Perception of Thermal Comfort during Skin Cooling and Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.1.1. Perception of Thermal Comfort
2.1.2. Regulation of Thermal Comfort
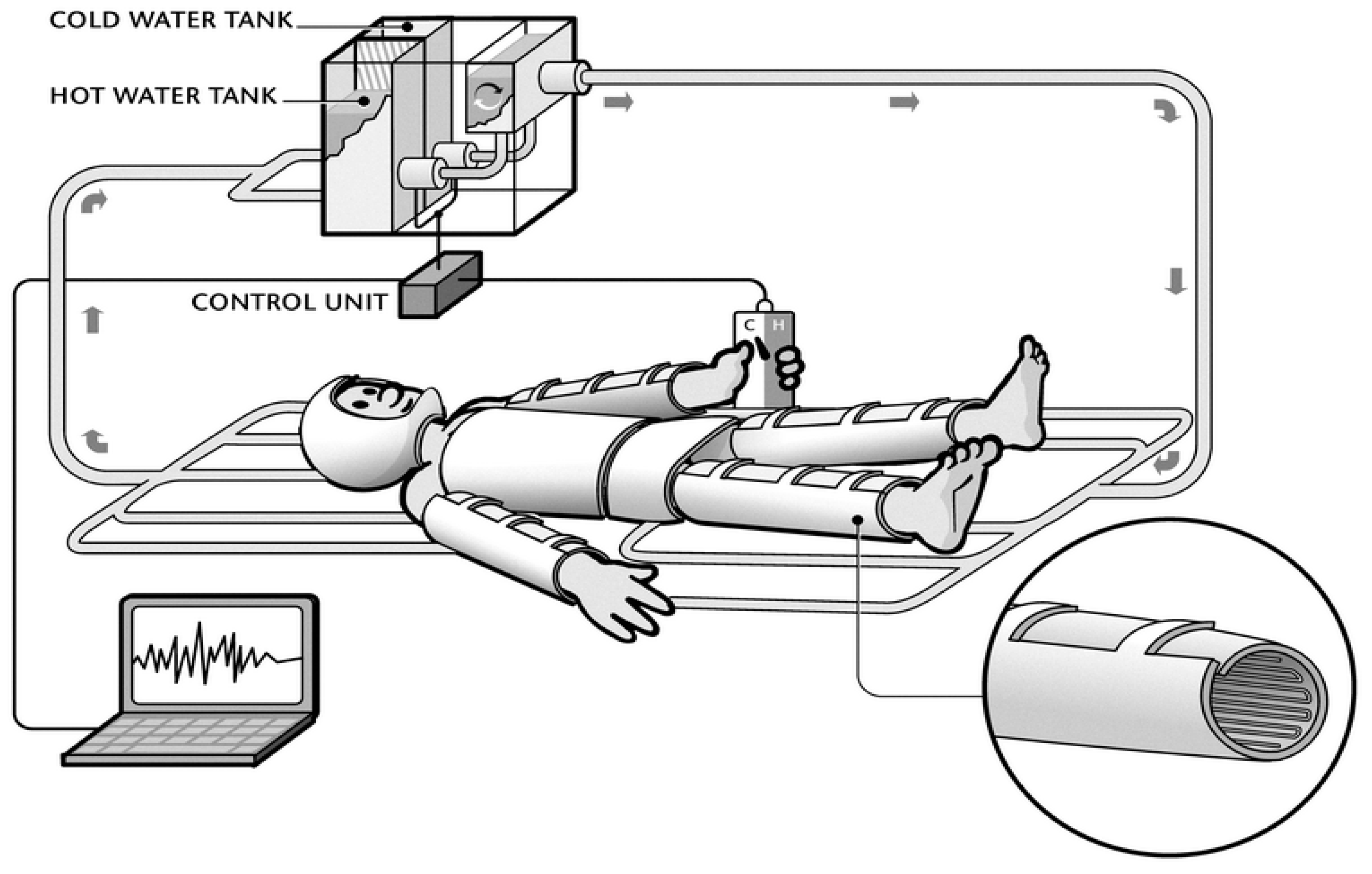
2.2. Instrumentation
2.2.1. Water Perfused Suit (WPS)
2.2.2. Temperature Control of WPS
2.2.3. Ambient Conditions
2.2.4. Skin and Core Temperature
2.3. Calculations and Data Analysis
3. Results
3.1. Characteristics of Temperature Changes in the WPS
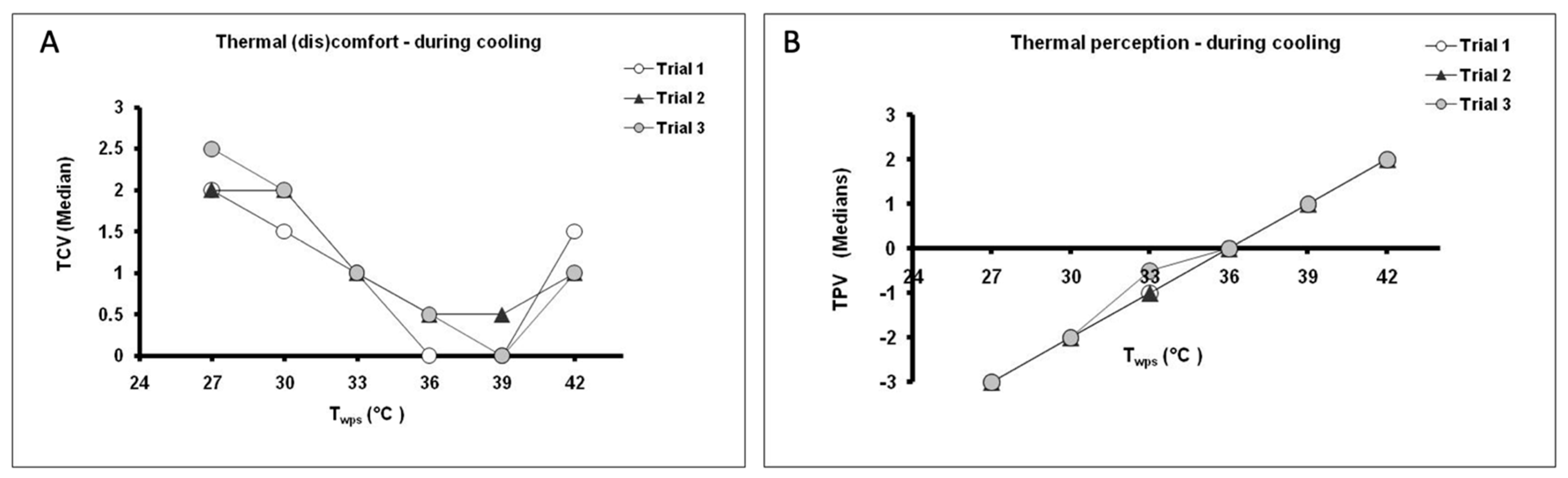
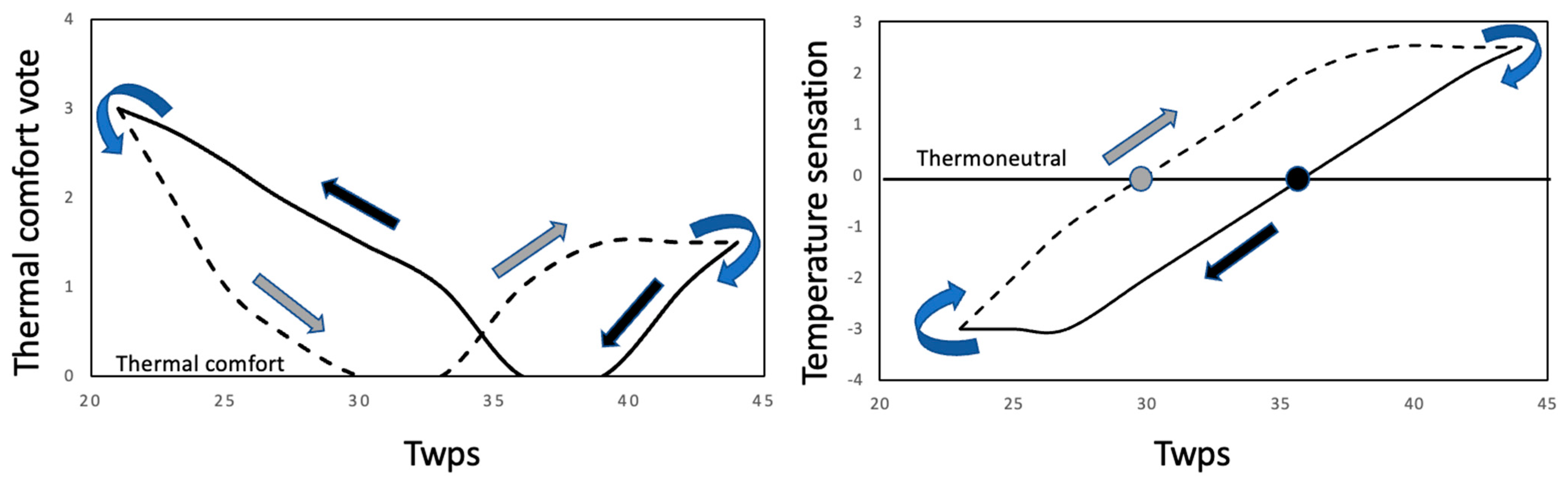
3.2. Thermal Comfort Vote (TCV) and Temperature Perception Vote (TPV) during Prescribed Conditions
3.3. Thermal Comfort Regulation during Individual Control
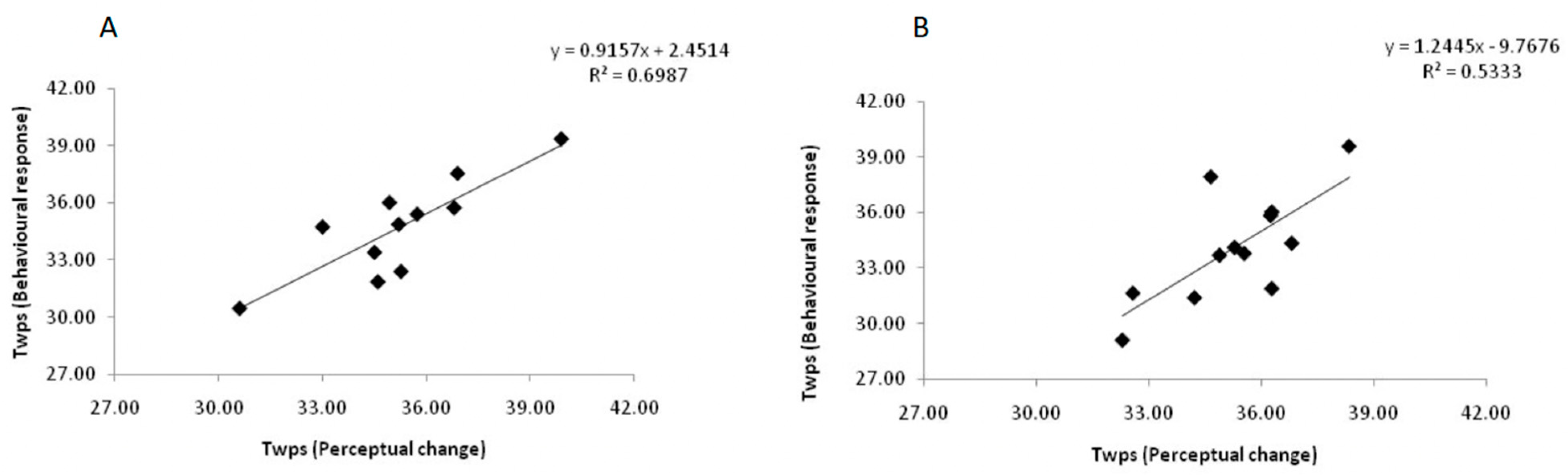
3.4. Correlation between Perceptual Change and Behavioral Response
4. Discussion
4.1. Visual Analog Scales as an Index of Behavioral Temperature Regulation
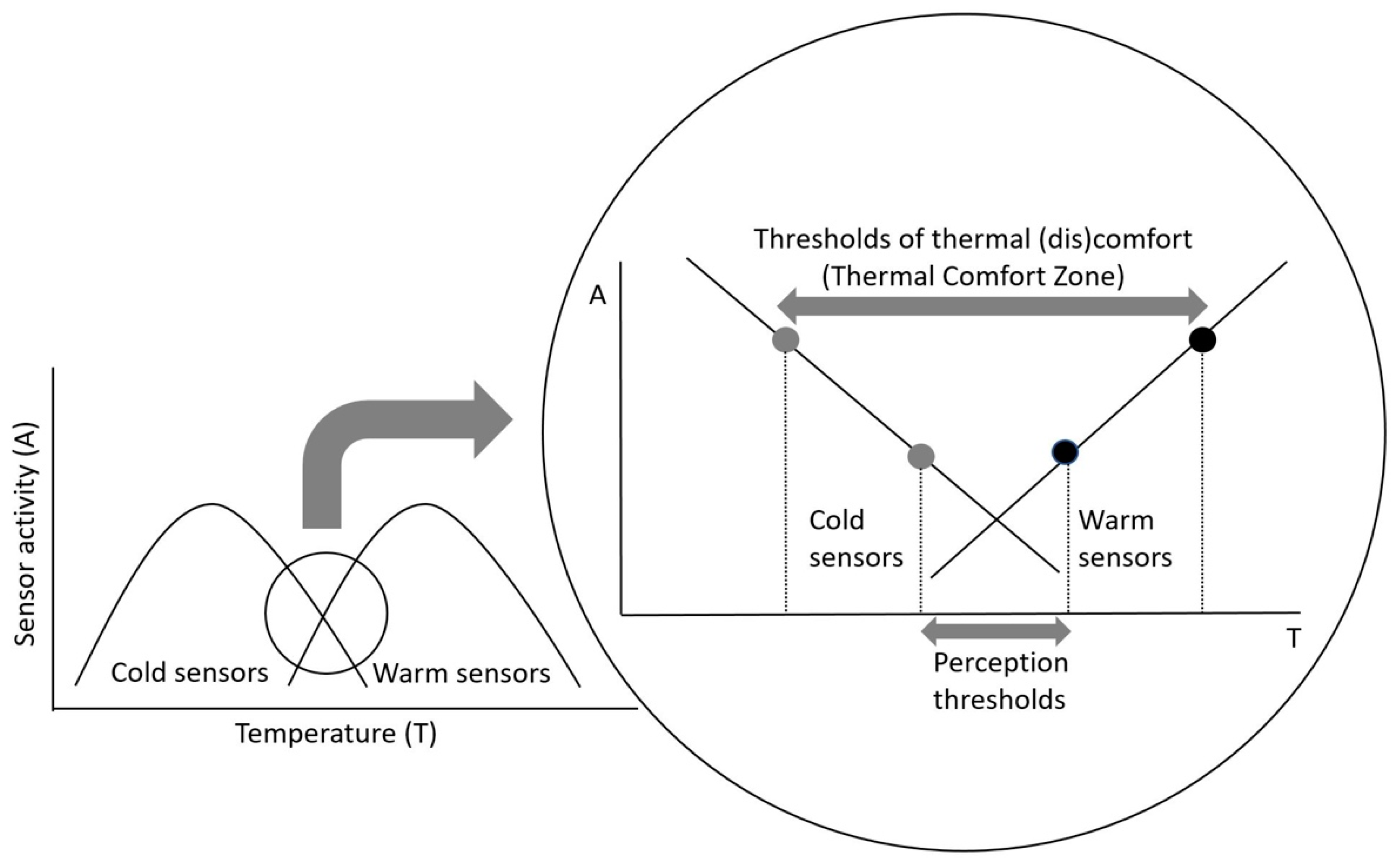
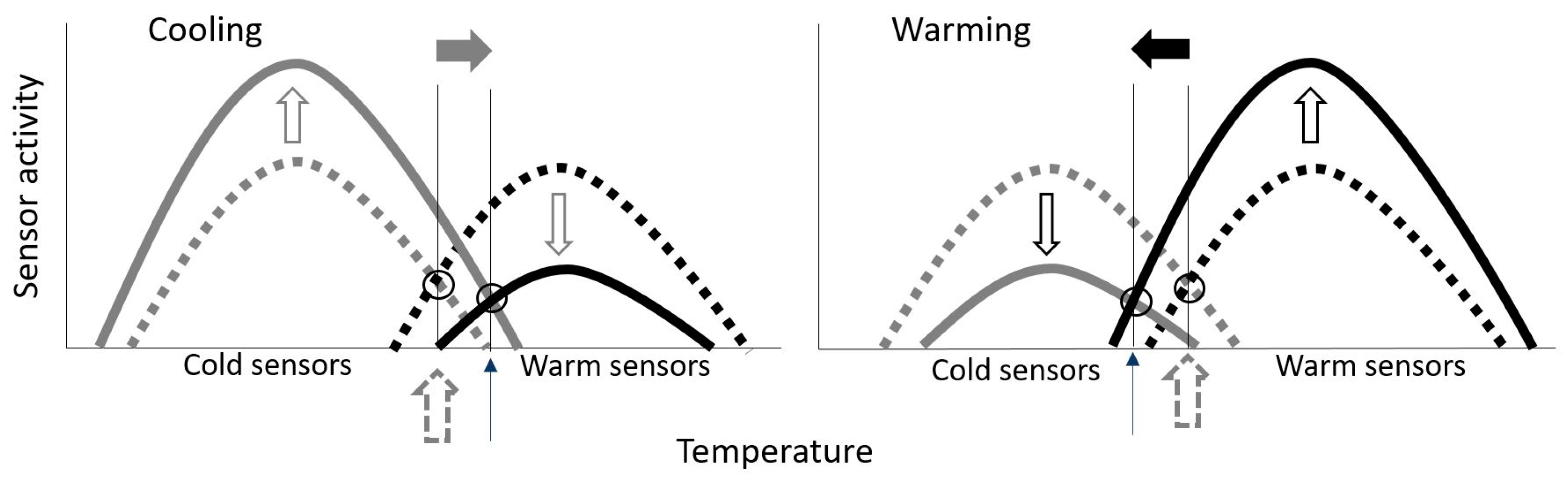
4.2. Neurophysiological Correlate of Thermal Comfort—A Hypothesis
4.3. Validity of Visual Analog Scales for Thermal Comfort
4.4. Anticipatory Response
4.5. Practical Implications
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabanac, M. Physiological role of pleasure. Science 1971, 173, 1103–1107. [Google Scholar] [CrossRef]
- Hensel, H. Correlations of neural activity and thermal sensation in man. In Sensory Functions of the Skin in Primates with Special Reference to Man; Zotterman, Y., Ed.; Oxford, Pergamon Press: London, UK, 1976; pp. 331–353. [Google Scholar]
