Google Scholar] [CrossRef]
- Omi, T.; Bjerring, P.; Sato, S.; Kawana, S.; Hankins, R.; Honda, M. 420 Nm Intense Continuous Light Therapy for Acne. J. Cosmet. Laser Ther. 2004, 6, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H.; Andriessen, A.; Biron, J.; Andriessen, H. Clinical efficacy of self-applied blue light therapy for mild-to-moderate facial acne. J. Clin. Aesthet. Derm. 2009, 2, 44–50. [Google Scholar] [CrossRef][Green Version]
- Morton, C.A.; Scholefield, R.D.; Whitehurst, C.; Birch, J. An Open Study to Determine the Efficacy of Blue Light in the Treatment of Mild to Moderate Acne. J. Dermatol. Treat. 2005, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Greaves, A.J. The Effects of Narrowbands of Visible Light Upon Some Skin Disorders: A Review. Int. J. Cosmet. Sci. 2016, 38, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.R.; Kim, S.J.; Sohn, K.C.; Seo, Y.J.; Lee, Y.; Whang, K.U.; Kim, C.D.; Lee, J.H.; Im, M. Regulation of Lipid Production by Light-Emitting Diodes in Human Sebocytes. Arch. Dermatol. Res. 2015, 307, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Queirós, C.; Garrido, P.M.; Silva, J.M.; Filipe, P. Photodynamic Therapy in Dermatology: Beyond Current Indications. Dermatol. Ther. 2020, 33, 1–7. [Google Scholar] [CrossRef]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.-M. Photodynamic Therapy in Dermatology: State-of-the-Art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Fluhr, J.W. Photodynamic Therapy in Dermatology: Past, Present, and Future. J. Biomed. Opt. 2012, 18, 061208. [Google Scholar] [CrossRef] [PubMed]
- Kostović, K.; Pastar, Z.; Ceović, R.; Mokos, Z.B.; Buzina, D.S.; Stanimirović, A. Photodynamic Therapy in Dermatology: Current Treatments and Implications. Coll. Antropol. 2012, 36, 1477–1481. [Google Scholar] [PubMed]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part one—photosensitizers, Photochemistry and Cellular Localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two-cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Klein, A.; Babilas, P.; Karrer, S.; Landthaler, M.; Szeimies, R.-M. Photodynamic Therapy in Dermatology—An Update 2008. J. Dtsch. Dermatol. Ges. 2008, 6, 839–845. [Google Scholar] [CrossRef]
- Piacquadio, D.J.; Chen, D.M.; Farber, H.F.; Fowler, J.F., Jr.; Glazer, S.D.; Goodman, J.J.; Hruza, L.L.; Jeffes, E.W.B.; Ling, M.R.; Phillips, T.J. Photodynamic Therapy With Aminolevulinic Acid Topical Solution and Visible Blue Light in the Treatment of Multiple Actinic Keratoses of the Face and Scalp: Investigator-Blinded, Phase 3, Multicenter Trials. Arch. Derm. 2004, 140, 41–46. [Google Scholar] [CrossRef] [PubMed]
| Skin Disease | Treatment Protocol | Irradiation Days Per Week | Irradiation Time (min) | Numer of Patients | Peak Wavelength (nm) | Intensity (mW/cm2)—Irradiance | Fluence (J/cm2)—Dose | Initial Seveity Index | Outcome | Reference | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Psoriasis vulgaris | 3 times per week for 4 weeks | 3 | No information | 17 | 417 | 8.5 | 10 | LPSI around 6,6 | No significant imporvement | [46] | 2003 |
| Psorisasis vulgaris | Every day (15 min) for 4 weeks | 7 | 15 | 40 | 420 (group 1), 453 (group 2) | 100 | 90 | LPSI 5 | Significant improvement in both groups | [39] | 2011 |
| Psoriais vulgaris | 3 (20 min) times per week for 4 weeks | 3 | 20 | 20 | 420 | 100 | “high dose” | LPSI 7,7 | 33,9 % improvement, | [40] | 2012 |
