The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry?
Abstract
1. Introduction
2. Methods
3. Results
4. CNS and Microbiota: The Gut–Brain Axis
5. Microbiota and Psychiatric Disorders
5.1. Mood Disorders
5.2. Obsessive-Compulsive Disorder and Related Conditions
5.3. Schizophrenia
5.4. Autism Spectrum Disorders
5.5. Miscellanea
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Stecher, B.; Hardt, W.D. Mechanisms controlling pathogen colonization of the gut. Curr. Opin. Microbiol. 2011, 14, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.G.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Ojeda, J.; Ávila, A.; Vidal, P.M. Gut microbiota interaction with the central nervous system throughout life. J. Clin. Med. 2021, 10, 1299. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and lifestyle: A special focus on diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends. Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 7. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Ono, S.; Karaki, S.I.; Kuwahara, A. Short-chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. Jpn. J. Physiol. 2004, 54, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut dysbiosis in patients with anorexia nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef]
- Bojović, K.; Ignjatović, Ð.I.; Soković Bajić, S.; Vojnović Milutinović, D.; Tomić, M.; Golić, N.; Tolinački, M. Gut microbiota dysbiosis associated with altered production of short chain fatty acids in children with neurodevelopmental disorders. Front. Cell. Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Halverson, T.; Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.J.; Xie, J.; Chen, J.J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Masand, P.S.; Keuthen, N.J.; Gupta, S.; Virk, S.; Yu-Siao, B.; Kaplan, D. Prevalence of irritable bowel syndrome in obsessive–compulsive disorder. CNS Spectr. 2006, 11, 21–25. [Google Scholar] [CrossRef]
- Wu, J.C. Psychological co-morbidity in functional gastrointestinal disorders: Epidemiology, mechanisms and management. J. Neurogastroenterol. Motil. 2012, 18, 13–18. [Google Scholar] [CrossRef]
- Sibelli, A.; Chalder, T.; Everitt, H.; Workman, P.; Windgassen, S.; Moss-Morris, R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol. Med. 2016, 46, 3065–3080. [Google Scholar] [CrossRef]
- Turna, J.; Kaplan, K.G.; Patterson, B.; Bercik, P.; Anglin, R.; Soreni, N.; Van Ameringen, M. Higher prevalence of irritable bowel syndrome and greater gastrointestinal symptoms in obsessive-compulsive disorder. J. Psychiatr. Res. 2019, 118, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.E.N.; Zhang, Z.; Gao, L.; Asatryan, L. Microbiome meets microglia in neuroinflammation and neurological disorders. Neuroimmunol. Neuroinflamm. 2020, 7, 215–233. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef]
- Mehrian-Shai, R.; Reichardt, J.K.V.; Harris, C.C.; Toren, A. The gut-brain axis, paving the way to brain cancer. Trends Cancer 2019, 5, 200–207. [Google Scholar] [CrossRef]
- Phillips, M.L.; Swartz, H.A. A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry 2014, 171, 829–843. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, L.; Qiu, C.; Jiang, T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci. Bull. 2015, 31, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.T.; Wang, M.; Andreescu, C.; Tudorascu, D.; Butters, M.A.; Karp, J.F.; Reynolds, C.F., 3rd; Aizenstein, H.J. Acute trajectories of neural activation predict remission to pharmacotherapy in late-life depression. Neuroimage Clin. 2018, 19, 831–839. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota-gut-brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Van Ameringen, M. “What’s bugging the gut in ocd?” A review of the gut microbiome in obsessive-compulsive disorder. Depress. Anxiety 2016, 33, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.A.