- Weiss, B.; Laties, V.G. Behavioral thermoregulation. Science 1961, 133, 1338–1344. [Google Scholar] [CrossRef]
- Mercer, J. Glossary of terms for thermal physiology—3rd edition (Reprinted from the Japanese Journal of Physiology). J. Therm. Biol. 2003, 26, 249–253. [Google Scholar] [CrossRef]
- De Dear, R.; Brager, G.S. Developing an adaptive model of thermal comfort and preference. ASHRAE 1998, 104, 145–167. [Google Scholar]
- ASHRAE. ASHRAE—Fundamentals; ASHRAE Inc.: Atlanta, GA, USA, 1997. [Google Scholar]
- Bedford, T. The warmth factor in comfort at work. In Industrial Health Research Board Report; HMSO: London, UK, 1936; p. 76. [Google Scholar]
- Fanger, P.O. Thermal Comfort: Analysis and Applications in Environmental Engineering; McGraw-Hill: New York, NY, USA, 1970. [Google Scholar]
- International Organization for Standardization. ISO 7730 Ergonomics of the Thermal Environment—Analytical Determination and Interpretation of Thermal Comfort Using Calculation of the PMV and PPD Indices and Local Thermal Comfort Criteria; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- Parsons, K.C. The effects of gender, acclimation state, the opportunity to adjust clothing and physical disability on requirements for thermal comfort. Energy Build. 2002, 34, 593–599. [Google Scholar] [CrossRef]
- Passias, T.C.; Meneilly, G.S.; Mekjavic, I.B. Effect of hypoglycemia on thermoregulatory responses. J. Appl. Physiol. 1996, 80, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S.; Ward, R.; Mekjavic, I.B. Gender differences in physiological reactions to thermal stress. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Hsieh, S.T.; Chiu, M.J.; Tseng, M.T.; Chang, Y.C. Effects of aging on contact heat-evoked potentials: The physiological assessment of thermal perception. Muscle Nerve 2007, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.M.; Tong, W.C. Thermal comfort study of young chinese people in Hong Kong. Build. Environ. 1990, 25, 317–328. [Google Scholar] [CrossRef]
- Paulson, P.E.; Minoshima, S.; Morrow, T.J.; Casey, K.L. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 1998, 76, 223–229. [Google Scholar] [CrossRef]
- Sarlani, E.; Farooq, N.; Greenspan, J.D. Gender and laterality differences in thermosensation throughout the perceptible range. Pain 2003, 106, 9–18. [Google Scholar] [CrossRef]
- Anderson, G.S.; Mekjavic, I.B. Thermoregulatory responses of circum-pubertal children. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 404–410. [Google Scholar] [CrossRef]
- Anderson, G.S.; Meneilly, G.S.; Mekjavic, I.B. Passive temperature lability in the elderly. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 278–286. [Google Scholar] [CrossRef]
- Collins, K.J.; Exton-Smith, A.N.; Doré, C. Urban hypothermia: Preferred temperature and thermal perception in old age. Brit. Med. J. 1981, 282, 175–177. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.A.; Allsopp, N.K.; Parkes, D.G. Preferred room temperature of young vs aged males: The influence of thermal sensation, thermal comfort, and affect. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M216–M221. [Google Scholar] [CrossRef]
