Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases?
Abstract
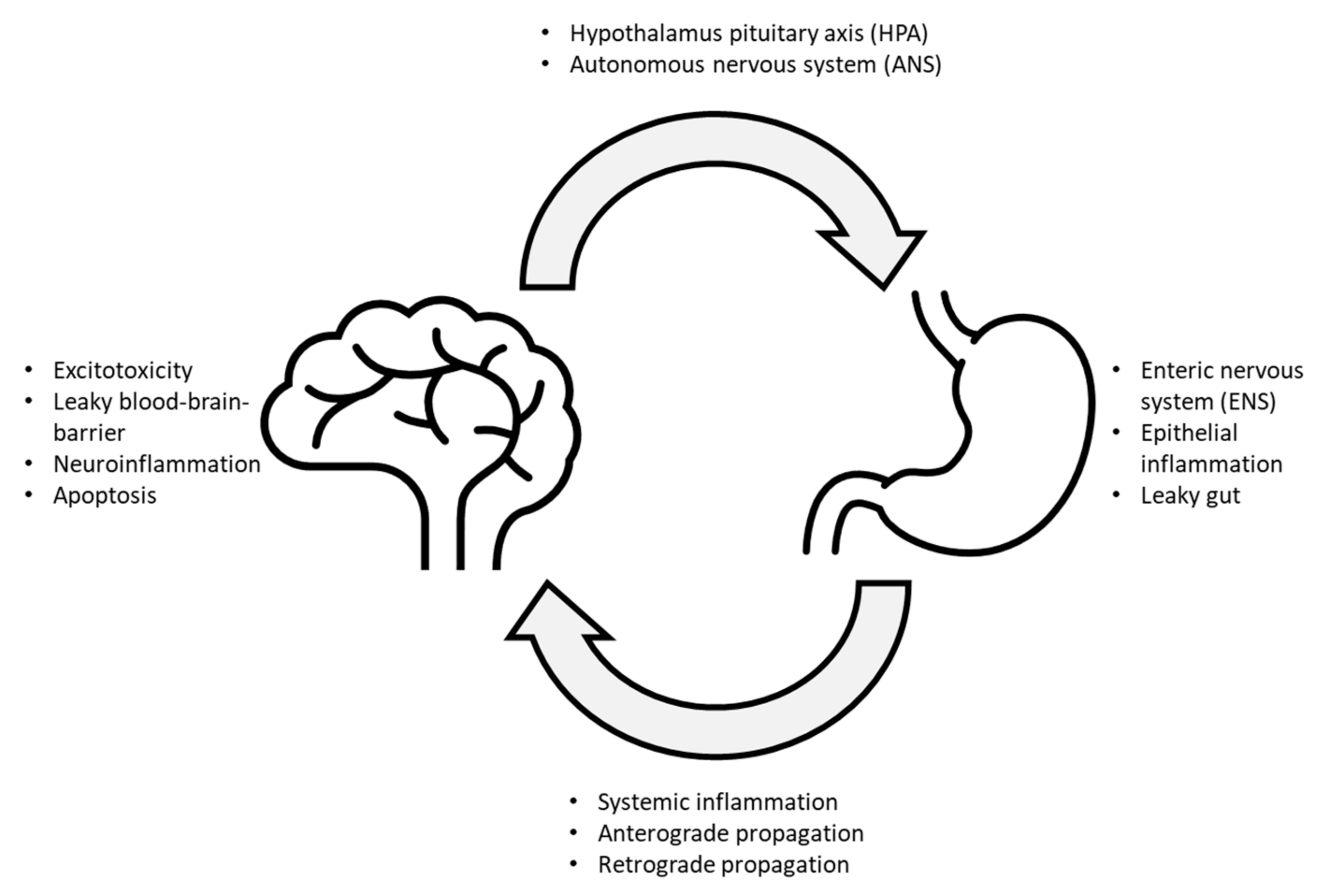
:1. Introduction
2. Gut Microbiome Dysregulation in Neurodegenerative Conditions
2.1. Parkinson’s Disease (PD)
2.1.1. PD Pathology
2.1.2. Microbes Associated with PD
2.1.3. Current Evidence for Microbe-Related Treatment for PD Patients
2.2. Alzheimer’s Disease (AD)
2.2.1. AD Pathology
2.2.2. Microbes Associated with AD
2.2.3. Current Evidence for Microbe-Related Treatment of AD
3. Impact of Diet on Gut Microbiome Composition
3.1. Diet(s) That Are Positively Associated with Neurodegenerative Diseases—Current Evidence for Microbe-Related Treatment of AD
3.2. Diets That Are Negatively Associated with Neurodegenerative Diseases
3.2.1. Ketogenic Diet (KD)
3.2.2. Mediterranean Diet
3.2.3. Plant-Based Diet
3.2.4. Caloric Restriction Diet (CR)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar-Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut Microbiome and Aging: Physiological and Mechanistic Insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Surathu, A.; Raplee, I.; Chockalingam, A.; Stewart, S.; Walker, L.; Sacks, L.; Patel, V.; Li, Z.; Rouse, R. The Effect of Antibiotics on the Gut Microbiome: A Metagenomics Analysis of Microbial Shift and Gut Antibiotic Resistance in Antibiotic Treated Mice. BMC Genom. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Budi, N.; Safdar, N.; Rose, W.E. Treatment Issues in Recurrent Clostridioides Difficile Infections and the Possible Role of Germinants. FEMS Microbes 2020, 1, xtaa001. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Solbiati, J.; Frias-Lopez, J. The Effect of the Stress Hormone Cortisol on the Metatranscriptome of the Oral Microbiome. NPJ Biofilms Microbiomes 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Ceppa, F.A.; Izzo, L.; Sardelli, L.; Raimondi, I.; Tunesi, M.; Albani, D.; Giordano, C. Human Gut-Microbiota Interaction in Neurodegenerative Disorders and Current Engineered Tools for Its Modeling. Front. Cell. Infect. Microbiol. 2020, 10, 297. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation with Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid. Based Integr. Med. 2020, 25, 2515690X2095722. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The Progress of Gut Microbiome Research Related to Brain Disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wang, S.; Jia, W. Calorie Restriction and Its Impact on Gut Microbial Composition and Global Metabolism. Front. Med. 2018, 12, 634–644. [Google Scholar] [CrossRef] [Green Version]
- Man, W.K.; Tahirbegi, B.; Vrettas, M.D.; Preet, S.; Ying, L.; Vendruscolo, M.; De Simone, A.; Fusco, G. The Docking of Synaptic Vesicles on the Presynaptic Membrane Induced by α-Synuclein Is Modulated by Lipid Composition. Nat. Commun. 2021, 12, 927. [Google Scholar] [CrossRef]
- Ma, J.; Gao, J.; Wang, J.; Xie, A. Prion-Like Mechanisms in Parkinson’s Disease. Front. Neurosci. 2019, 13, 552. [Google Scholar] [CrossRef]
- Baba, M.; Nakajo, S.; Tu, P.H.; Tomita, T.; Nakaya, K.; Lee, V.M.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of Alpha-Synuclein in Lewy Bodies of Sporadic Parkinson’s Disease and Dementia with Lewy Bodies. Am. J. Pathol. 1998, 152, 879–884. [Google Scholar] [PubMed]
- Cookson, M.R. Parkinsonism Due to Mutations in PINK1, Parkin, and DJ-1 and Oxidative Stress and Mitochondrial Pathways. Cold Spring Harb. Perspect. Med. 2012, 2, a009415. [Google Scholar] [CrossRef] [Green Version]
