Neuroimaging Studies of Nonsuicidal Self-Injury in Youth: A Systematic Review
Abstract
:1. Introduction
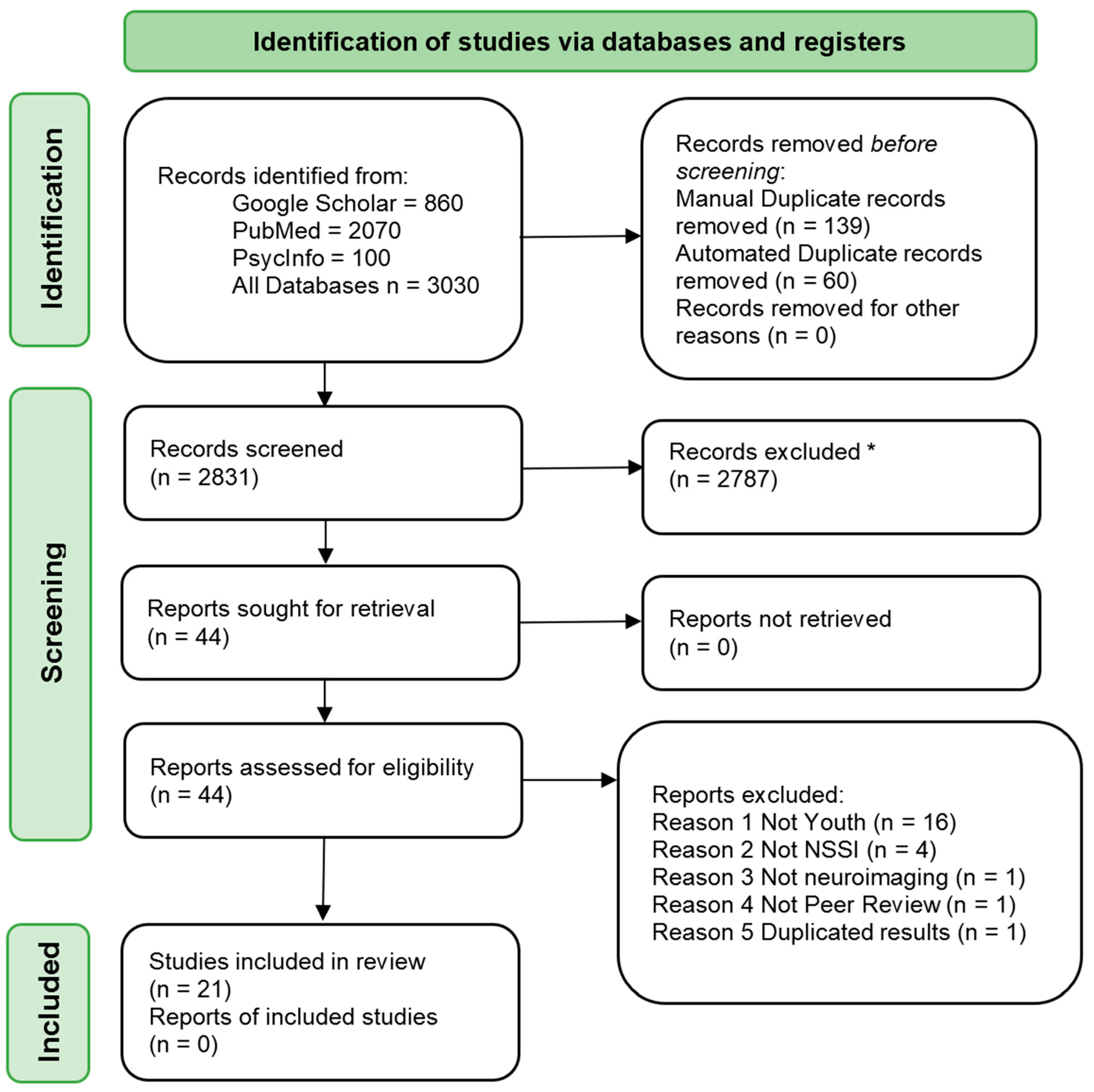
2. Materials and Methods
2.1. Search Strategy
2.2. PECO Strategy
- Population: samples with a mean age of up to 25 years old
- Exposure: NSSI
- Comparator: healthy control, psychiatric control, or none
- Outcome: neuroimaging (irrespective of the modality)
2.3. Screening of Abstracts for Eligibility
2.4. Study Selection
2.4.1. Inclusion Criteria
2.4.2. Exclusion Criteria for Studies
2.5. Data Extraction
2.6. Quality Appraisal
2.7. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Quality Assessment of Studies
3.3. Neuroimaging
3.3.1. Structural MRI Findings
3.3.2. Resting-State fMRI Findings
3.3.3. Task-Based fMRI Findings
Pain and Aversive Stimuli Processing
Reward Processing
Emotional Processing
Interpersonal and Self-Processing
Executive Function Processing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nock, M.K. Self-Injury. Annu. Rev. Clin. Psychol. 2010, 6, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Nock, M.K.; Joiner, T.E., Jr.; Gordon, K.H.; Lloyd-Richardson, E.; Prinstein, M.J. Non-Suicidal Self-Injury among Adolescents: Diagnostic Correlates and Relation to Suicide Attempts. Psychiatry Res. 2006, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.J.; Dixon-Gordon, K.L.; Austin, S.B.; Rodriguez, M.A.; Zachary Rosenthal, M.; Chapman, A.L. Non-Suicidal Self-Injury with and without Borderline Personality Disorder: Differences in Self-Injury and Diagnostic Comorbidity. Psychiatry Res. 2015, 230, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.; Shahrami, A.; Cali, S.; D’Mello, C.; Kako, M.; Palikucin-Reljin, A.; Savage, M.; Shaw, O.; Lunsky, Y. Self-Injury in Autism Spectrum Disorder and Intellectual Disability: Exploring the Role of Reactivity to Pain and Sensory Input. Brain Sci. 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westlund Schreiner, M.; Klimes-Dougan, B.; Begnel, E.D.; Cullen, K.R. Conceptualizing the Neurobiology of Non-Suicidal Self-Injury from the Perspective of the Research Domain Criteria Project. Neurosci. Biobehav. Rev. 2015, 57, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, R.C.; Plener, P.L. The Neurobiology of Non-Suicidal Self-Injury (NSSI): A Review. Suicidol. Online 2012, 3, 24–32. [Google Scholar]
- Glenn, C.R.; Klonsky, E.D. Nonsuicidal Self-Injury Disorder: An Empirical Investigation in Adolescent Psychiatric Patients. J. Clin. Child Adolesc. Psychol. 2013, 42, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association, American Psychiatric Association (Ed.) American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Swannell, S.V.; Martin, G.E.; Page, A.; Hasking, P.; St John, N.J. Prevalence of Nonsuicidal Self-Injury in Non-clinical Samples: Systematic Review, Meta-Analysis and Meta-Regression. Suicide Life Threat. Behav. 2014, 44, 273–303. [Google Scholar] [CrossRef]
- Whitlock, J.; Muehlenkamp, J.; Purington, A.; Eckenrode, J.; Barreira, P.; Baral Abrams, G.; Marchell, T.; Kress, V.; Girard, K.; Chin, C.; et al. Nonsuicidal Self-Injury in a College Population: General Trends and Sex Differences. J. Am. Coll. Health 2011, 59, 691–698. [Google Scholar] [CrossRef]
- Lloyd-Richardson, E.E.; Perrine, N.; Dierker, L.; Kelley, M.L. Characteristics and Functions of Non-Suicidal Self-Injury in a Community Sample of Adolescents. Psychol. Med. 2007, 37, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plener, P.L.; Schumacher, T.S.; Munz, L.M.; Groschwitz, R.C. The Longitudinal Course of Non-Suicidal Self-Injury and Deliberate Self-Harm: A Systematic Review of the Literature. Borderline Personal. Disord. Emot. Dysregulation 2015, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Kiekens, G.; Hasking, P.; Boyes, M.; Claes, L.; Mortier, P.; Auerbach, R.P.; Cuijpers, P.; Demyttenaere, K.; Green, J.G.; Kessler, R.C.; et al. The Associations between Non-Suicidal Self-Injury and First Onset Suicidal Thoughts and Behaviors. J. Affect. Disord. 2018, 239, 171–179. [Google Scholar] [CrossRef]