- Coleshaw, S.R.K.; Johnston, G.; Macdonald, A.G.; Pertwee, R.G. Effects of subanaesthetic soses of nitrous-oxide on thermal perception in man. J. Physiol. 1990, 427, P14. [Google Scholar]
- Fowler, B.; Ackles, K.N.; Porlier, G. Effects of inert gas narcosis on behavior—A critical review. Undersea Biomed. Res. 1985, 12, 369–402. [Google Scholar] [PubMed]
- Mekjavic, I.B.; Passias, T.; Sundberg, C.J.; Eiken, O. Perception of thermal comfort during narcosis. Undersea Hyperb. Med. 1994, 21, 9–19. [Google Scholar] [PubMed]
- Passias, T.C.; Mekjavic, I.B.; Eiken, O. The effect of 30% nitrous oxide on thermoregulatory responses in humans during hypothermia. Anesthesiology 1992, 76, 550–559. [Google Scholar] [CrossRef]
- Golja, P.; Mekjavic, I.B. Effect of hypoxia on preferred hand temperature. Aviat. Space Environ. Med. 2003, 74, 522–526. [Google Scholar] [PubMed]
- Golja, P.; Kacin, A.; Tipton, M.J.; Eiken, O.; Mekjavic, I.B. Hypoxia increases the cutaneous threshold for the sensation of cold. Eur. J. Appl. Physiol. 2004, 92, 62–68. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Botonic, P.G. Short-term sleep deprivation and human thermoregulatory function during thermal challenges. Exp. Physiol. 2021, 106, 1139–1148. [Google Scholar] [CrossRef]
- Ciuha, U.; Mekjavic, I.B. Regional thermal comfort zone in males and females. Physiol. Behav. 2016, 161, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Golja, P.; Kacin, A.; Tipton, M.J.; Eiken, O.; Mekjavic, I.B. Moderate hypoxia does not affect the zone of thermal comfort in humans. Eur. J. Appl. Physiol. 