Genome Characterization, Comparison and Phylogenetic Analysis of Complete Mitochondrial Genome of Evolvulus alsinoides Reveals Highly Rearranged Gene Order in Solanales
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Library Preparation and Genome Sequencing
2.2. Mitochondrial Genome Assembly
2.3. Mitochondrial Genome Annotation
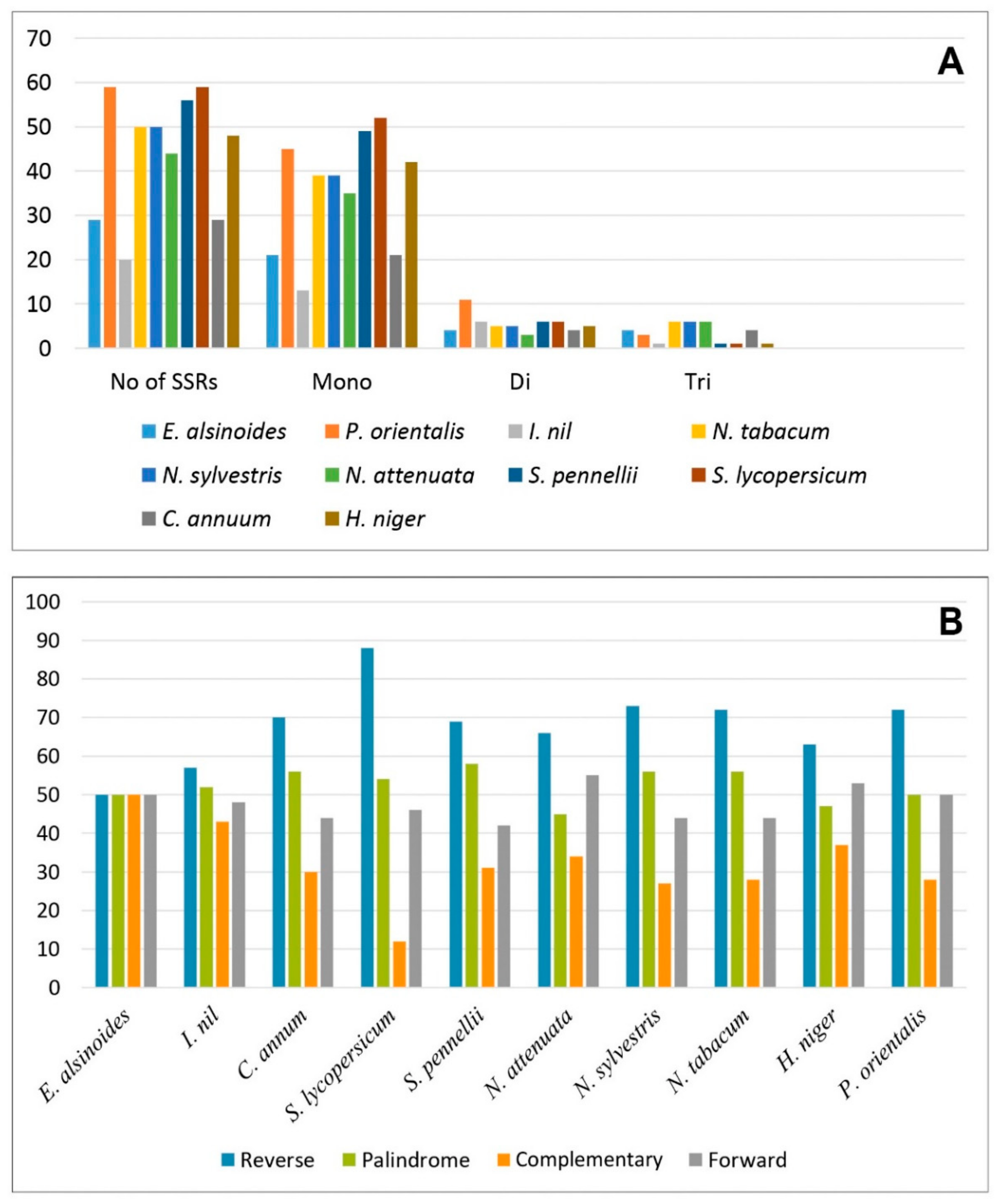
2.4. Repetitive Sequence Analysis
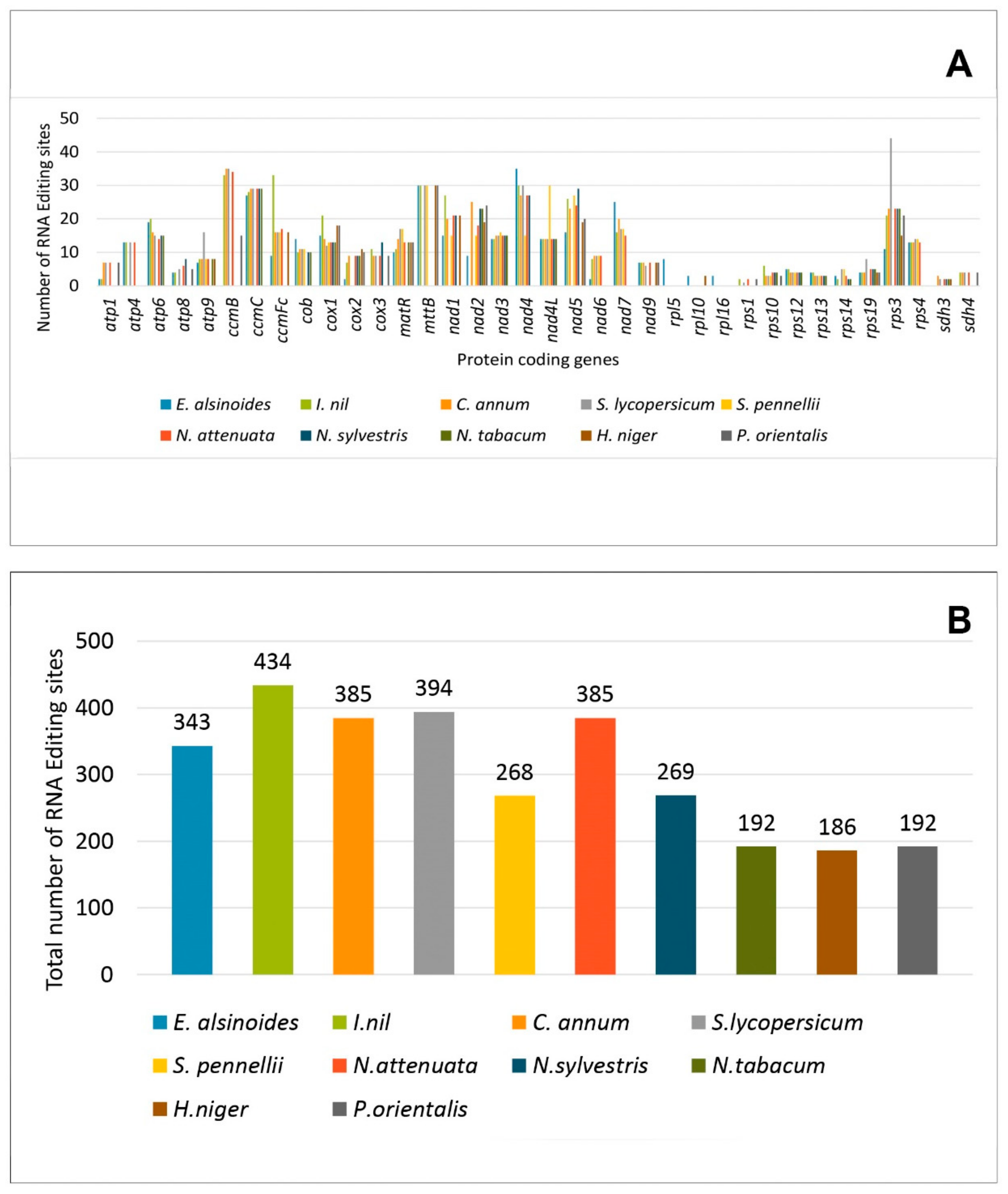
2.5. RNA Editing Site Prediction
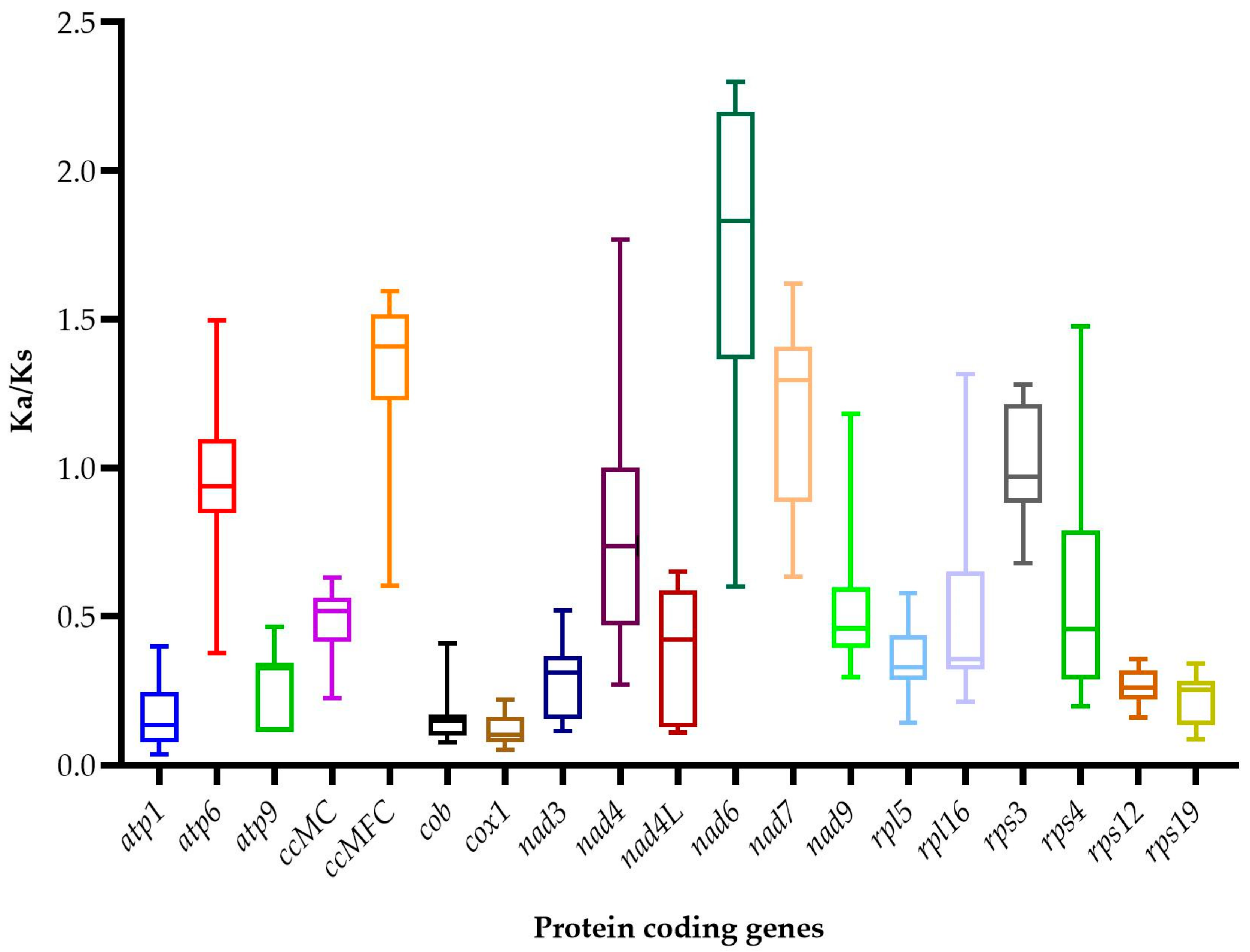
2.6. Synonymous and Nonsynonymous Substitution Ratio
2.7. Gene Arrangement and Synteny Analysis
2.8. Phylogenetic Analysis
3. Results and Discussion
3.1. Mitogenome Structure, Organization and Composition
3.2. Protein-Coding Genes
3.3. Transfer and Ribosomal RNA Genes
3.4. Comparative Analysis of Mitogenomes of Solanales
3.5. Repetitive Sequence Analysis
3.6. RNA Editing Site Prediction
3.7. Synonymous and Nonsynonymous Substitution Ratio
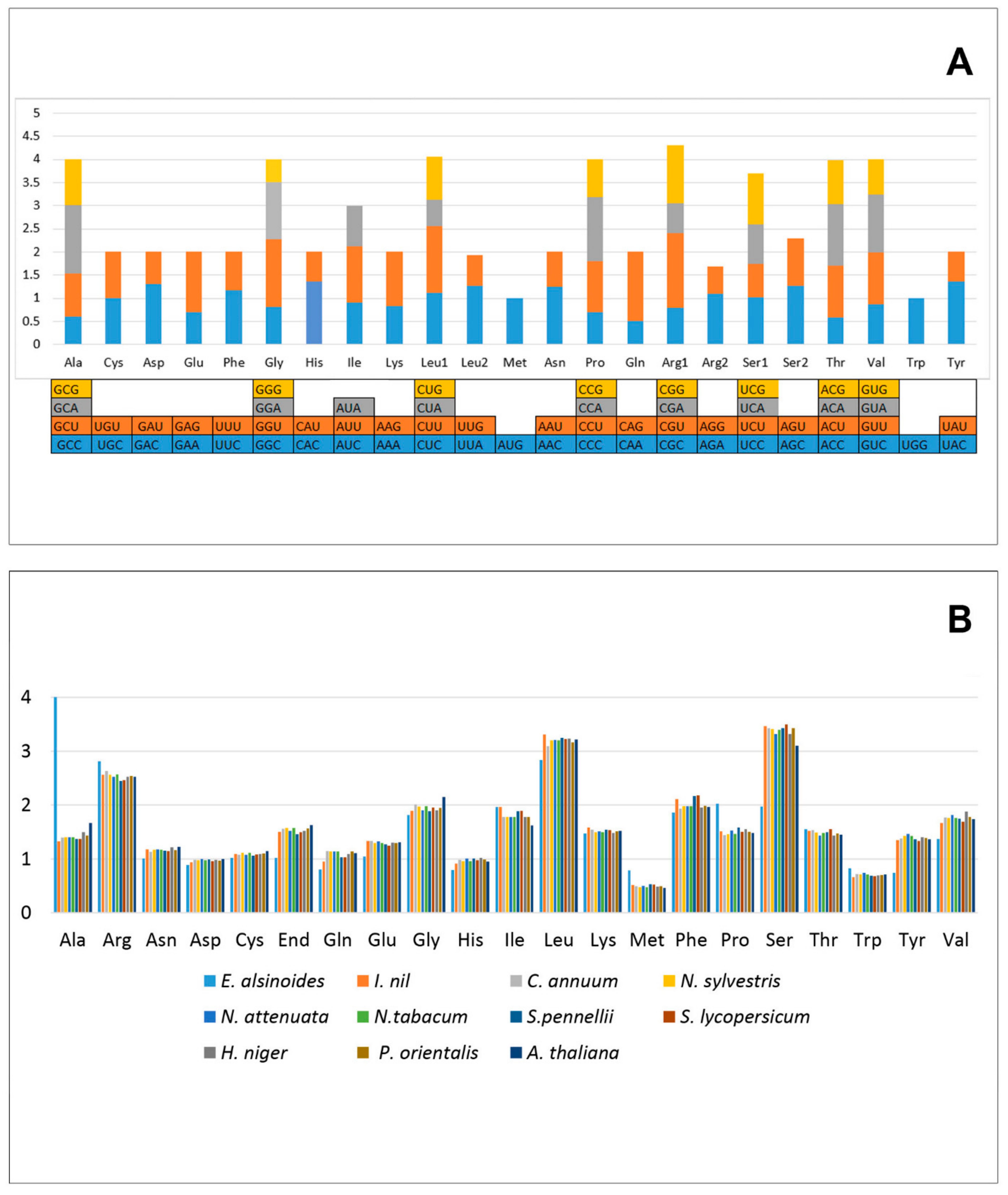
3.8. Codon Usage Analysis
3.9. Gene Arrangement and Synteny Analysis
3.10. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logan, D.C. The mitochondrial compartment. J. Exp. Bot. 2006, 57, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Bullerwell, C.E.; Gray, M.W. Evolution of the mitochondrial genome: Protist connections to animals, fungi and plants. Curr. Opin. Microbiol. 2004, 7, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P. Variation in protein gene and intron content among land plant mitogenomes. Mitochondrion 2020, 53, 203–213. [Google Scholar] [CrossRef]
