Phloretin Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Regulating the Inflammatory Response, Oxidative Stress and Apoptosis
Abstract
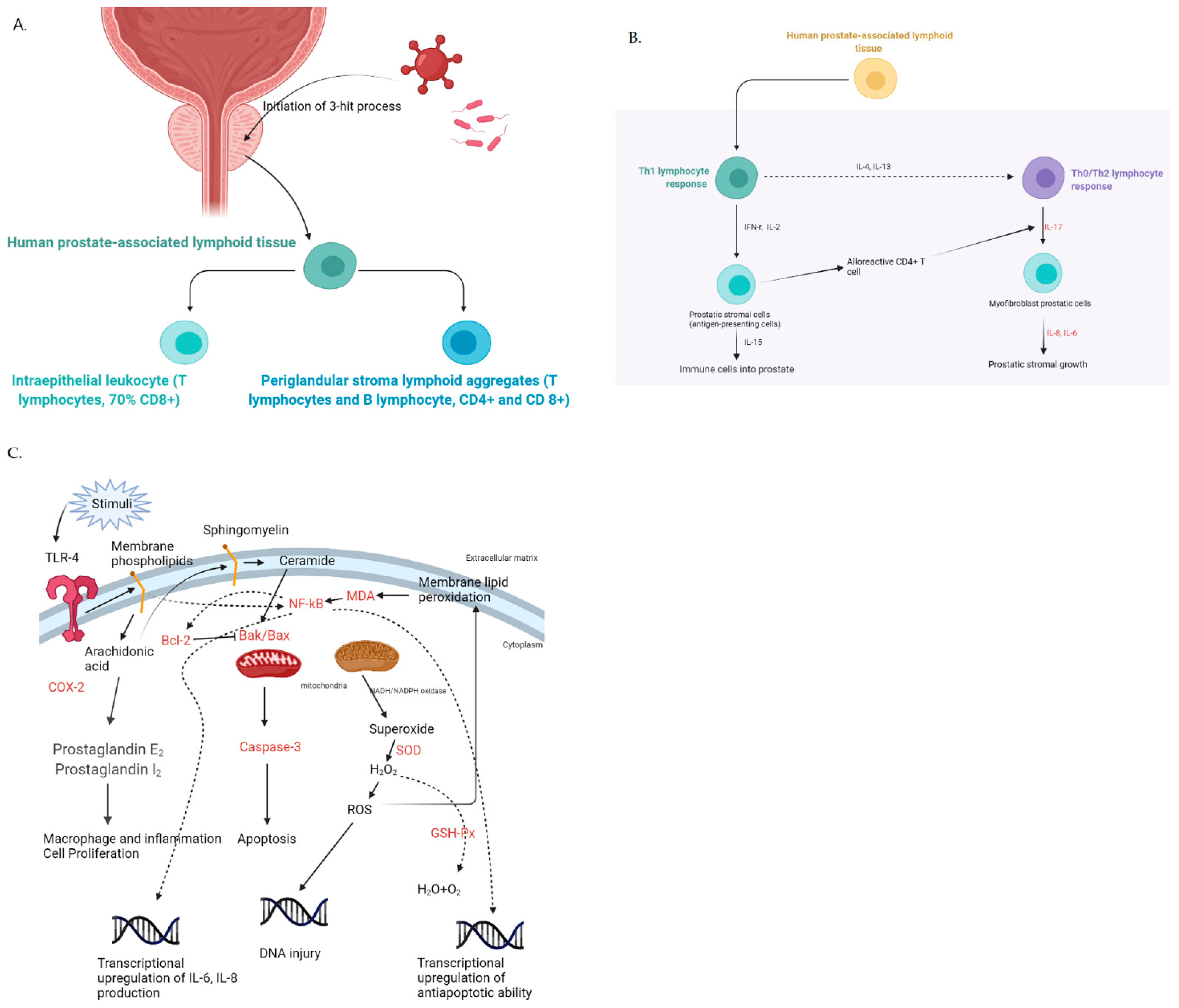
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. BPH induction and Dosage
2.3. Prostate Weight
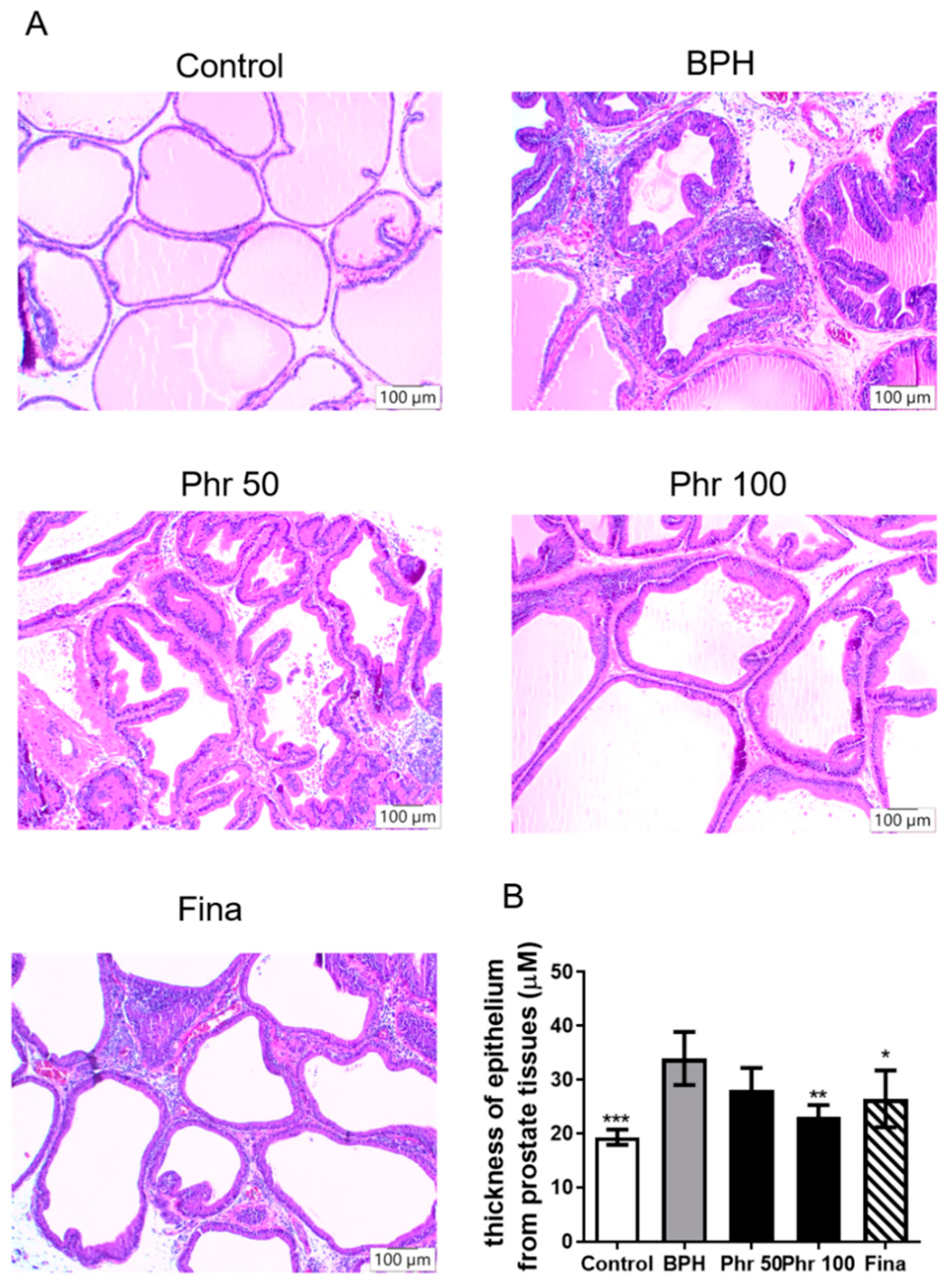
2.4. Histological Examination
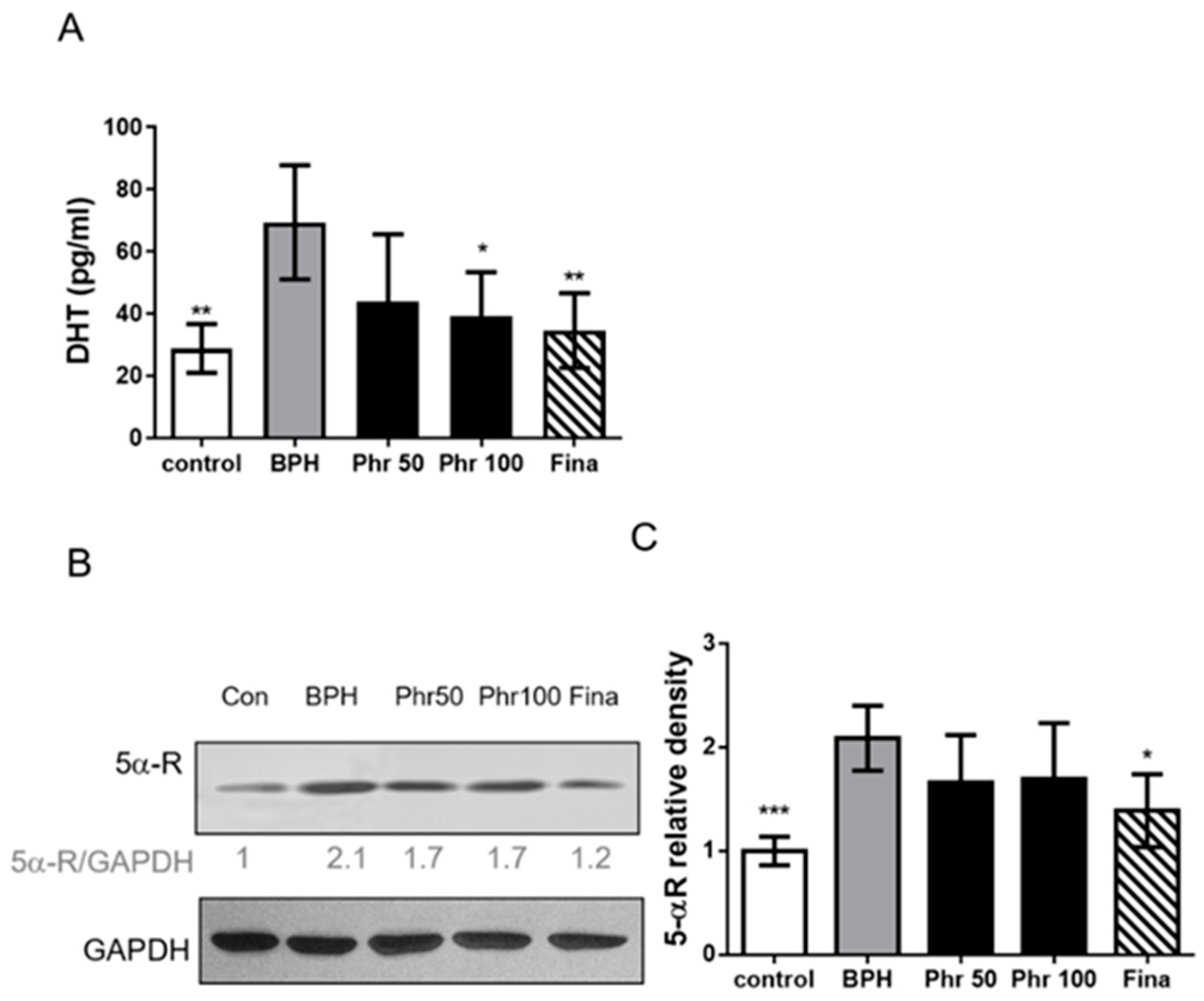
2.5. Determination of DHT in Serum
2.6. Assay for Antioxidant Markers in Prostate Tissue
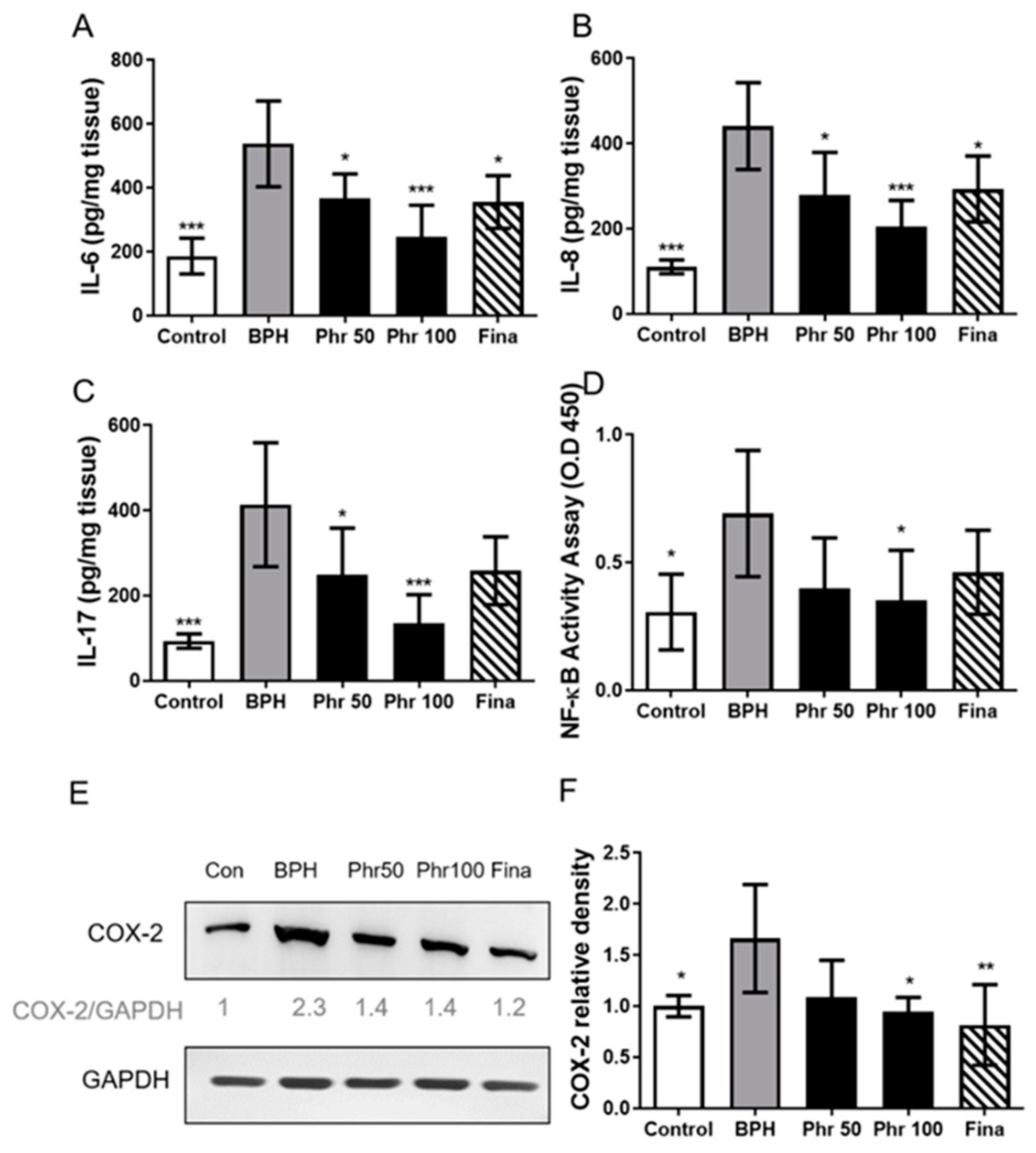
2.7. Assay for Cytokine Expressions in Prostate Tissue
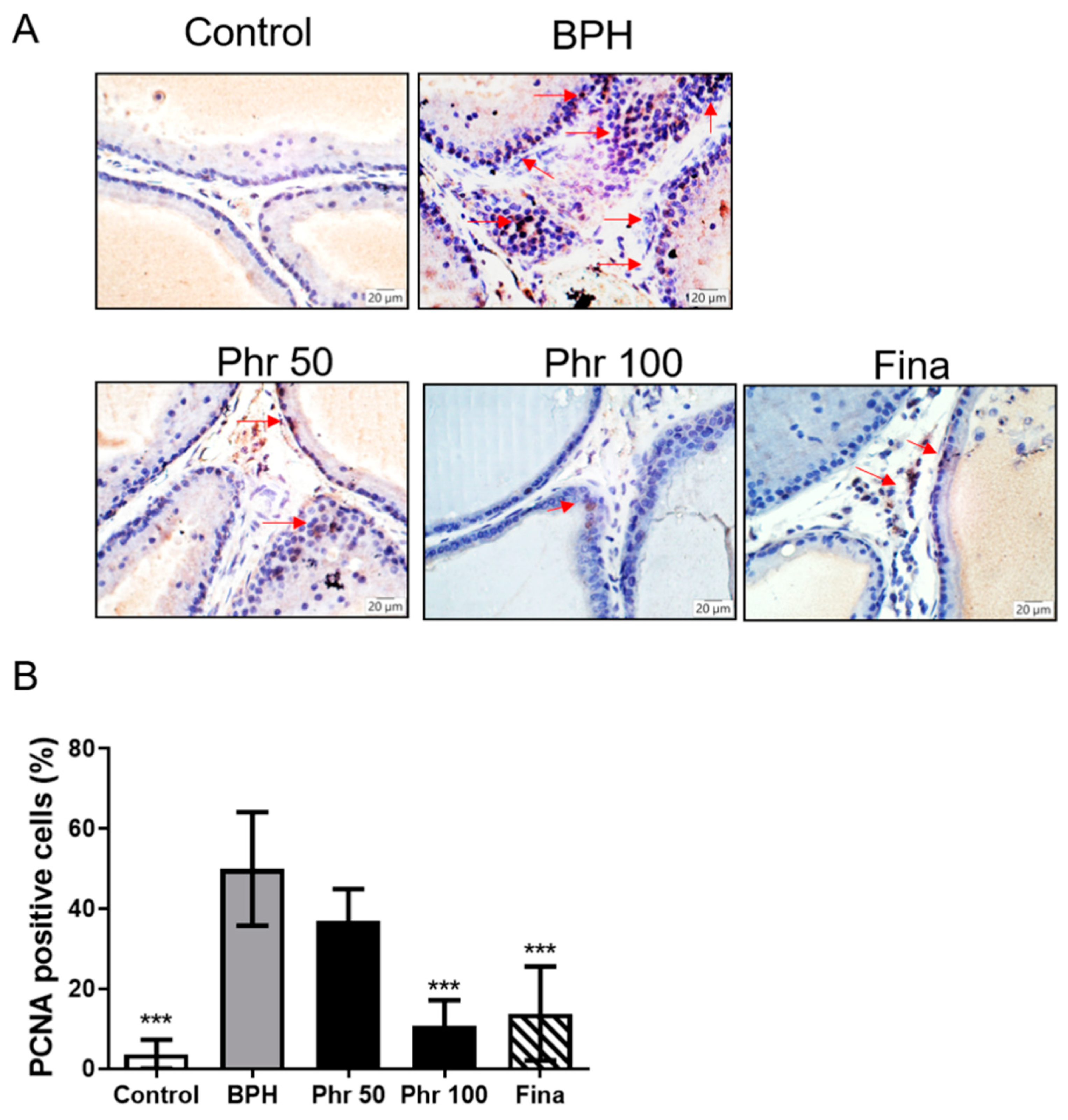
2.8. Immunohistochemistry
2.9. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Staining of Prostate Tissue
2.10. Assay for Cleaved Caspase-3 in Prostate Tissue
2.11. NF-κB Activity Assay
2.12. Western Blot Analysis
2.13. Statistical Analyses
3. Results
3.1. Phloretin Prevents Hyperplasia in Testosterone-Induced BPH Rats
3.2. Phloretin Suppressed the Hyperplastic Patterns in Prostate Tissue of BPH Rats
3.3. Daily 100 mg/kg Phloretin Significantly Decreased the Serum DHT Level in BPH Rats
3.4. Phloretin Regulated the Expression of Inflammatory Markers in BPH Rats
3.5. Phloretin Inhibited the Expression of PCNA in the Prostate
3.6. Effect of Phloretin on Prostatic MDA, SOD, and GSH-Px