- Nuytemans, K.; Theuns, J.; Cruts, M.; Van Broeckhoven, C. Genetic Etiology of Parkinson Disease Associated with Mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 Genes: A Mutation Update. Hum. Mutat. 2010, 31, 763–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s Disease: Possible Routes by Which Vulnerable Neuronal Types May Be Subject to Neuroinvasion by an Unknown Pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Reichmann, H.; Livermore, A.; Hummel, T. Olfactory Function in Idiopathic Parkinson’s Disease (IPD): Results from Cross-Sectional Studies in IPD Patients and Long-Term Follow-up of de-Novo IPD Patients. J. Neural Transm. 2002, 109, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Boesveldt, S.; Berendse, H.W.; Mackay-Sim, A.; Fleischmann, J.; Silburn, P.A.; Johnston, A.N.; Mellick, G.D.; Herting, B.; Reichmann, H.; et al. Prevalence of Smell Loss in Parkinson’s Disease—A Multicenter Study. Parkinsonism Relat. Disord. 2009, 15, 490–494. [Google Scholar] [CrossRef]
- Huang, H.; Xu, H.; Luo, Q.; He, J.; Li, M.; Chen, H.; Tang, W.; Nie, Y.; Zhou, Y. Fecal Microbiota Transplantation to Treat Parkinson’s Disease with Constipation: A Case Report. Medicine 2019, 98, e16163. [Google Scholar] [CrossRef]
- Frazzitta, G.; Ferrazzoli, D.; Folini, A.; Palamara, G.; Maestri, R. Severe Constipation in Parkinson’s Disease and in Parkinsonisms: Prevalence and Affecting Factors. Front. Neurol. 2019, 10, 621. [Google Scholar] [CrossRef]
- Yu, Q.-J.; Yu, S.-Y.; Zuo, L.-J.; Lian, T.-H.; Hu, Y.; Wang, R.-D.; Piao, Y.-S.; Guo, P.; Liu, L.; Jin, Z.; et al. Parkinson Disease with Constipation: Clinical Features and Relevant Factors. Sci. Rep. 2018, 8, 567. [Google Scholar] [CrossRef] [Green Version]
- Minguez-Castellanos, A.; Chamorro, C.E.; Escamilla-Sevilla, F.; Ortega-Moreno, A.; Rebollo, A.C.; Gomez-Rio, M.; Concha, A.; Munoz, D.G. Do Alpha-Synuclein Aggregates in Autonomic Plexuses Predate Lewy Body Disorders?: A Cohort Study. Neurology 2007, 68, 2012–2018. [Google Scholar] [CrossRef]
- Yan, F.; Chen, Y.; Li, M.; Wang, Y.; Zhang, W.; Chen, X.; Ye, Q. Gastrointestinal Nervous System α-Synuclein as a Potential Biomarker of Parkinson Disease. Medicine 2018, 97, e11337. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Hiniker, A.; Kuo, Y.-M.; Nussbaum, R.L.; Liddle, R.A. α-Synuclein in Gut Endocrine Cells and Its Implications for Parkinson’s Disease. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [Green Version]
- Park, B.S.; Lee, J.-O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Manning, B.J.; Wu, Q.D.; Blankson, S.; Bouchier-Hayes, D.; Redmond, H.P. Endotoxin/Lipopolysaccharide Activates NF-Kappa B and Enhances Tumor Cell Adhesion and Invasion through a Beta 1 Integrin-Dependent Mechanism. J. Immunol. 2003, 170, 795–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Inflammation and the Blood Microvascular System. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef] [PubMed]
- Püntener, U.; Booth, S.G.; Perry, V.H.; Teeling, J.L. Long-Term Impact of Systemic Bacterial Infection on the Cerebral Vasculature and Microglia. J. Neuroinflamm. 2012, 9, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Lv, G.; Lee, J.S.; Jung, B.C.; Masuda-Suzukake, M.; Hong, C.-S.; Valera, E.; Lee, H.-J.; Paik, S.R.; Hasegawa, M.; et al. Exposure to Bacterial Endotoxin Generates a Distinct Strain of α-Synuclein Fibril. Sci. Rep. 2016, 6, 30891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyvärinen, T.; Hagman, S.; Ristola, M.; Sukki, L.; Veijula, K.; Kreutzer, J.; Kallio, P.; Narkilahti, S. Co-Stimulation with IL-1β and TNF-α Induces an Inflammatory Reactive Astrocyte Phenotype with Neurosupportive Characteristics in a Human Pluripotent Stem Cell Model System. Sci. Rep. 2019, 9, 16944. [Google Scholar] [CrossRef]