- Smith, D.R.; Keeling, P.J. Mitochondrial and plastid genome architecture: Reoccurring themes, but significant differences at the extremes. Proc. Natl. Acad. Sci. USA 2015, 112, 10177–10184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauron, C.; Allen, J.; Clifton, S.; Newton, K. Plant mitochondrial genomes. In Molecular Biology and Biotechnology of Plant Organelles; Springer: Berlin, Germany, 2004; pp. 151–177. [Google Scholar]
- Gualberto, J.M.; Newton, K.J. Plant mitochondrial genomes: Dynamics and mechanisms of mutation. Annu. Rev. Plant Biol. 2017, 68, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, Y.; Zhang, S.; Zou, R.; Tang, J.; Mu, W.; Peng, Y.; Dong, S. Assembly and comparative analysis of the complete mitochondrial genome sequence of Sophora japonica ‘JinhuaiJ2’. PLoS ONE 2018, 13, e0202485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, S.; Wu, Z.; Zhang, H.; Zheng, Y.; Zhu, Z.; Liang, D.; Ding, Y. The mitochondrial genome map of Nelumbo nucifera reveals ancient evolutionary features. Sci. Rep. 2016, 6, 30158. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Yang, T.; Du, T.; Huang, Y.; Chen, J.; Yan, J.; He, J.; Guan, R. Mitochondrial genome sequencing helps show the evolutionary mechanism of mitochondrial genome formation in Brassica. BMC Genom. 2011, 12, 497. [Google Scholar] [CrossRef] [Green Version]
- Shedge, V.; Arrieta-Montiel, M.; Christensen, A.C.; Mackenzie, S.A. Plant mitochondrial recombination surveillance requires unusual RecA and MutS homologs. Plant Cell 2007, 19, 1251–1264. [Google Scholar] [CrossRef] [Green Version]
- Bendich, A.J. Reaching for the ring: The study of mitochondrial genome structure. Curr. Genet. 1993, 24, 279–290. [Google Scholar] [CrossRef]
- Ono, Y.; Sakai, A.; Takechi, K.; Takio, S.; Takusagawa, M.; Takano, H. NtPolI-like1 and NtPolI-like2, bacterial DNA polymerase I homologs isolated from BY-2 cultured tobacco [Nicotiana tabacum] cells, encode DNA polymerases engaged in DNA replication in both plastids and mitochondria. Plant Cell Physiol. 2007, 48, 1679–1692. [Google Scholar] [CrossRef] [Green Version]