3.7. Phloretin Regulated the Expression of Apoptosis Markers in BPH Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miñana, B.; Molero, J.M.; Rolán, A.A.; Martínez-Fornes, M.T.; Pinto, R.C.; Mingot, D.L.; Carreño, Á.; Palacios-Moreno, J.M. Real-world therapeutic management and evolution of patients with benign prostatic hyperplasia in primary care and urology in Spain. Int. J. Clin. Pract. 2021, 75, e14250. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, Y.; Wu, J.; Ding, R.; Cai, T.; Gao, Z. Meta-analysis of the efficacy and safety of combination of tamsulosin plus dutasteride compared with tamsulosin monotherapy in treating benign prostatic hyperplasia. BMC Urol. 2019, 19, 17. [Google Scholar] [CrossRef]
- Ng, M.; Baradhi, K.M. Benign Prostatic Hyperplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- La Vignera, S.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignozzi, L.; Rastrelli, G.; Corona, G.; Gacci, M.; Forti, G.; Maggi, M. Benign prostatic hyperplasia: A new metabolic disease? J. Endocrinol. Investig. 2014, 37, 313–322. [Google Scholar] [CrossRef]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of Inflammation in Benign Prostatic Hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar] [PubMed]
- Nickel, J.C.; Roehrborn, C.G.; Castro-Santamaria, R.; Freedland, S.J.; Moreira, D. Chronic Prostate Inflammation is Associated with Severity and Progression of Benign Prostatic Hyperplasia, Lower Urinary Tract Symptoms and Risk of Acute Urinary Retention. J. Urol. 2016, 196, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Ercan, M.; Alp, H.H.; Kocaturk, H.; Bakan, N.; Gul, M. Oxidative stress before and after surgery in benign prostatic hyperplasia patients. Andrologia 2019, 51, e13326. [Google Scholar] [CrossRef]
- Commisso, M.; Bianconi, M.; Poletti, S.; Negri, S.; Munari, F.; Ceoldo, S.; Guzzo, F. Metabolomic Profiling and Antioxidant Activity of Fruits Representing Diverse Apple and Pear Cultivars. Biology 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Alsanea, S.; Gao, M.; Liu, D. Phloretin Prevents High-Fat Diet-Induced Obesity and Improves Metabolic Homeostasis. AAPS J. 2017, 19, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Zuo, W.; Wu, Q.; Zhu, Q.; Liu, P. Inhibition of Specificity Protein 1 Is Involved in Phloretin-Induced Suppression of Prostate Cancer. BioMed Res. Int. 2020, 2020, 1358674. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-P.; Lin, S.-C.; Li, S.; Chao, Y.-H.; Hwang, G.-Y.; Lin, C.-C. Potent Antiarthritic Properties of Phloretin in Murine Collagen-Induced Arthritis. Evid. Based Complement. Altern. Med. 2016, 2016, 9831263. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Lin, S.-C.; Li, S.; Chiang, Y.-C.; Bracci, N.; Lehman, C.W.; Tang, K.-T.; Lin, C.-C. Phloretin alleviates dinitrochlorobenzene-induced dermatitis in BALB/c mice. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420929442. [Google Scholar] [CrossRef]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Laverny, G.; Gacci, M.; Crescioli, C.; Maggi, M.; Adorini, L. Human Benign Prostatic Hyperplasia Stromal Cells as Inducers and Targets of Chronic Immuno-Mediated Inflammation. J. Immunol. 2009, 182, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Corsiero, E.; Laverny, G.; Silvestrini, E.; Chavalmane, A.; Morelli, A.; Sarchielli, E.; Vannelli, G.B.; et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kB pathways. Prostate 2009, 69, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, E.M.; Amin, H.A.; Kamel, A.S.; Ibrahim, S.M.; Helmy, H.S. Immunomodulatory effect of diallyl sulfide on experimentally-induced benign prostate hyperplasia via the suppression of CD4+T/IL-17 and TGF-β1/ERK pathways. Inflammopharmacology 2020, 28, 1407–1420. [Google Scholar] [CrossRef]
- Suh, J.; Rabson, A.B. NF-κB activation in human prostate cancer: Important mediator or epiphenomenon? J. Cell. Biochem. 2004, 91, 100–117. [Google Scholar] [CrossRef]
- Chappell, W.H.; Candido, S.; Abrams, S.L.; Russo, S.; Ove, R.; Martelli, A.M.; Cocco, L.; Ramazzotti, G.; Cervello, M.; Montalto, G.; et al. Roles of p53, NF-κB and the androgen receptor in controlling NGAL expression in prostate cancer cell lines. Adv. Biol. Regul. 