2005, 93, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Yogev, D.; Eiken, O.; Pisot, R.; Biolo, G.; di Prampero, P.; Narici, M.; Mekjavic, I.B. Effect of 21 days of horizontal bed rest on behavioural thermoregulation. Eur. J. Appl. Physiol. 2010, 108, 281–288. [Google Scholar] [CrossRef]
- Almeida, M.C.; Steiner, A.A.; Branco, L.G.S.; Romanovsky, A.A. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur. J. Neurosci. 2006, 23, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. The role of behavioral thermoregulation as a thermoeffector during prolonged hypoxia in the rat. J. Therm. Biol. 1997, 22, 315–324. [Google Scholar] [CrossRef]
- Macdonald, A.G.; Marshall, N.R.; Pertwee, R.G. Behavioral thermoregulation in mice subjected to high pressure. J. Appl. Physiol. 1989, 66, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Marshall, N.R.; Macdonald, A.G. Behavioural thermoregulation in mice: Effects of low doses of general anaesthetics of different potency. Exp. Physiol. 1990, 75, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satinoff, E. Behavioral thermoregulation in response to local cooling of the rat brain. Am. J. Physiol. 1964, 206, 1389–1394. [Google Scholar] [CrossRef]
- Jeong, W.S. Clothing selection behavior of the aged women for thermal comfort. Appl. Hum. Sci. 1999, 18, 87–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.E.; Tokura, H. Effects of the menstrual cycle on dressing behavior in the cold. Physiol. Behav. 1995, 58, 699–703. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, W.S. Influence of illumination on autonomic thermoregulation and choice of clothing. Int. J. Biometeorol. 2002, 46, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.D. Thermal comfort. In Physiological and Behavioral Temperature Regulation; Hardy, J.D., Gagge, A.P., Stolwijk, J.A.J., Eds.; Charles C Thomas: Springfield, IL, USA, 1970; pp. 856–873. [Google Scholar]
- Webb, P.; Troutman, S.J., Jr.; Annis, J.F. Automatic cooling in water cooled space suits. Aerosp. Med. 1970, 41, 269–277. [Google Scholar]
- Hexamer, M.; Werner, J. Control of liquid cooling garments: Subjective versus technical control of thermal comfort. Appl. Hum. Sci. 1995, 14, 271–278. [Google Scholar] [CrossRef]
- Duclaux, R.; Kenshalo, D.R., Sr. Response characteristics of cutaneous warm receptors in the monkey. J. Neurophysiol. 1980, 43, 1–15. [Google Scholar] [CrossRef]
- Kenshalo, D.R.; Duclaux, R. Response characteristics of cutaneous cold receptors in the monkey. J. Neurophysiol. 1977, 40, 319–332. [Google Scholar] [CrossRef]
- Mekjavic, I.B.; Sun, J.; Lun, V.; Giesbrecht, G.G. Evaluation of an infrared tympanic thermometer during cold water immersion and rewarming. In Proceedings of the 5th International Conference on Environmental Ergonomics, Montebello, ON, Canada, 25–30 September 1992. [Google Scholar]
- Mekjavic, I.B.; Morrison, J.B. A model of shivering thermogenesis based on the neurophysiology of thermoreception. IEEE Trans. Biomed. Eng. 1985, 32, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Bazett, H.C. The regulation of body temperature. In Physiology of Heat Regulation and the Science of Clothing; Newburgh, L.H., Ed.; Saunders: Philadelphia, PA, USA, 1949; pp. 109–192. [Google Scholar]