- Cupp, J.D.; Nielsen, B.L. Minireview: DNA replication in plant mitochondria. Mitochondrion 2014, 19, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Zhang, Q.; Yin, P. RNA editing machinery in plant organelles. Sci. China Life Sci. 2018, 61, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Giudice, C.L.; Hernández, I.; Ceci, L.R.; Pesole, G.; Picardi, E. RNA editing in plants: A comprehensive survey of bioinformatics tools and databases. Plant Physiol. Biochem. 2019, 137, 53–61. [Google Scholar] [CrossRef]
- Ichinose, M.; Sugita, M. RNA editing and its molecular mechanism in plant organelles. Genes 2017, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Steinhauser, S.; Beckert, S.; Capesius, I.; Malek, O.; Knoop, V. Plant mitochondrial RNA editing. J. Mol. Evol. 1999, 48, 303–312. [Google Scholar] [CrossRef]
- Lurin, C.; Andrés, C.; Aubourg, S.; Bellaoui, M.; Bitton, F.; Bruyère, C.; Caboche, M.; Debast, C.; Gualberto, J.; Hoffmann, B. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 2004, 16, 2089–2103. [Google Scholar] [CrossRef] [Green Version]
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef]
- Kovar, L.; Nageswara-Rao, M.; Ortega-Rodriguez, S.; Dugas, D.V.; Straub, S.; Cronn, R.; Strickler, S.R.; Hughes, C.E.; Hanley, K.A.; Rodriguez, D.N. PacBio-based mitochondrial genome assembly of Leucaena trichandra (Leguminosae) and an intrageneric assessment of mitochondrial RNA editing. Genome Biol. Evol. 2018, 10, 2501–2517. [Google Scholar] [CrossRef]