- Wang, B.; Su, C.-J.; Liu, T.-T.; Zhou, Y.; Feng, Y.; Huang, Y.; Liu, X.; Wang, Z.-H.; Chen, L.-H.; Luo, W.-F.; et al. The Neuroprotection of Low-Dose Morphine in Cellular and Animal Models of Parkinson’s Disease Through Ameliorating Endoplasmic Reticulum (ER) Stress and Activating Autophagy. Front. Mol. Neurosci. 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Gross, A.; Vouri, S.M.; Camacho-Soto, A.; Willis, A.W.; Searles Nielsen, S. Immunosuppressants and Risk of Parkinson Disease. Ann. Clin. Transl. Neurol. 2018, 5, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.-M.; et al. Infiltration of CD4+ Lymphocytes into the Brain Contributes to Neurodegeneration in a Mouse Model of Parkinson Disease. J. Clin. Invest. 2009, 119, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of Inflammasome by Aggregated α-Synuclein, an Inflammatory Response in Synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef] [Green Version]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-Seeded α-Synuclein Fibrils Promote Gut Dysfunction and Brain Pathology Specifically in Aged Mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Milan Manani, S.; Virzì, G.M.; Giuliani, A.; Baretta, M.; Corradi, V.; De Cal, M.; Biasi, C.; Crepaldi, C.; Ronco, C. Lipopolysaccharide Evaluation in Peritoneal Dialysis Patients with Peritonitis. Blood Purif. 2020, 49, 434–439. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased Intestinal Permeability Correlates with Sigmoid Mucosa Alpha-Synuclein Staining and Endotoxin Exposure Markers in Early Parkinson’s Disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [Green Version]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A Gut Bacterial Amyloid Promotes α-Synuclein Aggregation and Motor Impairment in Mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Alhadhrami, G.; Huber, J.T.; Higginbotham, G.E.; Harper, J.M. Nutritive Value of High Moisture Alfalfa Hay Preserved with Urea. J. Dairy Sci. 1989, 72, 972–979. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural Changes in the Gut Microbiome of Constipated Patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef] [Green Version]
- Haikal, C.; Chen, Q.-Q.; Li, J.-Y. Microbiome Changes: An Indicator of Parkinson’s Disease? Transl. Neurodegener. 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Segain, J.-P. Butyrate Inhibits Inflammatory Responses through NFkappa B Inhibition: Implications for Crohn’s Disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium Butyrate Exerts Protective Effect against Parkinson’s Disease in Mice via Stimulation of Glucagon like Peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef]
- Qiao, C.-M.; Sun, M.-F.; Jia, X.-B.; Li, Y.; Zhang, B.-P.; Zhao, L.-P.; Shi, Y.; Zhou, Z.-L.; Zhu, Y.-L.; Cui, C.; et al. Sodium Butyrate Exacerbates Parkinson’s Disease by Aggravating Neuroinflammation and Colonic Inflammation in MPTP-Induced Mice Model. Neurochem. Res. 2020, 45, 2128–2142. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.R.; Marshall, B. Unidentified Curved Bacilli on Gastric Epithelium in Active Chronic Gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar]
- Polk, D.B.; Peek, R.M. Helicobacter Pylori: Gastric Cancer and Beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Çamcı, G.; Oğuz, S. Association between Parkinson’s Disease and Helicobacter Pylori. J. Clin. Neurol. 2016, 12, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Yamada, T. Viral Etiology for Parkinson’s Disease—A Possible Role of Influenza A Virus Infection. Jpn. J. Infect. Dis. 1999, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Boltz, D.A.; Webster, R.G.; Smeyne, R.J. Viral Parkinsonism. Biochim. Biophys. Acta 2009, 1792, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, L.A.; Vilensky, J.A. Encephalitis Lethargica: 100 Years after the Epidemic. Brain 2017, 140, 2246–2251. [Google Scholar] [CrossRef]
- Maurizi, C.P. Influenza Caused Epidemic Encephalitis (Encephalitis Lethargica): The Circumstantial Evidence and a Challenge to the Nonbelievers. Med. Hypotheses 2010, 74, 798–801. [Google Scholar] [CrossRef]