- Vendrik, A.J. The regulation of body temperature in man. Introduction. Ned. Tijdschr. Geneeskd. 1959, 103, 240–244. [Google Scholar] [PubMed]
- Zotterman, Y. Special senses: Thermal receptors. Ann. Rev. Physiol. 1953, 15, 357–372. [Google Scholar] [CrossRef]
- Bligh, J. Mammalian homeothermy: An integrative thesis. J. Therm. Biol. 1998, 23, 143–258. [Google Scholar] [CrossRef]
- Werner, J. System properties, feedback control and effector coordination of human temperature regulation. Eur. J. Appl. Physiol. 2010, 109, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.I.P.; Guillamóan, N.M.; Ortiz de Anda, R.M.C.; Psikuta, A.; Annaheim, S.; Rossi, M.R.; Salvador, J.M.C.; Perez-Soriano, P.; Palmer, R.S. Effect of perspiration on skin temperature measurements by infrared thermography and contact thermometry during aerobic cycling. Infrared Phys. Tech. 2015, 72, 68–76. [Google Scholar] [CrossRef]
- Ramanathan, N.L. A new weighting system for mean skin temperature of the body. J. Appl. Physiol. 1964, 19, 531–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciuha, U.; Tobita, K.; McDonnell, A.C.; Mekjavic, I.B. The effect of thermal transience on the perception of thermal comfort. Physiol. Behav. 2019, 210, 112623. [Google Scholar] [CrossRef] [PubMed]
- Mekjavic, I.B.; Morrison, J.B. Evaluation of predictive formulae for determining metabolic rate during cold water immersion. Aviat. Space Environ. Med. 1986, 57, 671–680. [Google Scholar]