- Negruk, V. Mitochondrial genome sequence of the legume Vicia faba. Front. Plant Sci. 2013, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Cardi, T.; Giegé, P.; Kahlau, S.; Scotti, N. Expression profiling of organellar genes. In Genomics of Chloroplasts and Mitochondria; Springer: Berlin, Germany, 2012; pp. 323–355. [Google Scholar]
- Sloan, D.B.; Wu, Z.; Sharbrough, J. Correction of persistent errors in Arabidopsis reference mitochondrial genomes. Plant Cell 2018, 30, 525–527. [Google Scholar] [CrossRef] [Green Version]
- Unseld, M.; Marienfeld, J.R.; Brandt, P.; Brennicke, A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 1997, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Meiotic studies of the Convolvulaceae Juss. from Indian Hot Desert. Chromosom. Bot. 2018, 12, 77–85. [Google Scholar]
- Priya, T. Antimicrobial Activity of Evovulus Alisinoids (L) Extract with Different Organic Solvents in Pathogenic Bacteria and Fungal Species. Int. J. Appl. Nat. Sci. 2017, 6, 47–54. [Google Scholar]
- Austin, D.F. Evolvulus alsinoides (Convolvulaceae): An American herb in the old world. J. Ethnopharmacol. 2008, 117, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, V.; Yoganarasimhan, N.; Gurudeva, M.R. Pharmacognostical studies on Sankhapushpi (Convolvulus microphyllus Sieb. ex Spreng. and Evolvulus alsinoides (L.) L. Indian J. Tradit. Knowl. 2008, 7, 529–541. [Google Scholar]
- Rajakaruna, N.; Harris, C.S.; Towers, G.H.N. Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka. Pharm. Biol. 2002, 40, 235–244. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Nahata, A.; Singh, P.K.; Mishra, S.H. Neuropharmacological evaluation on four traditional herbs used as nervine tonic and commonly available as Shankhpushpi in India. J. Ayurveda Integr. Med. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Kunwar, R.M.; Bussmann, R.W.; Paniagua-Zambrana, N.Y.; Turi, M.A. Evolvulus alsinoides (L.) L. Convolvulaceae. In Ethnobotany of the Himalayas; Kunwar, R.M., Sher, H., Bussmann, R.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–9. [Google Scholar]