- Valero-Pacheco, N.; Arriaga-Pizano, L.; Ferat-Osorio, E.; Mora-Velandia, L.M.; Pastelin-Palacios, R.; Villasís-Keever, M.Á.; Alpuche-Aranda, C.; Sánchez-Torres, L.E.; Isibasi, A.; Bonifaz, L.; et al. PD-L1 Expression Induced by the 2009 Pandemic Influenza A(H1N1) Virus Impairs the Human T Cell Response. Clin. Dev. Immunol. 2013, 2013, 989673. [Google Scholar] [CrossRef]
- Osborne, O.; Peyravian, N.; Nair, M.; Daunert, S.; Toborek, M. The Paradox of HIV Blood–Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies. Trends Neurosci. 2020, 43, 695–708. [Google Scholar] [CrossRef]
- Ren, R.; Racaniello, V.R. Poliovirus Spreads from Muscle to the Central Nervous System by Neural Pathways. J. Infect. Dis. 1992, 166, 747–752. [Google Scholar] [CrossRef]
- Young, V.A.; Rall, G.F. Making It to the Synapse: Measles Virus Spread in and among Neurons. Curr. Top. Microbiol. Immunol. 2009, 330, 3–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L.; et al. COVID-19 and Possible Links with Parkinson’s Disease and Parkinsonism: From Bench to Bedside. NPJ Parkinsons Dis. 2020, 6, 18. [Google Scholar] [CrossRef]
- Loewy, A.D. Viruses as Transneuronal Tracers for Defining Neural Circuits. Neurosci. Biobehav. Rev. 1998, 22, 679–684. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional Implications of Microbial and Viral Gut Metagenome Changes in Early Stage L-DOPA-Naïve Parkinson’s Disease Patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef]
- Mertsalmi, T.H.; Pekkonen, E.; Scheperjans, F. Antibiotic Exposure and Risk of Parkinson’s Disease in Finland: A Nationwide Case-Control Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 431–442. [Google Scholar] [CrossRef]
- Cleophas, M.C.P.; Ratter, J.M.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.S.; Netea, M.G.; Joosten, L.A.B. Effects of Oral Butyrate Supplementation on Inflammatory Potential of Circulating Peripheral Blood Mononuclear Cells in Healthy and Obese Males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; van Dijk, G.; El Aidy, S. Gut Bacterial Tyrosine Decarboxylases Restrict Levels of Levodopa in the Treatment of Parkinson’s Disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.-G.; Kim, T.K.; Suk, H.Y.; Jung, S.; Jo, D.-G. White Matter and Neurological Disorders. Arch. Pharm. Res. 2020, 43, 920–931. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The Genetic Landscape of Alzheimer Disease: Clinical Implications and Perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Attems, J.; Jellinger, K.A. The Overlap between Vascular Disease and Alzheimer’s Disease—Lessons from Pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Kapasi, A.; Schneider, J.A. Vascular Contributions to Cognitive Impairment, Clinical Alzheimer’s Disease, and Dementia in Older Persons. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2016, 1862, 878–886. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Drews, A.; Flint, J.; Shivji, N.; Jönsson, P.; Wirthensohn, D.; De Genst, E.; Vincke, C.; Muyldermans, S.; Dobson, C.; Klenerman, D. Individual Aggregates of Amyloid Beta Induce Temporary Calcium Influx through the Cell Membrane of Neuronal Cells. Sci. Rep. 2016, 6, 31910. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Zhang, M.; Gu, R.; Xu, G.; Wu, H. Activated Microglia Induce the Production of Reactive Oxygen Species and Promote Apoptosis of Co-Cultured Retinal Microvascular Pericytes. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 777–788. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Cuchillo-Ibanez, I.; Seereeram, A.; Byers, H.L.; Leung, K.; Ward, M.A.; Anderton, B.H.; Hanger, D.P. Phosphorylation of Tau Regulates Its Axonal Transport by Controlling Its Binding to Kinesin. FASEB J. 2008, 22, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Combs, B.; Mueller, R.L.; Morfini, G.; Brady, S.T.; Kanaan, N.M. Tau and Axonal Transport Misregulation in Tauopathies. Adv. Exp. Med. Biol. 2019, 1184, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Aducanumab Still Needs to Prove Itself, Researchers Say/ALZFORUM. Available online: https://www.alzforum.org/news/research-news/aducanumab-still-needs-prove-itself-researchers-say (accessed on 5 July 2021).