- Campisi, S.C.; Carducci, B.; Akseer, N.; Zasowski, C.; Szatmari, P.; Bhutta, Z.A. Suicidal Behaviours among Adolescents from 90 Countries: A Pooled Analysis of the Global School-Based Student Health Survey. BMC Public Health 2020, 20, 1102. [Google Scholar] [CrossRef]
- Garner, B.; Chanen, A.; Phillips, L.; Velakoulis, D.; Wood, S.; Jackson, H.; Pantelis, C.; Mcgorry, P. Pituitary Volume in Teenagers with First-Presentation Borderline Personality Disorder. Psychiatry Res. 2008, 156, 257–261. [Google Scholar] [CrossRef]
- Jovev, M.; Garner, B.; Phillips, L.; Velakoulis, D.; Wood, S.J.; Jackson, H.J.; Pantelis, C.; McGorry, P.D.; Chanen, A.M. An MRI Study of Pituitary Volume and Parasuicidal Behavior in Teenagers with First-Presentation Borderline Personality Disorder. Psychiatry Res. Neuroimaging 2008, 162, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Niedtfeld, I.; Schulze, L.; Kirsch, P.; Herpertz, S.C.; Bohus, M.; Schmahl, C. Affect Regulation and Pain in Borderline Personality Disorder: A Possible Link to the Understanding of Self-Injury. Biol. Psychiatry 2010, 68, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Rootes-Murdy, K.; Bastidas, D.M.; Nee, D.E.; Franklin, J.C. Brain Differences Associated with Self-Injurious Thoughts and Behaviors: A Meta-Analysis of Neuroimaging Studies. Sci. Rep. 2020, 10, 2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auerbach, R.P.; Pagliaccio, D.; Allison, G.O.; Alqueza, K.L.; Alonso, M.F. Neural Correlates Associated With Suicide and Nonsuicidal Self-Injury in Youth. Biol. Psychiatry 2021, 89, 119–133. [Google Scholar] [CrossRef]
- Domínguez-Baleón, C.; Gutiérrez-Mondragón, L.F.; Campos-González, A.I.; Rentería, M.E. Neuroimaging Studies of Suicidal Behavior and Non-Suicidal Self-Injury in Psychiatric Patients: A Systematic Review. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef]
- Carter, G.; Page, A.; Large, M.; Hetrick, S.; Milner, A.J.; Bendit, N.; Walton, C.; Draper, B.; Hazell, P.; Fortune, S.; et al. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guideline for the Management of Deliberate Self-Harm. Aust. N. Z. J. Psychiatry 2016, 50, 939–1000. [Google Scholar] [CrossRef]
- Hooley, J.M. Decreased Amygdalar Activation to NSSI-Stimuli in People Who Engage in NSSI: A Neuroimaging Pilot Study. Front. Psychiatry 2020, 11, 14. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handb. Syst. Rev. Interv. 2019. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.C.; Walker, J.C.; Teresi, G.I.; Kulla, A.; Kirshenbaum, J.S.; Gifuni, A.J.; Singh, M.K.; Gotlib, I.H. Default Mode and Salience Network Alterations in Suicidal and Non-Suicidal Self-Injurious Thoughts and Behaviors in Adolescents with Depression. Transl. Psychiatry 2021, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.M.; Perini, I.; Gustafsson, P.A.; Hamilton, J.P.; Kämpe, R.; Heilig, M.; Zetterqvist, M. Psychophysiological and Neural Support for Enhanced Emotional Reactivity in Female Adolescents With Nonsuicidal Self-Injury. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020. [Google Scholar] [CrossRef]
- Westlund Schreiner, M.; Mueller, B.A.; Klimes-Dougan, B.; Begnel, E.D.; Fiecas, M.; Hill, D.; Lim, K.O.; Cullen, K.R. White Matter Microstructure in Adolescents and Young Adults With Non-Suicidal Self-Injury. Front. Psychiatry 2019, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.A.; Schreiner, M.W.; Hunt, R.H.; Mueller, B.A.; Klimes-Dougan, B.; Thomas, K.M.; Cullen, K.R. Alexithymia Is Associated with Neural Reactivity to Masked Emotional Faces in Adolescents Who Self-Harm. J. Affect. Disord. 