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun. Integr. Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, L.; Yu, Y.; Cluette-Brown, J.; Martin, M.C.; Claud, C.E. Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci. Rep. 2018, 8, 5443. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16065. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Amireault, P.; Sibon, D.; Cote, F. Life without peripheral serotonin: Insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks. ACS Chem. Neurosci. 2013, 4, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Szulc, A. “Immune gate” of psychopathology—The role of gut derived immune activation in major psychiatric disorders. Front. Psychiatry 2018, 9, 205. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- Bangsgaard Bendtsen, K.M.; Krych, L.; Sørensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sørensen, S.J.; Hansen, A.K. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS ONE 2012, 7, e46231. [Google Scholar] [CrossRef]
- Brenner, D.J.; Fanning, G.R.; Johnson, K.E.; Citarella, R.V.; Falkow, S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J. Bacteriol. 1969, 98, 637–650. [Google Scholar] [CrossRef]
- Campisi, J.; Leem, T.H.; Fleshner, M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell. Stress Chaperones. 2003, 8, 272–286. [Google Scholar] [CrossRef]
- Kluge, M.; Schüssler, P.; Künzel, H.E.; Dresler, M.; Yassouridis, A.; Steiger, A. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J. Psychiatr. Res. 2007, 41, 928–933. [Google Scholar] [CrossRef]
- Zimomra, Z.R.; Porterfield, V.M.; Camp, R.M.; Johnson, J.D. Time-dependent mediators of HPA axis activation following live Escherichia coli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1648–R1657. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, J.; Banati, R.B.; Kreutzberg, G.W. Microglia in the immune surveillance of the brain: Human microglia constitutively express HLA-DR molecules. J. Neuroimmunol. 1993, 48, 189–198. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Priller, J.; Prinz, M. Targeting microglia in brain disorders. Science 2019, 365, 32–33. [Google Scholar] [CrossRef]
- Fazekas de St Groth, B. Regulatory T-cell abnormalities and the global epidemic of immuno-inflammatory disease. Immunol. Cell. Biol. 2012, 90, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Hollander, E.; Lensi, P.; Ravagli, S.; Cassano, G.B. Peripheral markers of serotonin and dopamine function in obsessive-compulsive disorder. Psychiatry Res. 1992, 42, 41–51. [Google Scholar] [CrossRef]
- Benros, M.E.; Eaton, W.W.; Mortensen, P.B. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol. Psychiatry 2014, 75, 300–306. [Google Scholar] [CrossRef]
- Al-Diwani, A.A.J.; Pollak, T.A.; Irani, S.R.; Lennox, B.R. Psychosis: An autoimmune disease? Immunology 2017, 152, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Gerentes, M.; Pelissolo, A.; Rajagopal, K.; Tamouza, R.; Hamdani, N. Obsessive-compulsive disorder: Autoimmunity and neuroinflammation. Curr. Psychiatry Rep. 2019, 21, 78. [Google Scholar] [CrossRef]
- Jeppesen, R.; Benros, M.E. Autoimmune diseases and psychotic disorders. Front. Psychiatry 2019, 10, 131. [Google Scholar] [CrossRef]
- Marazziti, D.; Mucci, F.; Fontanelle, L.F. Immune system and obsessive-compulsive disorder. Psychoneuroendocrinology 2018, 93, 39–44. [Google Scholar] [CrossRef]
- Rogers, J.P.; Pollak, T.A.; Blackman, G.; David, A.S. Catatonia and the immune system: A review. Lancet Psychiatry 2019, 6, 620–630. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability–A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog. 2016, 8, 33. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.E.M. Leaky gut, leaky brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Terruzzi, V. The role of intestinal dysbiosis in the pathogenesis of autism: Minireview. Int. J. Microbiol. Adv. Immunol. 2014, 2, 41–44. [Google Scholar] [CrossRef]