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Jackson, R.; Paul, G.; Shi, J.; Sabbagh, M. Why Do Trials for Alzheimer’s Disease Drugs Keep Failing? A Discontinued Drug Perspective for 2010–2015. Expert Opin. Investig. Drugs 2017, 26, 735–739. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Killin, L.O.J.; Starr, J.M.; Shiue, I.J.; Russ, T.C. Environmental Risk Factors for Dementia: A Systematic Review. BMC Geriatr. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griciuc, A.; Patel, S.; Federico, A.N.; Choi, S.H.; Innes, B.J.; Oram, M.K.; Cereghetti, G.; McGinty, D.; Anselmo, A.; Sadreyev, R.I.; et al. TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron 2019, 103, 820–835.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota Is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. JAD 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [Green Version]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.R. Role of Escherichia coli Curli Operons in Directing Amyloid Fiber Formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherny, I.; Rockah, L.; Levy-Nissenbaum, O.; Gophna, U.; Ron, E.Z.; Gazit, E. The Formation of Escherichia Coli Curli Amyloid Fibrils Is Mediated by Prion-like Peptide Repeats. J. Mol. Biol. 2005, 352, 245–252. [Google Scholar] [CrossRef]
- Reichhardt, C.; Lim, J.Y.; Rice, D.; Fong, J.N.; Cegelski, L. Structure and Function of Bacterial Biofilms by Solid-State NMR. Biophys. J. 2014, 106, 192a. [Google Scholar] [CrossRef] [Green Version]
- Lundmark, K.; Westermark, G.T.; Olsen, A.; Westermark, P. Protein Fibrils in Nature Can Enhance Amyloid Protein a Amyloidosis in Mice: Cross-Seeding as a Disease Mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 6098–6102. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis Elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimers Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Friedland, R.P.; Chapman, M.R. The Role of Microbial Amyloid in Neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Mezö, C.; Dokalis, N.; Mossad, O.; Staszewski, O.; Neuber, J.; Yilmaz, B.; Schnepf, D.; de Agüero, M.G.; Ganal-Vonarburg, S.C.; Macpherson, A.J.; et al. Different Effects of Constitutive and Induced Microbiota Modulation on Microglia in a Mouse Model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 119. [Google Scholar] [CrossRef]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal Metabolite of a Gnotobiotic Mouse Transplanted with Gut Microbiota from a Patient with Alzheimer’s Disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; Sun, D.; Chen, S. Gut Microbiota: Implications in Alzheimer’s Disease. J. Clin. Med. 2020, 9, 42. [Google Scholar] [CrossRef]
- Wang, T.; Hu, X.; Liang, S.; Li, W.; Wu, X.; Wang, L.; Jin, F. Lactobacillus Fermentum NS9 Restores the Antibiotic Induced Physiological and Psychological Abnormalities in Rats. Benef. Microbes 2015, 6, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does Severity of Alzheimer’s Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic Supplementation in Patients with Alzheimer’s Dementia—An Explorative Intervention Study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-Producing Bacteria Supplemented in Vitro to Crohn’s Disease Patient Microbiota Increased Butyrate Production and Enhanced Intestinal Epithelial Barrier Integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef] [Green Version]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Schafer, M.J.; Sohn, J.; Vincentini, J.; Weiner, H.L.; Ginsberg, S.D.; Blaser, M.J. Calorie Restriction Slows Age-Related Microbiota Changes in an Alzheimer’s Disease Model in Female Mice. Sci. Rep. 2019, 9, 17904. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Frith, M.; Sidebottom, A.; Cao, Y.; Koval, J.; Chang, E.; Sisodia, S.S. Synergistic Depletion of Gut Microbial Consortia, but Not Individual Antibiotics, Reduces Amyloidosis in APPPS1-21 Alzheimer’s Transgenic Mice. Sci. Rep. 2020, 10, 8183. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Riccio, P.; Rossano, R. Diet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis. Neurotherapeutics 2018, 15, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tang, K.; Ma, J.; Zhou, L.; Liu, J.; Zeng, L.; Zhu, L.; Xu, P.; Chen, J.; Wei, K.; et al. Ketogenesis-Generated β-Hydroxybutyrate Is an Epigenetic Regulator of CD8+ T-Cell Memory Development. Nat. Cell Biol. 2020, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Barborka, C.J. Ketogenic Diet Treatment of Epilepsy in Adults. J. Am. Med. Assoc. 1928, 91, 73. [Google Scholar] [CrossRef]
- Maiorana, A.; Manganozzi, L.; Barbetti, F.; Bernabei, S.; Gallo, G.; Cusmai, R.; Caviglia, S.; Dionisi-Vici, C. Ketogenic Diet in a Patient with Congenital Hyperinsulinism: A Novel Approach to Prevent Brain Damage. Orphanet J. Rare Dis. 2015, 10, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Adherence to Mediterranean Diet and Risk of Developing Cognitive Disorders: An Updated Systematic Review and Meta-Analysis of Prospective Cohort Studies. Sci. Rep. 2017, 7, 41317. [Google Scholar] [CrossRef]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The Gut Microbiome Modulates the Protective Association between a Mediterranean Diet and Cardiometabolic Disease Risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.D.; Yanckello, L.M.; Chlipala, G.; Hammond, T.C.; McCulloch, S.D.; Parikh, I.; Sun, S.; Morganti, J.M.; Green, S.J.; Lin, A.-L. Dietary Inulin Alters the Gut Microbiome, Enhances Systemic Metabolism and Reduces Neuroinflammation in an APOE4 Mouse Model. PLoS ONE 2019, 14, e0221828. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-Virgin Olive Oil and the Gut-Brain Axis: Influence on Gut Microbiota, Mucosal Immunity, and Cardiometabolic and Cognitive Health. Nutr. Rev. 2021, nuaa148. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary Fats and the Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 194. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- McCarty, M.F. Does a Vegan Diet Reduce Risk for Parkinson’s Disease? Med. Hypotheses 2001, 57, 318–323. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary Fiber Intervention on Gut Microbiota Composition in Healthy Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Tidjani-Alou, M.; Khelaifia, S.; Bachar, D.; Lagier, J.-C.; Dione, N.; Brah, S.; Hugon, P.; Lombard, V.; Armougom, F.; et al. Increased Gut Redox and Depletion of Anaerobic and Methanogenic Prokaryotes in Severe Acute Malnutrition. Sci. Rep. 2016, 6, 26051.e21. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Armstrong, N.; Khelaifia, S.; Guilhot, E.; Richez, M.; Lagier, J.-C.; Dubourg, G.; Chabriere, E.; Raoult, D. The Antioxidants Glutathione, Ascorbic Acid and Uric Acid Maintain Butyrate Production by Human Gut Clostridia in The Presence of Oxygen In Vitro. Sci. Rep. 2020, 10, 7705. [Google Scholar] [CrossRef]
- Liu, H.-X.; Rocha, C.S.; Dandekar, S.; Wan, Y.-J.Y. Functional Analysis of the Relationship between Intestinal Microbiota and the Expression of Hepatic Genes and Pathways during the Course of Liver Regeneration. J. Hepatol. 2016, 64, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Hegelmaier, T.; Lebbing, M.; Duscha, A.; Tomaske, L.; Tönges, L.; Holm, J.B.; Bjørn Nielsen, H.; Gatermann, S.G.; Przuntek, H.; Haghikia, A. Interventional Influence of the Intestinal Microbiome Through Dietary Intervention and Bowel Cleansing Might Improve Motor Symptoms in Parkinson’s Disease. Cells 2020, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Clavel, T.; Fallani, M.; Lepage, P.; Levenez, F.; Mathey, J.; Rochet, V.; Sérézat, M.; Sutren, M.; Henderson, G.; Bennetau-Pelissero, C.; et al. Isoflavones and Functional Foods Alter the Dominant Intestinal Microbiota in Postmenopausal Women. J. Nutr. 2005, 135, 2786–2792. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xie, Q.; Kong, P.; Liu, L.; Sun, S.; Xiong, B.; Huang, B.; Yan, L.; Sheng, J.; Xiang, H. Polyphenol- and Caffeine-Rich Postfermented Pu-Erh Tea Improves Diet-Induced Metabolic Syndrome by Remodeling Intestinal Homeostasis in Mice. Infect. Immun. 2017, 86, e00601-17. [Google Scholar] [CrossRef] [Green Version]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Chen, Y. Polyphenol Supplementation Benefits Human Health via Gut Microbiota: A Systematic Review via Meta-Analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Hidalgo-Liberona, N.; González-Domínguez, R.; Vegas, E.; Riso, P.; Del Bo’, C.; Bernardi, S.; Peron, G.; Guglielmetti, S.; Gargari, G.; Kroon, P.A.; et al. Increased Intestinal Permeability in Older Subjects Impacts the Beneficial Effects of Dietary Polyphenols by Modulating Their Bioavailability. J. Agric. Food Chem. 2020, 68, 12476–12484. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.-E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent Fasting Interventions for Treatment of Overweight and Obesity in Adults: A Systematic Review and Meta-Analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural Modulation of Gut Microbiota in Life-Long Calorie-Restricted Mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, C.; Schubert, D. Heat Shock Partially Protects Rat Pheochromocytoma PC12 Cells from Amyloid β Peptide Toxicity. Neurosci. Lett. 1993, 154, 1–4. [Google Scholar] [CrossRef]
- Ehrenfried, J.A.; Evers, B.M.; Chu, K.U.; Townsend, C.M.; Thompson, J.C. Caloric Restriction Increases the Expression of Heat Shock Protein in the Gut. Ann. Surg. 1996, 223, 592–597. [Google Scholar] [CrossRef]
- Graff, J.; Kahn, M.; Samiei, A.; Gao, J.; Ota, K.T.; Rei, D.; Tsai, L.-H. A Dietary Regimen of Caloric Restriction or Pharmacological Activation of SIRT1 to Delay the Onset of Neurodegeneration. J. Neurosci. 2013, 33, 8951–8960. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Wang, D.; Ren, H.; Cai, K.; Chen, P.; Fang, C.; Shi, Z.; Zhang, P.; Wang, J.; Yang, H.; et al. Effect of Caloric Restriction on BMI, Gut Microbiota, and Blood Amino Acid Levels in Non-Obese Adults. Nutrients 2020, 12, 631. [Google Scholar] [CrossRef] [Green Version]
- Pifferi, F.; Terrien, J.; Marchal, J.; Dal-Pan, A.; Djelti, F.; Hardy, I.; Chahory, S.; Cordonnier, N.; Desquilbet, L.; Hurion, M.; et al. Caloric Restriction Increases Lifespan but Affects Brain Integrity in Grey Mouse Lemur Primates. Commun. Biol. 2018, 1, 30. [Google Scholar] [CrossRef] [Green Version]
- Redman, L.M.; Ravussin, E. Caloric Restriction in Humans: Impact on Physiological, Psychological, and Behavioral Outcomes. Antioxid. Redox Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Georgescu, D.; Ancusa, O.; Georgescu, L.; Ionita, I.; Reisz, D. Nonmotor Gastrointestinal Disorders in Older Patients with Parkinson&rsquos Disease: Is There Hope? Clin. Interv. Aging 2016, Volume 11, 1601–1608. [Google Scholar] [CrossRef] [Green Version]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of Probiotics for the Treatment of Constipation in Parkinson’s Disease Patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar] [PubMed]