2019, 249, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Perini, I.; Gustafsson, P.A.; Hamilton, J.P.; Kämpe, R.; Mayo, L.M.; Heilig, M.; Zetterqvist, M. Brain-Based Classification of Negative Social Bias in Adolescents With Nonsuicidal Self-Injury: Findings From Simulated Online Social Interaction. EClinicalMedicine 2019, 13, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, J.A.; Thompson, J.C.; Forbes, E.E.; Chaplin, T.M. Adolescents’ Reward-Related Neural Activation: Links to Thoughts of Nonsuicidal Self-Injury. Suicide Life Threat. Behav. 2019, 49, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.R.; Schreiner, M.W.; Klimes-Dougan, B.; Eberly, L.E.; LaRiviere, L.L.; Lim, K.O.; Camchong, J.; Mueller, B.A. Neural Correlates of Clinical Improvement in Response to N-Acetylcysteine in Adolescents with Non-Suicidal Self-Injury. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109778. [Google Scholar] [CrossRef]
- Santamarina-Perez, P.; Romero, S.; Mendez, I.; Leslie, S.M.; Packer, M.M.; Sugranyes, G.; Picado, M.; Font, E.; Moreno, E.; Martinez, E.; et al. Fronto-Limbic Connectivity as a Predictor of Improvement in Nonsuicidal Self-Injury in Adolescents Following Psychotherapy. J. Child Adolesc. Psychopharmacol. 2019, 29, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, M.K. Prefrontal Cortex Activation during Cognitive Interference in Nonsuicidal Self-Injury. Psychiatry Res. 2018, 277, 28–38. [Google Scholar] [CrossRef]
- Malejko, K.; Neff, D.; Brown, R.C.; Plener, P.L.; Bonenberger, M.; Abler, B.; Grön, G.; Graf, H. Somatosensory Stimulus Intensity Encoding in Borderline Personality Disorder. Front. Psychol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Reichl, C.; Scheu, F.; Bykova, A.; Parzer, P.; Resch, F.; Brunner, R.; Kaess, M. Regional Grey Matter Volume Reduction in Adolescents Engaging in Non-Suicidal Self-Injury. Psychiatry Res. Neuroimaging 2018, 280, 48–55. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Sauder, C.L.; Derbidge, C.M.; Uyeji, L.L. Self-Injuring Adolescent Girls Exhibit Insular Cortex Volumetric Abnormalities That Are Similar to Those Seen in Adults with Borderline Personality Disorder. Dev. Psychopathol. 2019, 31, 1203–1212. [Google Scholar] [CrossRef]
- Westlund Schreiner, M.; Klimes-Dougan, B.; Mueller, B.A.; Eberly, L.E.; Reigstad, K.M.; Carstedt, P.A.; Thomas, K.M.; Hunt, R.H.; Lim, K.O.; Cullen, K.R. Multi-Modal Neuroimaging of Adolescents with Non-Suicidal Self-Injury: Amygdala Functional Connectivity. J. Affect. Disord. 2017, 221, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C. Differential Neural Processing of Social Exclusion and Inclusion in Adolescents with Non-Suicidal Self-Injury and Young Adults with Borderline Personality Disorder. Front. Psychiatry 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groschwitz, R.C. Differential Neural Processing of Social Exclusion in Adolescents with Non-Suicidal Self-Injury: An FMRI Study. Psychiatry Res. 2016, 255, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Quevedo, K. The Neurobiology of Self-Knowledge in Depressed and Self-Injurious Youth. Psychiatry Res. 2016, 254, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Sauder, C.L.; Derbidge, C.M.; Beauchaine, T.P. Neural Responses to Monetary Incentives among Self-Injuring Adolescent Girls. Dev. Psychopathol. 2016, 28, 277–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonenberger, M.; Plener, P.L.; Groschwitz, R.C.; Grön, G.; Abler, B. Differential Neural Processing of Unpleasant Haptic Sensations in Somatic and Affective Partitions of the Insula in Non-Suicidal Self-Injury (NSSI). Psychiatry Res. Neuroimaging 2015, 234, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Osuch, E. Functional MRI of Pain Application in Youth Who Engaged in Repetitive Non-Suicidal Self-Injury vs. Psychiatric Controls. Psychiatry Res. 2014, 223, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Plener, P.L.; Bubalo, N.; Fladung, A.K.; Ludolph, A.G.; Lulé, D. Prone to Excitement: Adolescent Females with Non-Suicidal Self-Injury (NSSI) Show Altered Cortical Pattern to Emotional and NSS-Related Material. Psychiatry Res. 2012, 203, 146–152. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 30 June 2021).