- Reddy, B.L.; Saier, M.H. Autism and our intestinal microbiota. J. Mol. Microbiol. Biotechnol. 2015, 25, 51–55. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Wang, Y.P. Gut microbiota-brain axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. Cross talk: The microbiota and neurodevelopmental disorders. Front. Neurosci. 2017, 11, 490. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Press: Washington, DC, USA, 2013. [Google Scholar]
- Akiskal, H.S. A developmental perspective on recurrent mood disorders: A review of studies in man. Psychopharmacol. Bull. 1986, 22, 579–586. [Google Scholar]
- Engel, C. Mood Disorders in Adolescent medicine, 1st ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; Chapter 36; pp. 282–290. [Google Scholar]
- Krishnan, V.; Nestler, E.J. Linking molecules to mood: New insight into the biology of depression. Am. J. Psychiatry 2010, 167, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Pitchot, W.; Scantamburlo, G.; Ansseau, M.; Souery, D. Bipolar disorder: A multifactorial disease. Rev. Med. Liege 2012, 67, 366–373. [Google Scholar] [PubMed]
- Price, J.L.; Drevets, W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012, 16, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gonda, X.; Petschner, P.; Eszlari, N.; Baksa, E.; Edes, A.; Antal, P.; Juhasz, G.; Bagdy, G. Genetic variants in major depressive disorder: From pathophysiology to therapy. Pharmacol. Ther. 2019, 194, 22–43. [Google Scholar] [CrossRef]
- Rowland, T.A.; Marwaha, S. Epidemiology and risk factors for bipolar disorder. Ther. Adv. Psychopharmacol. 2018, 8, 251–269. [Google Scholar] [CrossRef]
- McNamara, R.K.; Lotrich, F.E. Elevated immune-inflammatory signaling in mood disorders: A new therapeutic target? Expert Rev. Neurother. 2012, 12, 1143–1161. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Derecki, N.C.; Lovenberg, T.W.; Drevets, W.C. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology 2016, 233, 1623–1636. [Google Scholar] [CrossRef]
- Colpo, G.D.; Leboyer, M.; Dantzer, R.; Trivedi, M.H.; Teixeira, A.L. Immune-based strategies for mood disorders: Facts and challenges. Expert Rev. Neurother. 2018, 18, 139–152. [Google Scholar] [CrossRef]
- Mucci, F.; Marazziti, D.; Della Vecchia, A.; Baroni, S.; Morana, P.; Carpita, B.; Mangiapane, P.; Morana, F.; Morana, B.; Dell’Osso, L. State-of-the-Art: Inflammatory and metabolic markers in mood disorders. Life 2020, 10, 82. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Nemeroff, C.B. Depression as a systemic disease. Personalized Med. Psychiatry 2017, 1–2, 11–25. [Google Scholar] [CrossRef][Green Version]
- Sousa, S. Depression as a Systemic Illness Edited by James J. Strain, Michael Blumenfield Oxford University Press. 2018. 336 pp. ISBN 9780190603342. Br. J. Psychiatry 2020, 217, 463. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693 Pt B, 128–133. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, K. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Flowers, S.A.; Evans, S.J.; Ward, K.M.; McInnis, M.G.; Ellingrod, V.E. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy 2017, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Lai, J.B.; Du, Y.L.; Xu, Y.; Ruan, L.M.; Hu, S.H. Current understanding of gut microbiota in mood disorders: An update of human studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut microbiota, bacterial translocation, and interactions with diet: Pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Maier, E.; Anderson, R.C.; Roy, N.C. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients 2015, 7, 45–73. [Google Scholar] [CrossRef]