| Psoriasis vulgaris | Every day (30 min) for 4 weeks and 3 times per week for the next 8 weeks | 7 for 4 weeks and 3 for 8 weeks | 30 | 47 | 453 | 100 and 200 | 90 | LPSI 5,17 (200 mW/cm2 group) LPASI 5,52 (100 mW/cm2 group) | improvement of LPSI in both groups. 29.2% achieved reduction of LPSI between ≥25 and <50%, 33.3% between ≥50 and <75%, and 16.7% of more than 75% | [47] | 2015 |
| Psoriasis vulgaris | 15 min or 30 min daily for 12 weeks | 7 | 15 or 30 | 51 | 453 | 600 | 38 and 76 | LPSI 5,31 (group 1), LPSI 4,8 (group 2) | improvement of about 50% in both groups | [48] | 2019 |
| Atopic dermatitis/hand and foot eczema | 30 min 3 times per week for 4 weeks | 3 | 30 | 10 | 40% between 400–500, 26% between 400–450 | 23 | 42 | DASI (dyshidrosis area and severity index) 33,85 | Significant improvement (DASI 23,3) | [50] | 2005 |
| Atopic dermatitis | 1 cycle = 5 consecutive irradiations. 2–5 cycles for maximum 24 weeks | 5 | 24 min of each side of the body | 36 | 66% between 400–500 nm | No information | 28,9 | EASI 20,6 (6,8–54) | Improvement of about 54% | [51] | 2011 |
| Eczema | 3 times per week for 4 weeks | 3 | No information | 21 | 453 | No information | 90J | Local ESI (local Eczema Severity Index) 4 | significant improvement of Local ESI (1,9) | [52] | 2016 |
| Skin Disorder | Treatment Protocol | Irradiation Days Per Week | Irradiation Time | Wavelength, Intensity | Number of Patients | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Acne vulgaris (mild to moderate, facial, inflammatory) | every other day for 8 weeks, with final assessment 4 weeks post-treatment. | 7 | No information | 414 nm | 26 (treatment group), 15 (control group) | Reduction of inflammatory lesion by 50.02%. Maximum effect at week 8–12. After week 12 all patients achieved improvement. | [53] |
| Acne vulgaris (mild to moderate) | twice a week, with an interval of two days, for 4 weeks | 2 | 20 min | 405 +/− 10 nm blue light at the power of 30 mW/cm2 | 10 (blue light), 10 (red light) | Reduction of lesions by 71.4%. The mean number of lesions decreased from 19.2 to 5.5 after 8 irradiations. | [54] |
| Acne vulgaris | twice a week up to 5 weeks | 2 | No information | 407–420 nm, 90 mW/cm2 | 30 | Reduction of lesions by 64%. Within week 5 77% of patients achieved improvement. | [55] |
| Acne vulgaris | once or twice a week. | 1–2 | 30 min (face), 45 min (back) | 405 and 420 nm | 10 | Improvement was observed in 80% of patients | [56] |
| Acne vulgaris (mild to moderate facial acne) | twice a week for 4 weeks | 2 | 14 min | 415–425 nm (peak 420 nm) | 21 | Significant reduction of lesions | [57] |
| Acne vulgaris (facial acne) | Twice a week for 4 weeks | 2 | 15 min | 420 nm | 28 | Lesions improved by 64.7% | [58] |
| Acne vulgaris (mild-to-moderate inflammatory acne on the face) | Once a day for 8 weeks | 7 | 6 min | 414 nm | 21 | Reduction of open and closed comedomes, papules | [59] |
| Acne vulgaris (mild to moderate) | Twice a week for 4 weeks | 2 | 10/20 min | 409–419 nm at 40 mW/cm2 | 30 | Reduction of lesions (at week 8–12) | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, M.; Narbutt, J.; Lesiak, A. Blue Light in Dermatology. Life 2021, 11, 670. https://doi.org/10.3390/life11070670
Sadowska M, Narbutt J, Lesiak A. Blue Light in Dermatology. Life. 2021; 11(7):670. https://doi.org/10.3390/life11070670
Chicago/Turabian StyleSadowska, Magdalena, Joanna Narbutt, and Aleksandra Lesiak. 2021. "Blue Light in Dermatology" Life 11, no. 7: 670. https://doi.org/10.3390/life11070670
APA StyleSadowska, M., Narbutt, J., & Lesiak, A. (2021). Blue Light in Dermatology. Life, 11(7), 670. https://doi.org/10.3390/life11070670