- Tarnutzer, A.A.; Straumann, D.; Brugger, P.; Feddermann-Demont, N. Persistent Effects of Playing Football and Associated (Subconcussive) Head Trauma on Brain Structure and Function: A Systematic Review of the Literature. Br. J. Sports Med. 2017, 51, 1592–1604. [Google Scholar] [CrossRef]
- McPheeters, M.L.; Kripalani, S.; Peterson, N.B.; Idowu, R.T.; Jerome, R.N.; Potter, S.A.; Andrews, J.C. Closing the Quality Gap: Revisiting the State of the Science (Vol. 3: Quality Improvement Interventions to Address Health Disparities). Evid. ReportTechnol. Assess. 2012, 208, 1–475. [Google Scholar]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: North Adelaide, SA, Australia, 2020; ISBN 978-0-648-84880-6. Available online: https://doi.org/10.46658/JBIMES-20-04 (accessed on 30 June 2021).
- Craig, A.D.B. Significance of the Insula for the Evolution of Human Awareness of Feelings from the Body. Ann. N. Y. Acad. Sci. 2011, 1225, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Kross, E.; Berman, M.G.; Mischel, W.; Smith, E.E.; Wager, T.D. Social Rejection Shares Somatosensory Representations with Physical Pain. Proc. Natl. Acad. Sci. USA 2011, 108, 6270–6275. [Google Scholar] [CrossRef] [Green Version]
- Wessa, M.; Lois, G. Brain Functional Effects of Psychopharmacological Treatment in Major Depression: A Focus on Neural Circuitry of Affective Processing. Curr. Neuropharmacol. 2015, 13, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, J.A.; O’Doherty, J.; Dolan, R.J. Encoding Predictive Reward Value in Human Amygdala and Orbitofrontal Cortex. Science 2003, 301, 1104–1107. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.D.; Cheung, C.K.; Choi, W. Cyberostracism: Effects of Being Ignored over the Internet. J. Pers. Soc. Psychol. 2000, 79, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Hannay, H.J.; Howieson, D.B.; Loring, D.W. Neuropsychological Assessment, 4th ed.; Oxford Univ. Press: Oxford, UK, 2004; ISBN 978-0-19-511121-7. [Google Scholar]
- Ougrin, D.; Tranah, T.; Stahl, D.; Moran, P.; Asarnow, J.R. Therapeutic Interventions for Suicide Attempts and Self-Harm in Adolescents: Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 97–107.e2. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, C.P.; Strauman, T.J. Neuroimaging for Psychotherapy Research: Current Trends. Psychother. Res. J. Soc. Psychother. Res. 2015, 25, 185–213. [Google Scholar] [CrossRef] [Green Version]
- Calder, A.J.; Lawrence, A.D.; Young, A.W. Neuropsychology of Fear and Loathing. Nat. Rev. Neurosci. 2001, 2, 352–363. [Google Scholar] [CrossRef]
- Stevens, F.L. Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. The Orbitofrontal Cortex: Reward, Emotion and Depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- Hooley, J.M.; Franklin, J.C. Why Do People Hurt Themselves? A New Conceptual Model of Nonsuicidal Self-Injury. Clin. Psychol. Sci. 2018, 6, 428–451. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Krause-Utz, A.; Frost, R.; Winter, D.; Elzinga, B.M. Dissociation and Alterations in Brain Function and Structure: Implications for Borderline Personality Disorder. Curr. Psychiatry Rep. 2017, 19, 6. [Google Scholar] [CrossRef] [Green Version]
- Calati, R.; Bensassi, I.; Courtet, P. The Link between Dissociation and Both Suicide Attempts and Non-Suicidal Self-Injury: Meta-Analyses. Psychiatry Res. 2017, 251, 103–114. [Google Scholar] [CrossRef]
- Linehan, M.M. Cognitive-Behavioral Treatment of Borderline Personality Disorder; Guilford Publications: New York, NY, USA, 2018; ISBN 978-1-4625-3920-8. [Google Scholar]
- Choi-Kain, L.; Wilks, C.; Ilagan, G.; Iliakis, E. Dialectical Behavior Therapy for Early Life Trauma. Curr. Treat. Options Psychiatry 2021. [Google Scholar] [CrossRef]
- Kleindienst, N.; Priebe, K.; Görg, N.; Dyer, A.; Steil, R.; Lyssenko, L.; Winter, D.; Schmahl, C.; Bohus, M. State Dissociation Moderates Response to Dialectical Behavior Therapy for Posttraumatic Stress Disorder in Women with and without Borderline Personality Disorder. Eur. J. Psychotraumatology 2016, 7. [Google Scholar] [CrossRef]
- Hamza, C.A.; Willoughby, T.; Heffer, T. Impulsivity and Nonsuicidal Self-Injury: A Review and Meta-Analysis. Clin. Psychol. Rev. 2015, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Van Leijenhorst, L.; Zanolie, K.; Van Meel, C.S.; Westenberg, P.M.; Rombouts, S.A.R.B.; Crone, E.A. What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cereb. Cortex N. Y. N 1991 2010, 20, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MPhil, S.U. Two Pathways to Self-Harm in Adolescence. Adolesc. Psychiatry 2021, 10. [Google Scholar] [CrossRef]
- Victor, S.E.; Scott, L.N.; Stepp, S.D.; Goldstein, T.R. I Want You to Want Me: Interpersonal Stress and Affective Experiences as Within-Person Predictors of Nonsuicidal Self-Injury and Suicide Urges in Daily Life. Suicide Life Threat. Behav. 2019, 49, 1157–1177. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.; Pane, H.; Ha, C.; Venta, A.; Patel, A.B.; Sturek, J.; Fonagy, P. Theory of Mind and Emotion Regulation Difficulties in Adolescents With Borderline Traits. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevets, W.C.; Savitz, J.; Trimble, M. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef]