- Schiepers, O.J.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.X.; Xia, J.J.; Deng, F.L.; Liang, W.W.; Wu, J.; Yin, B.M.; Dong, M.X.; Chen, J.J.; Ye, F.; Wang, H.Y. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiatry 2018, 8, 130. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Prevot, T.; Sibille, E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol. Psychiatry. 2020, 26, 151–167. [Google Scholar] [CrossRef]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2015, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafans, E.; Schweinfurth, L.A.; Savage, C.L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef]
- Macedo, D.; Filho, A.; Soares de Sousa, C.N.; Quevedo, J.; Barichello, T.; Júnior, H.; Freitas de Lucena, D. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association Press: Washington, DC, USA, 2000. [Google Scholar]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013, 25, 521–528. [Google Scholar] [CrossRef]
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79. [Google Scholar] [CrossRef]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Clinical Excellence (NICE). Obsessive-Compulsive Disorder (OCD) and Body Dysmorphic Disorder (BDD); British Psychological Society: Leicester, UK, 2005. [Google Scholar]
- Sanikhani, N.S.; Modarressi, M.H.; Jafari, P.; Vousooghi, N.; Shafei, S.; Akbariqomi, M.; Heidari, R.; Lavasani, P.S.; Yazarlou, F.; Motevaseli, E.; et al. The effect of Lactobacillus casei consumption in improvement of obsessive-compulsive disorder: An animal study. Probiotics Antimicrob. Proteins 2020, 12, 1409–1419. [Google Scholar] [CrossRef]
- Kobliner, V.; Mumper, E.; Baker, S.M. Reduction in obsessive compulsive disorder and self-injurious behavior with Saccharomyces boulardii in a child with autism: A case report. Integr. Med. (Encinitas) 2018, 17, 38–41. [Google Scholar]
- Altemus, M.; Pigott, T.; Kalogeras, K.T.; Demitrack, M.; Dubbert, B.M.; Murphy, D.L.; Gold, P.W. Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Arch. Gen. Psychiatry 1992, 49, 9–20. [Google Scholar] [CrossRef]
- Monteleone, P.; Catapano, F.; Tortorella, A.; Maj, M. Cortisol response to d-fenfluramine in patients with obsessive-compulsive disorder and in healthy subjects: Evidence for a gender-related effect. Neuropsychobiology 1997, 36, 8–12. [Google Scholar] [CrossRef]
- Ullrich, M.; Weber, M.; Post, A.M.; Popp, S.; Grein, J.; Zechner, M.; Guerrero González, H.; Kreis, A.; Schmitt, A.G.; Üçeyler, N.; et al. OCD-like behavior is caused by dysfunction of thalamo-amygdala circuits and upregulated TrkB/ERK-MAPK signaling as a result of SPRED2 deficiency. Mol. Psychiatry 2018, 23, 444–458. [Google Scholar] [CrossRef]
- Rees, J.C. Obsessive-compulsive disorder and gut microbiota dysregulation. Med. Hypotheses 2014, 82, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Savage, D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 1974, 9, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Tullberg, C.; Ahrné, S.; Hamberg, K.; Lazou Ahrén, I.; Molin, G.; Sonesson, M.; Håkansson, Å. Oral administration of Lactobacillus plantarum 299v reduces cortisol levels in luman saliva during examination induced stress: A randomized, double-blind controlled trial. Int. J. Microbiol. 2016, 8469018. [Google Scholar] [CrossRef]
- Swedo, S.E.; Leonard, H.L.; Garvey, M.; Mittleman, B.; Allen, A.J.; Perlmutter, S.; Lougee, L.; Dow, S.; Zamkoff, J.; Dubbert, B.K. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am. J. Psychiatry 1998, 155, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bellido, J.L.; Munoz-Criado, S.; Garcìa-Rodrìguez, J.A. Antimicrobial activity of psychotropic drugs: Selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 2000, 14, 177–180. [Google Scholar] [CrossRef]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Zwicker, A. Etiology in psychiatry: Embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry 2017, 16, 121–129. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zimbron, J.; Lewis, G.; Jones, P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013, 43, 239–257. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zammit, S.; Lewis, G.; Jones, P.B. A population-based study of atopic disorders and inflammatory markers in childhood before psychotic experience in adolescence. Schizophr. Res. 2014, 152, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lachance, L.R.; McKenzie, K. Biomarkers of gluten sensitivity in patients with non-affective psychosis: A meta-analysis. Schizophr. Res. 2014, 152, 521–527. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2017, 353, 772–777. [Google Scholar] [CrossRef]
- Birnbaum, R.; Jaffe, A.E.; Chen, Q.; Shin, J.H.; Kleinman, J.E.; Hyde, T.M.; Weinberger, D.R. Investigating the neuroimmunogenic architecture of schizophrenia. Mol. Psychiatry 2017, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- García-Bueno, B.; Bioque, M.; Mac-Dowell, K.S.; Barcones, M.F.; Martínez-Cengotitabengoa, M.; Pina-Camacho, L.; Rodríguez-Jiménez, R.; Sáiz, P.A.; Castro, C.; Lafuente, A.; et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: Toward an integrative inflammatory hypothesis of schizophrenia. Schizophr. Bull. 2014, 40, 376–387. [Google Scholar] [CrossRef]
- García-Bueno, B.; Bioque, M.; Mac-Dowell, K.S.; Santabárbara, J.; Martínez-Cengotitabengoa, M.; Moreno, C.; Sáiz, P.A.; Berrocoso, E.; Gassó, P.; Fe Barcones, M.; et al. Pro-/antiinflammatory dysregulation in early psychosis: Results from a 1-year follow-up study. Int. J. Neuropsychopharmacol. 2015, 18, pyu037. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Leboyer, M.; Oliveira, J.; Tamouza, R.; Groc, L. Is it time for immunopsychiatry in psychotic disorders? Psychopharmacology 2016, 233, 1651–1660. [Google Scholar] [CrossRef]
- Gumusoglu, S.B.; Stevens, H.E. Maternal inflammation and neurodevelopmental programming: A review of preclinical outcomes and implications for translational psychiatry. Biol. Psychiatry 2018, 85, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Golofast, B.; Vales, K. The connection between microbiome and schizophrenia. Neurosci. Biobehav. Rev. 2020, 108, 712–731. [Google Scholar] [CrossRef]
- Schwarz, E.; Maukonen, J.; Hyytiäinen, T.; Kieseppä, T.; Orešič, M.; Sabunciyan, S.; Mantere, O.; Saarela, M.; Yolken, R.; Suvisaari, J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 2018, 192, 398–403. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H.; et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, J.; Li, Z.; Huang, Y.; Yuan, Y.; Wang, J.; Zhang, M.; Hu, S.; Liang, Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr. Res. 2018, 197, 470–477. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, P.; Wang, Y.; Liu, Y.; Li, X.; Kumar, B.U.; Hei, G.; Lv, L.; Huang, X.F.; Fan, X.; et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr. Res. 2018, 201, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Asif, H.; Dai, L.; He, Y.; Zheng, W.; Wang, D.; Ren, H.; Tang, J.M.; Li, C.; Jin, K.; et al. Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. J. Psych. Res. 2020, 123, 136–144. [Google Scholar] [CrossRef]
- Khodaie-Ardakani, M.R.; Mirshafiee, O.; Farokhnia, M.; Tajdini, M.; Hosseini, S.M.; Modabbernia, A.; Rezaei, F.; Salehi, B.; Yekehtaz, H.; Ashrafi, M.; et al. Minocycline add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: Randomized double-blind placebo-controlled study. Psychiatry Res. 2014, 215, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.C.; Spitzer, S.; Anthony, D.C.; Lennox, B.; Burnet, P. Prebiotic attenuation of olanzapine-induced weight gain in rats: Analysis of central and peripheral biomarkers and gut microbiota. Transl. Psychiatry 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Pełka-Wysiecka, J.; Kaczmarczyk, M.; Bąba-Kubiś, A.; Liśkiewicz, P.; Wroński, M.; Skonieczna-Żydecka, K.; Marlicz, W.; Misiak, B.; Starzyńska, T.; Kucharska-Mazur, J.; et al. Analysis of gut microbiota and their metabolic potential in patients with schizophrenia treated with olanzapine: Results from a six-week observational prospective cohort study. J. Clin. Med. 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. The gut microbiome and treatment-resistance in schizophrenia. Psychiatr Q. 2020, 91, 127–136. [Google Scholar] [CrossRef]