- Mekjavic, I.B.; Tipton, M.J.; Eiken, O. Thermal considerations in diving. In Bennett and Elliott’s Physiology and Medicine of Diving; Brubakk, A.O., Neuman, T.S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Mekjavic, I.B.; Eiken, O. Contribution of thermal and nonthermal factors to the regulation of body temperature in humans. J. Appl. Physiol. 2006, 100, 2065–2072. [Google Scholar] [CrossRef] [Green Version]
- Charkoudian, N.; Stachenfeld, N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton. Neurosci. Basic Clin. 2016, 196, 75–80. [Google Scholar] [CrossRef]
- Kocjan, N. The Effect of Female Hormones on the Cutaneous Thermal Sensitivity and the Thermal Comfort Zone. Master’s Thesis, Jožef Stefan International Postgraduate Schoolo, Ljubljana, Slovenia, 2007. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekjavic, I.B.; Yogev, D.; Ciuha, U. Perception of Thermal Comfort during Skin Cooling and Heating. Life 2021, 11, 681. https://doi.org/10.3390/life11070681
Mekjavic IB, Yogev D, Ciuha U. Perception of Thermal Comfort during Skin Cooling and Heating. Life. 2021; 11(7):681. https://doi.org/10.3390/life11070681
Chicago/Turabian StyleMekjavic, Igor B., Daniel Yogev, and Urša Ciuha. 2021. "Perception of Thermal Comfort during Skin Cooling and Heating" Life 11, no. 7: 681. https://doi.org/10.3390/life11070681
APA StyleMekjavic, I. B., Yogev, D., & Ciuha, U. (2021). Perception of Thermal Comfort during Skin Cooling and Heating. Life, 11(7), 681. https://doi.org/10.3390/life11070681