- Stanley, B.; Sher, L.; Wilson, S.; Ekman, R.; Huang, Y.; Mann, J.J. Non-Suicidal Self-Injurious Behavior, Endogenous Opioids and Monoamine Neurotransmitters. J. Affect. Disord. 2010, 124, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Marín, O. Developmental Timing and Critical Windows for the Treatment of Psychiatric Disorders. Nat. Med. 2016, 22, 1229–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fair, D.A.; Dosenbach, N.U.F.; Church, J.A.; Cohen, A.L.; Brahmbhatt, S.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. Development of Distinct Control Networks through Segregation and Integration. Proc. Natl. Acad. Sci. USA 2007, 104, 13507–13512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, J.R.; Salum, G.A.; Gadelha, A.; Picon, F.A.; Pan, P.M.; Vieira, G.; Zugman, A.; Hoexter, M.Q.; Anés, M.; Moura, L.M.; et al. Age Effects on the Default Mode and Control Networks in Typically Developing Children. J. Psychiatr. Res. 2014, 58, 89–95. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | N (F) NSSI/Controls | Age (Mean ± SD) NSSI/Controls | Study Design | Imaging Modality | Paradigm | NSSI Scale | Mental Conditions | Medications | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Ho et al. 2021 [26] | 29 (20F)/21 (12F) | 16.34 ± 1.37/16.15 ± 1.29 | Case-control, HC | fMRI/whole brain | Resting-state | SITBI | MDD 100%, suicidal ideation 69%, suicide attempt 38% | 48% on medications | ↓ coherence in the ventral and anterior default mode and the insula salience networks / ↑ connectivity between the central executive and the default mode networks / ↓ coherence in the default mode and the insula salience networks was associated with higher past-month NSSI |
| Mayo et al. 2020 [27] | 30 (30F)/30 (30F) | 15.9 ± 0.79/16.4 ± 1.00 | Case-control, HC | fMRI/whole brain | Affective and NSSI-related pictures task | CANDI | Lifetime suicidal ideation 100%, lifetime suicide attempt 36.7%, MDD 50.0%, ADHD 50.0%, BPD 43.3%, social anxiety disorder 30.0%, autism spectrum disorder (high functioning) 13.3%, panic disorder 10.0%, eating disorder (unspecified) 10.0%, ODD/CD 10.0%, GAD 6.67%, atypical anorexia nervosa 6.67%, agoraphobia 3.30%, anxiety disorder (unspecified) 3.30%, PTSD 3.30%, anorexia nervosa 3.30% | No medication 63.3%, SSRI/SNRI 26.7%, SSRI/SNRI + methylphenidate 3.30%, antipsychotic 3.30%, SSRI/SNRI + neuroleptic 3.30% | No differences in self-reported affect during task / ↑ positive (e.g., zygomatic) and negative (e.g., corrugator) reactivity during task / + correlation between anterior insula response and the averaged electromyography magnitude |
| Hooley et al. 2020 [22] | 15 (15F)/15 (15F) | 21.27 ± 3.67/22.80 ± 3.28 | Case-control, HC | fMRI/ROI /amygdala, CC, OFC, NAcc, and VTA | Affective and NSSI-related pictures task | SITBI | BPD 86.67%, mood disorders 80.00%, anxiety disorders 53.33%, eating disorders 6.67%, past alcohol dependence 6.67%, suicide attempt 26.67% | NA | ↓ amygdala and ↑ CC and OFC activation to NSSI and negative images / ↑ amygdala and OFC activation to positive images |
| Schreiner et al. 2020 [28] | 28 (28F)/22 (22F) | 17.53 ± 2.36/17.69 ± 2.26 | Case-control, HC | Diffusion MRI/structural (white matter (TBSS)/whole brain | — | DSHI, ISAS | MDD 57%, GAD 29%, depression NOS 18%, PTSD 18%, Specific Phobia 11%, panic disorder 11%, anxiety disorder NOS 7%, OCD 7%, ADHD 7%, alcohol dependence 7%, social phobia 4%, eating disorder NOS 4%, no current disorder 18% | Currently medicated 43%, antidepressants 32%, stimulants 7%, antipsychotics 4%, anxiolytics/benzodiazepines 14%, other psychotropics 4% | ↓ generalized fractional anisotropy (GFA) in several white matter tracts, including the uncinate fasciculus, cingulum, bilateral superior and inferior longitudinal fasciculi, anterior thalamic radiation, callosal body, and corticospinal tract / ↓ GFA in the left cingulum was associated with NSSI duration / ↓ GFA in the left uncinate fasciculus was associated with higher levels of attentional impulsivity |
| Demers et al. 2019 [29] | 25 (25F)/No controls | 17.30 ± 2.35/No controls | Cross-sectional, No controls | fMRI/whole brain | Masked emotional facial expressions | DSHI, ISAS | Mood disorder 84%, anxiety disorder 48%, alcohol use disorder 4%, eating disorder 12.5%, PTSD 4% | Almost half the participants on medications (no specification) | Externally-oriented thinking, a facet of alexithymia, was related to differential reactivity to masked emotional faces in clusters in the right supramarginal and right inferior frontal gyri |