- Ng, Q.X.; Soh, A.; Venkatanarayanan, N.; Ho, C.; Lim, D.Y.; Yeo, W.S. A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology 2019, 78, 1–6. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93 (Suppl. 1), S13–S25. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, P.; Zhang, X.K.; Yang, Y.; Yang, M.; Wang, T.; Lu, H.; Tian, H.; Wang, H.; Liu, J. Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food Funct. 2021, 12, 1156–1175. [Google Scholar] [CrossRef]
- Li, S.; Song, J.; Ke, P.; Kong, L.; Lei, B.; Zhou, J.; Huang, Y.; Li, H.; Li, G.; Chen, J.; et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci. Rep. 2021, 11, 9743. [Google Scholar] [CrossRef]
- Yuan, X.; Kang, Y.; Zhuo, C.; Huang, X.F.; Song, X. The gut microbiota promotes the pathogenesis of schizophrenia via multiple pathways. Biochem. Biophys. Res. Commun. 2019, 512, 373–380. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Park, C.Y.; Theesfeld, C.L.; Wong, A.K.; Yuan, Y.; Scheckel, C.; Fak, J.J.; Funk, J.; Yao, K.; Tajima, Y.; et al. Whole-genome deep learning analysis identifies contribution of noncoding mutations to autism risk. Nat. Genet. 2019, 51, 973–980. [Google Scholar] [CrossRef]
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tildon, J.T. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 1999, 135, 559–563. [Google Scholar] [CrossRef]
- White, J.F. Intestinal pathophysiology in autism. Exp. Biol. Med. 2003, 228, 639–649. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennetta, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Donovan, S.M. Microbiome and nutrition in autism spectrum disorder: Current knowledge and research needs. Nutr. Rev. 2016, 74, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 1–24. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; Mcdonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe 2011, 17, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002, 35 (Suppl. 1), S6–S16. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Young, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Cox, S.B.; Meyer, D.; Austin, D.W. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012, 5, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary intake, nutrient status, and growth parameters in children with autism spectrum disorder and severe food selectivity: An electronic medical record review. J. Acad. Nutr. Diet. 2018, 118, 1943–1950. [Google Scholar] [CrossRef]
- Yule, S.; Wanik, J.; Holm, E.M.; Bruder, M.B.; Shanley, E.; Sherman, C.Q.; Fitterman, M.; Lerner, J.; Marcello, M.; Parenchuck, N.; et al. Nutritional deficiency disease secondary to ARFID symptoms associated with autism and the broad autism phenotype: A qualitative systematic review of case reports and case series. J. Acad. Nutr. Diet. 2021, 121, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: Comorbidity or causative mechanisms? Bioessays 2014, 36, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child. Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child. Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Gagliardi, M.; Guarino, M.; Siniscalchi, M.; Ciacci, C.; Iovino, P. Eating disorders and gastrointestinal diseases. Nutrients 2019, 11, 3038. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Belheouane, M.; Schulz, N.; Dempfle, A.; Baines, J.F.; Herpertz-Dahlmann, B. The impact of starvation on the microbiome and gut-brain interaction in anorexia nervosa. Front. Endocrinol 2019, 10, 41. [Google Scholar] [CrossRef]