- Healey, A.; Furtado, A.; Cooper, T.; Henry, R.J. Protocol: A simple method for extracting next-generation sequencing quality genomic DNA from recalcitrant plant species. Plant Methods 2014, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, S.A.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Boetzer, M.; Pirovano, W. SSPACE-LongRead: Scaffolding bacterial draft genomes using long read sequence information. BMC Bioinform. 2014, 15, 211. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F. BLAST algorithm. e LS 2001. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA genes in genomic sequences. In Gene Prediction; Springer: Berlin, Germany, 2019; pp. 1–14. [Google Scholar]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Unequal base frequencies and the estimation of substitution rates. Mol. Biol. Evol. 1995, 12, 359. [Google Scholar]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [Green Version]
- Mower, J.P. PREP-Mt: Predictive RNA editor for plant mitochondrial genes. BMC Bioinform. 2005, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Sánchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernt, M.; Merkle, D.; Ramsch, K.; Fritzsch, G.; Perseke, M.; Bernhard, D.; Schlegel, M.; Stadler, P.F.; Middendorf, M. CREx: Inferring genomic rearrangements based on common intervals. Bioinformatics 2007, 23, 2957–2958. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Lin, Y.; Tang, J. MLGO: Phylogeny reconstruction and ancestral inference from gene-order data. BMC Bioinform. 2014, 15, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ling, C.; Luo, A.; Gao, J. MrBayes 3.2. 