| Perini et al. 2019 [30] | 30 (30F)/30 (30F) | 15.9 ± 0.79/16.4 ± 1.00 | Case-control, HC | fMRI/whole brain | Simulated online game | CANDI | Suicidal ideation 100%, suicidal attempt 36.7%, MDD 50.0%, anxiety disorder 43.3%, PTSD 3.3%, borderline traits 43.3%, eating disorder 20.0%, ADHD 50.0%, autism (high functioning13.3%), ODD/CD 10.0% | SSRI/SNRI 26.7%, SSRI/SNRI + methylphenidate 3.3%, neuroleptic 3.3%, SSRI/SNRI + neuroleptic 3.3%, no medication 63.3% | Negative bias in processing social feedback from others / Brain regions that classified NSSI subjects and controls included dmPFC and sgACC cortices |
| Poon et al. 2019 [31] | 71 (33F)/No controls | 12.56 ± 0.65/No controls | Cross-sectional, No controls | fMRI/ROI/caudate, putamen, NAcc, vmPFC | Monetary reward | SITBI | No diagnosis 94.4%, MDD 2.8%, GAD 1.4%, ODD 1.4% | Medication use was an exclusion criterion. | ↑ activation in the bilateral putamen associated with NSSI thoughts |
| Cullen et al. 2019 [32] | 18 (18F)/No controls | 17.11 ± 2.07/No controls | Open-label trial, No Controls | fMRI/ROI/amygdala and the nucleus accumbens | Resting-state | DSHI, ISAS | NA | N-acetylcysteine 100%, other medications 22% | Reduction in NSSI frequency was associated with: ↓ in left amygdala connectivity between right SMA / ↑ in right amygdala connectivity between right inferior frontal cortex / ↓ in connectivity between right nucleus accumbens and left superior medial frontal cortex |
| Santamarina-Perez et al. 2019 [33] | 24 (21F)/16 (13F) | 15.42 ± 0.97/15.50 ± 1.10 | Open-label trial, HC | fMRI/ROI/amygdala and the mPFC | Resting-state | C-SSRS (specific items), SIQ-Jr | MDD 87%, anxiety disorder (any) 46%, eating disorder 33%, PTSD 29%, bipolar disorder 12,5%, PTSD 29% | Antipsychotics 75%, antidepressants 63%, lithium 8% | ↓ connectivity between the amygdala and the ACC, subcallosal cortex, and paracingulate gyrus / ↓ connectivity between the amygdala and the right planum temporale and right insula / ↓ connectivity between the mPFC and the precentral and postcentral gyri and the left insula / Stronger negative amygdala–prefrontal connectivity was associated with greater posttreatment improvement in NSSI / Greater positive baseline amygdala–brainstem connectivity was associated with NSSI improvement |
| Dahlgren et al. 2018 [34] | 15 (15F)/15 (15F) | 21.27 ± 3.67/22.80 ± 3.28 | Case-control, HC | fMRI/ROI/CC and dlPFC | Multi-source interference task | SITBI | BPD 86.7%, mood disorders 80.0%, anxiety disorders 53.34%, eating disorders 6.7%, alcohol dependence 6.7%, suicide attempt 26.67% | Antidepressants 16.6%, antipsychotics 6.7%, anxiolytics 6.7%, stimulants 3.34% | ↑ activation in CC and ↓ activation in dlPFC during task / dlPFC activation inversely correlated with emotional reactivity and impulsivity |
| Malejko et al. 2018 [35] | 15 (15F) (BPD)/15 (15F) (HC) | 23.33 ± 1.07/23.27 ± 1.11 | Case-control, HC | fMRI/whole brain | Unpleasant (but not painful) electric stimulation | FASM | BPD 100%, MDD 86.7%, PTSD 53.3%, dysthymia 13.3% | Antidepressants 86.7%, lithium 6.7% | Significant intensity-encoding neural activations were observed within the primary and secondary somatosensory cortex, the posterior insula, the posterior midcingulate cortex, and SMA in both HC and BPD / No significant between-group differences in intensity-encoding neural activations, even at lowered significance thresholds |
| Ando et al. 2018 [36] | 29 (29F)/21 (21F) | 15.9 ± 1.3/15.8 ± 1.1 | Case-control, HC | MRI (structural)/volume (Freesurfer)/ROI/lateral and medial PFC, OFC, ACC, insula, thalamus, hippocampus, and amygdala | — | SITBI | Mood disorders 82.8%; neurotic, stress-related and somatoform disorders 58.6%; suicide attempt 55.2%, BPD 45%; behavioral and emotional disorders with onset in childhood and adolescence 31.0%; mental and behavioral disorders due to psychoactive substance use 27.6%; behavioral syndromes associated with physiological disturbances and physical factors 17.2% | NA | ↓ regional grey matter volume in insula / Suggestion (not survived correction for multiple comparisons) of ↓volume in ACC / Even smaller ACC volume in adolescents engaging in NSSI with a history of suicide attempt in comparison to those with no history of suicide attempt |
| Beauchaine et al. 2018 [37] | 20 (20F)/20 (20F) | 15.70 ± 1.77/15.93 ± 2.03 | Case-control, HC | MRI (structural)/volume (VBM)/whole brain | — | L-SASI | 1.25 suicide attempts, 3 symptoms of BPD (averages) | NA | ↓ gray matter volumes in the insular cortex bilaterally and in the right inferior frontal gyrus / Insular and inferior frontal gyrus gray matter volumes correlated inversely with self-reported emotion dysregulation, over-and-above effects of psychopathology. |