2018, 69, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, D.; Su, X.; Yan, H.; Ma, A.; Li, L.; Wu, J.; Sun, Z. The prostaglandin synthases, COX-2 and L-PGDS, mediate prostate hyperplasia induced by low-dose bisphenol A. Sci. Rep. 2020, 10, 13108. [Google Scholar] [CrossRef] [PubMed]
- Gokkaya, C.S.; Aktas, B.K.; Ozden, C.; Bulut, S.; Karabakan, M.; Erkmen, A.E.; Memis, A. Flurbiprofen alone and in combination with alfuzosin for the management of lower urinary tract symptoms. Central Eur. J. Urol. 2015, 68, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Juszczak, K.; Drewa, T. The cardiovascular and gastrointestinal adverse effects of cyclooxygenase inhibitors seems to be a major concern that restricts their use in the treatment of urinary bladder dysfunction. Central Eur. J. Urol. 2015, 68, 57–59. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Takahashi, N.; Uramoto, H.; Okada, Y. Chloride Channel Inhibition Prevents ROSdependentApoptosis Induced by Ischemia-Reperfusion in Mouse Cardiomyocytes. Cell. Physiol. Biochem. 2005, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Malekova, L.; Tomaskova, J.; Novakova, M.; Stefanik, P.; Kopacek, J.; Lakatos, B.; Pastorekova, S.; Krizanova, O.; Breier, A.; Ondrias, K. Inhibitory effect of DIDS, NPPB, and phloretin on intracellular chloride channels. Pflugers Arch. 2007, 455, 349–357. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]