- Seitz, J.; Trinh, S.; Herpertz-Dahlmann, B. The microbiome and eating disorders. Psychiatr. Clin. N. Am. 2019, 42, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Treasure, J. Psychopharmacological advances in eating disorders. Expert Rev. Clin. Pharmacol. 2018, 11, 95–108. [Google Scholar] [CrossRef] [PubMed]
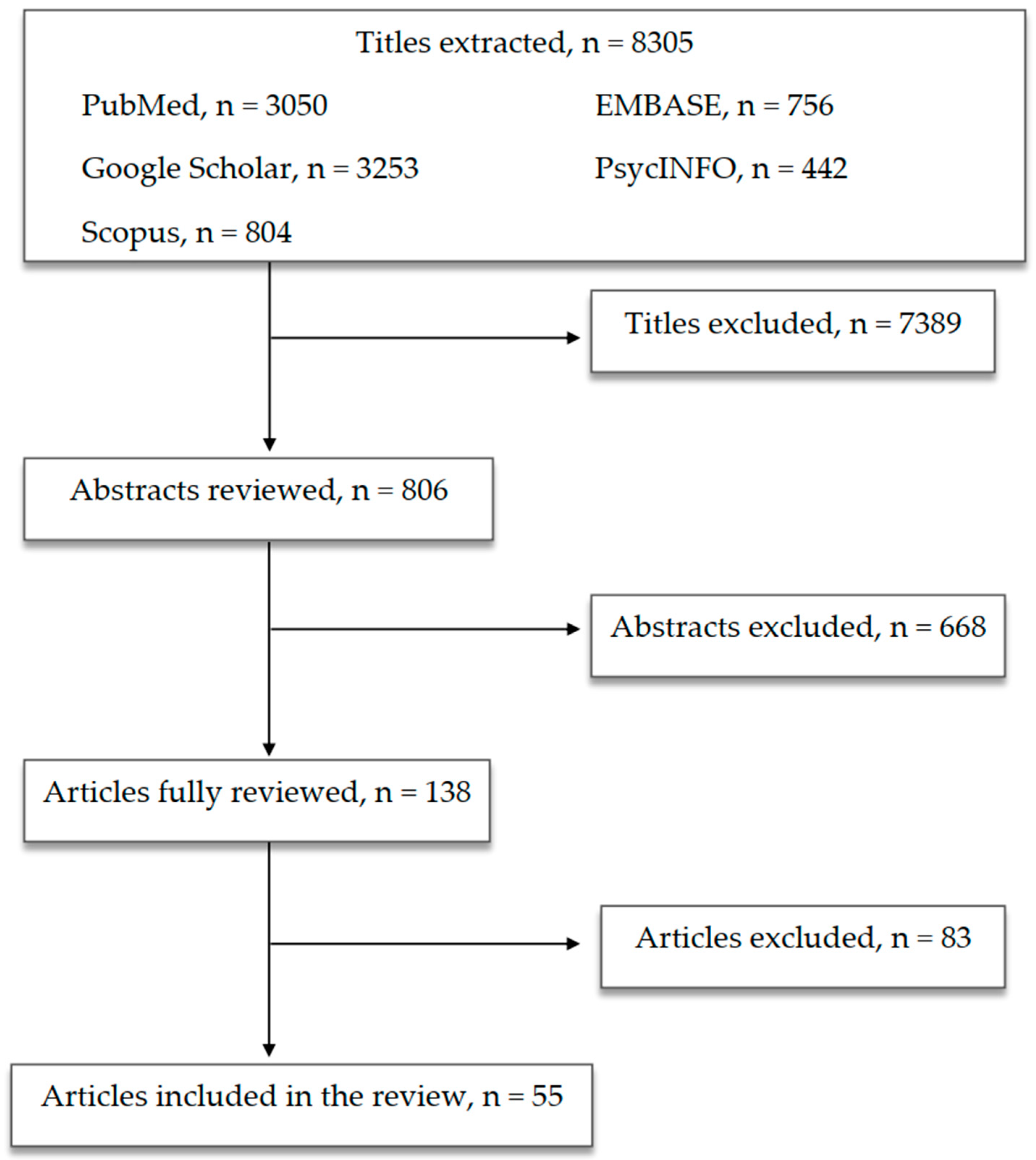
| Authors and Year | Type of Study | Population | Methods | Findings |
|---|---|---|---|---|
| Mangiola et al., 2016 [84] | Review | - | Selected studies on the role of gut microbiota and the use of microbiota-modulating strategies in MDs/ASD |
|
| Colpo et al., 2017 [101] | Review | - | Selected studies on the role of inflammation and immune-based therapeutic strategies in MDs |
|
| Jiang et al., 2015 [106] | Cross-sectional study | 46 depressed patients (active MDD and responded MDD) and 30 HC | Comparing blood samples and faecal samples using high-throughput pyrosequencing |
|
| Aizawa et al., 2016 [107] | Cross-sectional study | 43 MDD patients and 57 HC | Comparing faecal samples using bacterial rRNA-targeted reverse transcription-quantitative PCR |
|
| Zheng et al., 2016 [108] | Cross-sectional study; animal study (mice) | GF and SPF Kunming mice | Open-field test, Y-maze, tail suspension test, forced swimming test; 16S rRNA gene sequencing on faecal samples from MDD patients and HC; FMT |
|
| Evans et al., 2017 [109] | Cross-sectional study | 115 BD patients and 64 HC | Comparing faecal samples using 16S rRNA gene sequence analysis; psychometric evaluations |
|
| Flowers et al., 2017 [110] | Cross-sectional study | 117 BD patients (AAP-treated or non-AAP-treated) | Comparing faecal samples using 16S ribosomal sequencing |
|
| Painold et al., 2019 [112] | Cross-sectional study | 32 BD patients and 10 HC | Comparing blood samples and faecal samples using 16S rRNA gene sequencing |
|
| Huang et al., 2019 [113] | Review | - | 12 selected human studies |
|
| Maes et al., 2008 [114] | Cross-sectional study | MDD patients and HC | Comparing blood samples |
|
| Slyepchenko et al., 2017 [115] | Narrative review | - | 2016 selected studies on the role of intestinal dysbiosis in the pathophysiology of MDD and somatic comorbidities |
|
| Kelly et al., 2016 [116] | Cross-sectional study; animal study (rats) | 34 MDD patients and 33 matched HC | Comparing blood, salivary and faecal samples; FMT to a microbiota-deficient rat model |
|
| Yang et al., 2020 [117] | Cross-sectional study | 156 MDD patients and 155 HC | Whole-genome shotgun metagenomic and untargeted metabolomic methods |
|
| Patterson et al., 2019 [122] | Animal study (mice) | Diet-induced obese and metabolically dysfunctional mice | Daily administration of GABA-producing L. brevis (L. brevis DPC6108 or L. brevis DSM32386) for 12 weeks |
|
| Naseribafrouei et al., 2014 [125] | Cross-sectional study | 37 depressed patients and 18 HC | Comparing faecal samples using 16S rRNA gene sequencing |
|
| Severance et al., 2016 [127] | Cross-sectional study | Two cohorts totaling 947 individuals with SZ and BD, as well as HC | Comparing blood samples in patients with SZ and BD, as well as HC |
|
| Dickerson et al., 2017 [128] | Review | - | Selected human studies on the relationship between immune alterations and microbiome in SZ and BD |
|
| Macedo et al., 2017 [129] | Narrative review | - | 120 selected articles on the mutual relationship between stress, depression and gut microbiota composition and antimicrobial effect of ADs and vice versa |
|
| Authors and Year | Type of Study | Findings |
|---|---|---|
| Kantak et al., 2014 [132] | Animal study (BALB/cJ house mice) |
|
| Sanikhani et al., 2020 [135] | Animal study (rats) |
|