| Schreiner at al. 2017 [38] | 25 (25F)/20 (20F) (RSFC); 24 (24F)/17(17F) (Task) | 17.57 ± 2.49/18.01 ± 2.08 (RSFC) 17.34 ± 2.44/17.98 ± 2.00 (Task) | Case-control, HC | fMRI/ROI/amygdala | Resting-state and emotion face-matching functional connectivity | DSHI, ISAS | RSFC and task, respectively: MDD 52%, 58%; depressive disorder NOS 20%, 13%; GAD 24%, 21%; anxiety disorder NOS 4%, 8%; social phobia 4%, 8%; specific phobia 12%, 8%; panic disorder (8%) (8%; PTSD 12%, 13%; OCD 8%, 4%; eating disorder NOS 4%, 4%; ADHD 4%, 4%; alcohol dependence 8%, 4%; no current disorder 20%, 21% | RSFC and task data, respectively: currently medicated 42%, 44%; antidepressants 29%, 35%; stimulants 4%,4%; antipsychotics 4%, 4%; anxiolytics/benzodiazepines 13%, 13%; other psychotropics 4%, 4% | Atypical amygdala–frontal connectivity driven by depression symptoms (RSFC and task) / Hyperconnectivity between amygdala and SMA independent of depression symptoms (RSFC) / Widespread amygdala–cortical connectivity anomalies (RSFC and task) |
| Brown et al. 2017 [39] | NSSI: 13 (10F) NSSI and BPD: 14 (14F) HC (adolescents): 15 (12F) HC (young adults): 17 (17F) | NSSI: 15.5 ± 2.0 NSSI and BPD: 23.6 ± 4.1 HC (adolescents): 14.5 ± 1.7 HC (young adults): 23.2 ± 4.4 | Case-control, HC, and Psychiatric Controls | fMRI/whole brain | “Cyberball” | SITBI | NSSI and BPD, respectively: major depression 100%, 100%; hyperkinetic disorder 23%, 0; eating disorder 15%, 7%; anxiety disorder 15%, 14%; PTSD 0, 50% | NSSI and BPD, respectively: antidepressants 15%, 86%; mood stabilizers 0, 7% | NSSI and BPD showed enhanced feelings of social exclusion as compared with HC / ↑ activation in the putamen during social exclusion versus inclusion in NSSI / ↑ activation in the ventral anterior CC during social exclusion in NSSI and BPD / ↑ activation in DLPFC, dmPFC, and the anterior insula during social inclusion as compared with a passive watching condition in BPD |
| Groschwitz et al. 2016 [40] | NSSI and depression: 14 (11F) Depression: 14 (11F) HC: 15 (12F) | NSSI and depression: 15.4 ± 1.9 Depression: 15.9 ± 1.6 HC: 14.5 ± 1.7 | Case-control, HC, and Psychiatric Controls | fMRI/whole brain | “Cyberball” | SITBI | NSSI and depression, and depression, respectively: major depression 100%, 100%; anxiety disorder 7%, 14%; PTSD 0, 29%; eating disorder 14%, 21%; ADHD 14%, 7%; conduct disorder 21%, 7% | NSSI and depression, respectively: antidepressants 14%, 50%; psychostimulants 7%, 0 | ↑ activation of the mPFC and the VLPFC in depressed adolescents with NSSI compared with depressed adolescents without NSSI and also compared with HC |
| Quevedo et al. 2016 [41] | NSSI: 50 (32F) Depression: 36 (17F) HC: 37 (18F) | NSSI: 14.94 ± 1.54 Depression: 14.77 ± 1.86 HC: 14.49 ± 1.53 | Case-control, HC, and Psychiatric Controls | fMRI/whole brain | Interpersonal self-processing task | K-SADS (item) | Depression: 100% in both clinical groups suicide ideation: NSSI > depression group | HC, depression and NSSI, respectively: antidepressants 0, 33.3%, 48%; antipsychotic 0, 0, 12%; mood stabilizers 5%, 0, 2%; stimulants 0, 11%, 12%; anxiolytic 0, 3%, 10% | ↑ activation in limbic areas, and anterior and posterior cortical midline structures in NSSI versus DEP and HC / ↑ activity in rostrolateral, frontal pole, and occipital cortex in HC versus NSSI and DEP / ↑ responses in amygdala, hippocampus, parahippocampus, and fusiform in NSSI when taking their mother’s perspective, which was negatively correlated with self-reports of the mother’s support of adolescent’s emotional distress in the NSSI group / ↑ precuneus and posterior cingulate cortex activity in NSSI during indirect self-processing from their classmates’ perspective |
| Sauder et al. 2016 [42] | 19 (19F)/19 (19F) | 15.93 ± 2.03/15.70 ± 1.77 | Case-control, HC | fMRI/ROI/striatum (caudate + putamen) and OFC | Monetary incentive delay task | L-SASI | Depression 47%, substance use disorder 16% | SSRI 26% | ↓ activation in striatal and OFC regions during anticipation of reward / ↓ bilateral amygdala activation during reward anticipation |
| Bonenberger et al. 2015 [43] | 14 (14F)/16 (16F) | 21.1 ± 2.51/23.4 ± 3.72 | Case-control, HC | fMRI/ROI/insula and somatosensory cortex | Unpleasant haptic electric stimulation | SITBI | BPD 21.4%, MDD 14.2%, agoraphobia 14.2% | No medication | Activation of the anterior insula was significantly modulated only in HC, but not in subjects with NSSI |
| Osuch et al. 2014 [44] | NSSI: 13 (10F) Non-NSSI: 15 (13F) | NSSI: 20 ± 2.4 Non-NSSI: 21 ± 1.8 | Case-control, Psychiatric Controls | fMRI/whole brain | Painfully “cold” and “cool” stimulus | SIMSv2; OSI | NSSI and non-NSSI, respectively: MDD 46%, 27%; bipolar type I 15%, 13%; bipolar type II 8%, 0; OCD 15%, 7%; GAD 8%, 20%; panic disorder 15%, 13%; social phobia 31%, 27%; specific phobia 15%, 7%; hypochondriasis 8%, 7%; PTSD 23%, 0; bulimia 0, 7%; adjustment disorder (past) 0, 7%; alcohol abuse 0, 7%; alcohol dependence 8%, 7%; drug abuse 15%, 13%; acute stress 0, 7% | NSSI: antidepressants (61.5%); mood stabilizer (7.7%); atypical antipsychotic (7.7%) Non-NSSI: antidepressants (53.3%); mood stabilizer (6.7%); MAO+modafinil (6.7%); benzodiazepine (6.7%) | ↑ activation in right midbrain/pons, culmen, amygdala, OFC and parahippocampal, inferior frontal and superior temporal gyri in NSSI / ↑ activation associated with a subjective sense of “relief” in areas associated with reward/pain and addiction, including thalamus, dorsal striatum, and anterior precuneus / ↓ functional connectivity between right OFC and anterior CC in NSSI youth (post hoc analysis) |