- Ren, D.; Liu, Y.; Zhao, Y.; Yang, X. Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem. Toxicol. 2016, 94, 203–212. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, D.A.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a Marker of Oxidative Stress in Periodontitis Patients. J. Pharm. Bioallied Sci. 2019, 11, S297–S300. [Google Scholar] [CrossRef]
- Kangari, P.; Farahany, T.Z.; Golchin, A.; Ebadollahzadeh, S.; Salmaninejad, A.; Mahboob, S.A.; Nourazarian, A. Enzymatic antioxidant and lipid peroxidation evaluation in the newly diagnosed breast cancer patients in Iran. Asian Pac. J. Cancer Prev. 2018, 19, 3511–3515. [Google Scholar] [CrossRef] [Green Version]
- Kaya, E.; Ozgok, Y.; Zor, M.; Eken, A.; Bedir, S.; Erdem, O.; Ebiloglu, T.; Ergin, G. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: A prospective controlled study. Adv. Clin. Exp. Med. 2017, 26, 1095–1099. [Google Scholar] [CrossRef] [Green Version]
- Sajjaboontawee, N.; Supasitthumrong, T.; Tunvirachaisakul, C.; Nantachai, K.; Snabboon, T.; Reiche, E.M.V.; Simão, A.N.C.; Maes, M. Lower thiol, glutathione, and glutathione peroxidase levels in prostate cancer: A meta-analysis study. Aging Male 2020, 23, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. Effects of inflammatory responses, apoptosis, and STAT3/NF-κB- and Nrf2-mediated oxidative stress on benign prostatic hyperplasia induced by a high-fat diet. Aging 2019, 11, 5570–5578. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.Y.; Lin, Y.S.; Weng, W.C.; Panny, L.; Chen, H.L.; Tung, M.C.; Ou, Y.C.; Lin, C.C.; Yang, C.H. Phloretin Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Regulating the Inflammatory Response, Oxidative Stress and Apoptosis. Life 2021, 11, 743. https://doi.org/10.3390/life11080743
Hsu CY, Lin YS, Weng WC, Panny L, Chen HL, Tung MC, Ou YC, Lin CC, Yang CH. Phloretin Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Regulating the Inflammatory Response, Oxidative Stress and Apoptosis. Life. 2021; 11(8):743. https://doi.org/10.3390/life11080743
Chicago/Turabian StyleHsu, Chao Yu, Yi Sheng Lin, Wei Chun Weng, Lauren Panny, Hsiang Lai Chen, Min Che Tung, Yen Chuan Ou, Chi Chien Lin, and Che Hsueh Yang. 2021. "Phloretin Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Regulating the Inflammatory Response, Oxidative Stress and Apoptosis" Life 11, no. 8: 743. https://doi.org/10.3390/life11080743
APA StyleHsu, C. Y., Lin, Y. S., Weng, W. C., Panny, L., Chen, H. L., Tung, M. C., Ou, Y. C., Lin, C. C., & Yang, C. H. (2021). Phloretin Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Regulating the Inflammatory Response, Oxidative Stress and Apoptosis. Life, 11(8), 743. https://doi.org/10.3390/life11080743