| Kobliner et al., 2018 [136] | Case report | S. boulardii administration, aimed at reducing GI symptoms, resulted in an amelioration of OCD and SIB in a boy with ASD, OCD, tics, SIB, a history of GI disturbances and global immune dysregulation |
| Rees et al., 2014 [140] | Review | Antibiotics altering the composition of intestinal flora could be the causative factor of PANDAS rather than GABHS |
| Authors and Year | Type of Study | Methods | Findings |
|---|---|---|---|
| Zheng et al., 2019 [160] | Cross-sectional study; animal study | Comparing gut microbiota between 63 treated and untreated SZ patients and 69 HCs; GF mice received SZ FMT |
|
| Shen et al., 2018 [161] | Cross-sectional study | Comparing gut microbiota between 64 SZ patients and 53 HC using 16S rRNA sequencing |
|
| Yuan et al., 2018 [162] | Cross-sectional study | Comparing gut microbiota between 41 first-episode SZ patients and 41 HCs; testing 24-week risperidone treatment effects |
|
| Li et al., 2021 [171] | Cross-sectional study | Investigating faecal microbiota differences between 38 SZ patients and 38 HC, as well as exploring whether such differences were associated with brain structure and function, through 16S rRNA sequencing, sMRI and rs-fMRI |
|
| Authors and Year | Type of Study | Participants (N) | Methods | Findings |
|---|---|---|---|---|
| McElhanon et al., 2014 [181] | Systematic meta-analysis | ASD group: 2215; comparison group: 50664 | 15 studies included in the systematic review | Greater prevalence of GI symptoms among children with ASD compared with control children |
| Adams et al., 2011 [182] | Cross-sectional study | 58 ASD children; 39 healthy controls | GI symptoms: assessed with a modified six-item GI Severity Index (6-GSI) questionnaire; autistic symptoms: assessed with the Autism Treatment Evaluation Checklist (ATEC) | Correlations between GI symptoms and autism severity |
| Kang et al., 2017 [183] | Open-label trial | 18 ASD-diagnosed children | MTT |
|
| Gondalia et al., 2012 [196] | Cross-sectional study | 28 autistic children with GI dysfunction; 23 autistic children without GI dysfunction; 53 neurotypical siblings | Comparing gut microbiota | No significant difference between groups |
| Sandler et al., 2000 [201] | Open-label trial | 11 children with regressive-onset autism | Administration of vancomycin | Short-term behavioural improvement |
| Authors and Year | Type of Study | Findings |
|---|---|---|
| Cenit et al., 2017 [89] | Review |
|
| Cenit et al., 2017 [202] | Review |
|
| Authors and Year | Type of Study | Findings |
|---|---|---|
| Santonicola et al., 2019 [203] | Review |
|
| Seitz et al., 2019 [204] | Review |
|
| Seitz et al., 2019 [205] | Review |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marazziti, D.; Buccianelli, B.; Palermo, S.; Parra, E.; Arone, A.; Beatino, M.F.; Massa, L.; Carpita, B.; Barberi, F.M.; Mucci, F.; et al. The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry? Life 2021, 11, 760. https://doi.org/10.3390/life11080760
Marazziti D, Buccianelli B, Palermo S, Parra E, Arone A, Beatino MF, Massa L, Carpita B, Barberi FM, Mucci F, et al. The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry? Life. 2021; 11(8):760. https://doi.org/10.3390/life11080760
Chicago/Turabian StyleMarazziti, Donatella, Beatrice Buccianelli, Stefania Palermo, Elisabetta Parra, Alessandro Arone, Maria Francesca Beatino, Lucia Massa, Barbara Carpita, Filippo M. Barberi, Federico Mucci, and et al. 2021. "The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry?" Life 11, no. 8: 760. https://doi.org/10.3390/life11080760
APA StyleMarazziti, D., Buccianelli, B., Palermo, S., Parra, E., Arone, A., Beatino, M. F., Massa, L., Carpita, B., Barberi, F. M., Mucci, F., & Dell’Osso, L. (2021). The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry? Life, 11(8), 760. https://doi.org/10.3390/life11080760