| Plener et al. 2012 [45] | 9 (9F)/9 (9F) | 15.2 ± 1.5/15.0± 0.9 | Case-control, HC | fMRI/whole brain | Affective and NSSI-related pictures task | OSI; FASM; SHBQ | MDD 66.7%, suicide attempt 54%, mild depression disorder 11.1%, dysthymia 11.1%, PTSD 11.1%, BPD 22.2%, combined personality disorder 11.1% | Antidepressant 11.1% | NSSI group rated pictures with self-injurious reference as significantly more arousing than controls / ↑ activation in amygdala, hippocampus, and anterior cingulate cortex bilaterally during emotional pictures / Depression explained differences between groups in the limbic area / ↑ activation in the middle OFC, and inferior and middle frontal cortex during NSSI pictures / ↓ activation in the occipital cortex in correlation to arousal and inferior frontal cortex in correlation to valence when watching emotional pictures |
| Selection | Comparability | Exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | 1 Is the Case Definition Adequate? | 2 Representativeness of the Cases | 3 Selection of Controls | 4 Definition of Controls | 1 Comparability of Cases and Controls on the Basis of the Design or Analysis | 1 Ascertainment of Exposure | 2 Same Method of Ascertainment for Cases and Controls | 3 Non-Response Rate | TOTAL STARS (0–9) | Quality Overall Score |
| Ho et al. 2021 [26] | * | * | * | * | ** | * | * | * | 9 | good |
| Mayo et al. 2020 [27] | * | * | * | * | ** | * | * | ◯ | 8 | good |
| Hooley et al. 2020 [22] | ◯ | ◯ | * | * | ** | * | * | * | 7 | good |
| Schreiner et al. 2020 [28] | * | * | * | * | ** | * | * | * | 9 | good |
| Demers et al. 2019 [29] | * | * | ◯ | ◯ | ◯ | * | ◯ | * | 4 | fair |
| Perini et al. 2019 [30] | * | * | * | * | ** | * | * | * | 9 | good |
| Poon et al. 2019 [31] | ◯ | ◯ | ◯ | ◯ | ◯ | * | ◯ | * | 2 | poor |
| Dahlgren et al. 2018 [34] | * | * | * | * | ** | * | * | * | 9 | good |
| Malejko et al. 2018 [35] | * | * | * | * | ** | * | * | * | 9 | good |
| Ando et al. 2018 [36] | * | * | * | * | ** | * | * | ◯ | 8 | good |
| Beauchaine et al. 2018 [37] | * | * | * | * | ** | * | * | * | 9 | good |
| Schreiner et al. 2017 [38] | * | * | * | * | ** | * | * | * | 9 | good |
| Brown et al. 2017 [39] | * | * | * | * | ** | * | * | ◯ | 8 | good |
| Groschwitz et al. 2016 [40] | * | * | * | * | ** | * | * | ◯ | 8 | good |
| Quevedo et al. 2016 [41] | * | * | * | * | ** | * | * | ◯ | 8 | good |
| Sauder et al. 2016 [42] | * | * | * | * | ** | * | * | * | 9 | good |
| Bonenberger et al. 2015 [43] | * | * | * | * | ** | * | * | ◯ | 8 | good |
| Osuch et al. 2014 [44] | * | * | ◯ | ◯ | ** | * | * | * | 7 | good |
| Plener et al. 2012 [45] | * | * | * | * | ** | * | * | ◯ | 9 | good |
| Author, Year | ||
|---|---|---|
| Questions | Cullen et al. 2019 [32] | Santamarina-Perez et al. 2019 [33] |
| 1. Is it clear in the study what is the “cause” and what is the “effect” (i.e., there is no confusion about which variable comes first)? | Yes | Yes |
| 2. Were the participants included in any comparisons similar? | Yes | Yes |
| 3. Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? | Not Applicable | Yes |
| 4. Was there a control group? | No | Yes |
| 5. Were there multiple measurements of the outcome, both pre and post the intervention/exposure? | Yes * | No ** |
| 6. Was follow-up complete, and if not, were differences between groups in terms of their follow-up adequately described and analyzed? | Yes | Yes |
| 7. Were the outcomes of participants included in any comparisons measured in the same way? | Yes | Yes |
| 8. Were outcomes measured in a reliable way? | Yes | Yes |
| 9. Was appropriate statistical analysis used? | Yes | Yes |
| Total | 7 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brañas, M.J.A.A.; Croci, M.S.; Ravagnani Salto, A.B.; Doretto, V.F.; Martinho, E., Jr.; Macedo, M.; Miguel, E.C.; Roever, L.; Pan, P.M. Neuroimaging Studies of Nonsuicidal Self-Injury in Youth: A Systematic Review. Life 2021, 11, 729. https://doi.org/10.3390/life11080729
Brañas MJAA, Croci MS, Ravagnani Salto AB, Doretto VF, Martinho E Jr., Macedo M, Miguel EC, Roever L, Pan PM. Neuroimaging Studies of Nonsuicidal Self-Injury in Youth: A Systematic Review. Life. 2021; 11(8):729. https://doi.org/10.3390/life11080729
Chicago/Turabian StyleBrañas, Marcelo J. A. A., Marcos S. Croci, Ana Beatriz Ravagnani Salto, Victoria F. Doretto, Eduardo Martinho, Jr., Marcos Macedo, Euripedes C. Miguel, Leonardo Roever, and Pedro M. Pan. 2021. "Neuroimaging Studies of Nonsuicidal Self-Injury in Youth: A Systematic Review" Life 11, no. 8: 729. https://doi.org/10.3390/life11080729
APA StyleBrañas, M. J. A. A., Croci, M. S., Ravagnani Salto, A. B., Doretto, V. F., Martinho, E., Jr., Macedo, M., Miguel, E. C., Roever, L., & Pan, P. M. (2021). Neuroimaging Studies of Nonsuicidal Self-Injury in Youth: A Systematic Review. Life, 11(8), 729. https://doi.org/10.3390/life11080729