- Lugtenberg, B. Composition and Function of the Outer Membrane of Escherichia Coli. Trends Biochem. Sci. 1981, 6, 262–266. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia Coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; de Souza, F.S.J.; Soto, G.; Meyer, C.; Alonso, G.D.; Flawiá, M.M.; Bravo-Almonacid, F.; Ayub, N.D.; Rodríguez-Concepción, M. Selective Pressure against Horizontally Acquired Prokaryotic Genes as a Driving Force of Plastid Evolution. Sci. Rep. 2016, 6, 19036. [Google Scholar] [CrossRef] [Green Version]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and Probiotics Attenuate the Development of Alzheimer’s Disease in Transgenic Mice: Role of Microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, A.; Solana, E.; Corpas, R.; Bartrés-Faz, D.; Pallàs, M.; Vina, J.; Sanfeliu, C.; Gomez-Cabrera, M.C. Long-Term Exercise Training Improves Memory in Middle-Aged Men and Modulates Peripheral Levels of BDNF and Cathepsin B. Sci. Rep. 2019, 9, 3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Fitzsimmons, B.; Vanajakumari, M.U.; Lee, K.; Jayaraman, A. Effect of Norepinephrine on Gut Bacterial Community Structure and Function. Faseb J. 2019, 33. [Google Scholar] [CrossRef]
- Antoni, M.H.; Cruess, D.G.; Cruess, S.; Lutgendorf, S.; Kumar, M.; Ironson, G.; Klimas, N.; Fletcher, M.A.; Schneiderman, N. Cognitive–Behavioral Stress Management Intervention Effects on Anxiety, 24-Hr Urinary Norepinephrine Output, and T-Cytotoxic/Suppressor Cells over Time among Symptomatic HIV-Infected Gay Men. J. Consult. Clin. Psychol. 2000, 68, 31–45. [Google Scholar] [CrossRef]
- Mudd, A.T.; Berding, K.; Wang, M.; Donovan, S.M.; Dilger, R.N. Serum Cortisol Mediates the Relationship between Fecal Ruminococcus and Brain N-Acetylaspartate in the Young Pig. Gut Microbes 2017, 8, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Abelson, J.L.; Liberzon, I.; Young, E.A.; Khan, S. Cognitive Modulation of the Endocrine Stress Response to a Pharmacological Challenge in Normal and Panic Disorder Subjects. Arch. Gen. Psychiatry 2005, 62, 668–675. [Google Scholar] [CrossRef] [Green Version]

| Disease | Upregulated/Downregulated | Mechanistic Pathway | Microbe Species |
|---|---|---|---|
| PD | Upregulated | Pro-inflammatory (Secrete LPS) | Lactobacillus [41] Akkermansia [41] Bifidobacterium [41] Enterobacteriaceae [42,43] |
| Methane production | Christensenella spp. [45] Methanobrevibacter [45] | ||
| Bacterial amyloid production | Escherichia coli [44] | ||
| Downregulated | Anti-inflammatory (Secrete SCFA) | Prevotellaceae [48] | |
| Butyrate production | Roseburia [49] Faecalibacterium [49] Blautia [49] | ||
| Mucin-degrading | Dorea [49] | ||
| AD | Upregulated | Pro-inflammatory (Secrete LPS) | H. pylori [86] A. muciniphila [86] Enterobacteriaceae [86,87] Lactobacillus [86] O. splanchnicus [87] B. fragilis [87] |
| Bacterial Aβ crosslink | Klebsiella spp. [87] Enterobacteriaceae, E. coli [88,89,90] | ||
| Mucin-degrading | Dorea [97] | ||
| Downregulated | Anti-inflammatory (Secrete SCFA) | Prevotellaceae [87] | |
| Butyrate production | Clostridium [87] Coprococcus [87] Roseburia [87] Faecalibacterium [87] | ||
| Modulation of gut microbiome profile | Lactobacillus fermentus [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.Y.; Yeo, X.Y.; Bae, H.-G.; Lee, D.P.S.; Ho, R.C.; Kim, J.E.; Jo, D.-G.; Jung, S. Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life 2021, 11, 698. https://doi.org/10.3390/life11070698
Tan LY, Yeo XY, Bae H-G, Lee DPS, Ho RC, Kim JE, Jo D-G, Jung S. Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life. 2021; 11(7):698. https://doi.org/10.3390/life11070698
Chicago/Turabian StyleTan, Li Yang, Xin Yi Yeo, Han-Gyu Bae, Delia Pei Shan Lee, Roger C. Ho, Jung Eun Kim, Dong-Gyu Jo, and Sangyong Jung. 2021. "Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases?" Life 11, no. 7: 698. https://doi.org/10.3390/life11070698
APA StyleTan, L. Y., Yeo, X. Y., Bae, H.-G., Lee, D. P. S., Ho, R. C., Kim, J. E., Jo, D.-G., & Jung, S. (2021). Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life, 11(7), 698. https://doi.org/10.3390/life11070698