6 on Tianhe-1A: A high performance and distributed implementation of phylogenetic analysis. In Proceedings of the 2016 IEEE 22nd International Conference on Parallel and Distributed Systems (ICPADS), Wuhan, China, 13–16 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1181–1186. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901. [Google Scholar] [CrossRef] [Green Version]
- Lanfear, R.; Hua, X.; Warren, D.L. Estimating the effective sample size of tree topologies from Bayesian phylogenetic analyses. Genome Biol. Evol. 2016, 8, 2319–2332. [Google Scholar] [CrossRef] [Green Version]
- Stefanović, S.; Rice, D.W.; Palmer, J.D. Long branch attraction, taxon sampling, and the earliest angiosperms: Amborella or monocots? BMC Evol. Biol. 2004, 4, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Soltis, D.E.; Albert, V.A.; Savolainen, V.; Hilu, K.; Qiu, Y.-L.; Chase, M.W.; Farris, J.S.; Stefanović, S.; Rice, D.W.; Palmer, J.D. Genome-scale data, angiosperm relationships, and ‘ending incongruence’: A cautionary tale in phylogenetics. Trends Plant Sci. 2004, 9, 477–483. [Google Scholar] [CrossRef]
- Ma, P.-F.; Guo, Z.-H.; Li, D.-Z. Rapid sequencing of the bamboo mitochondrial genome using Illumina technology and parallel episodic evolution of organelle genomes in grasses. PLoS ONE 2012, 7, e30297. [Google Scholar] [CrossRef]
- Bergsten, J. A review of long-branch attraction. Cladistics 2005, 21, 163–193. [Google Scholar] [CrossRef]
- Siddall, M.E.; Whiting, M.F. Long-branch abstractions. Cladistics 1999, 15, 9–24. [Google Scholar] [CrossRef]
- Ma, Q.; Li, S.; Bi, C.; Hao, Z.; Sun, C.; Ye, N. Complete chloroplast genome sequence of a major economic species, Ziziphus jujuba (Rhamnaceae). Curr. Genet. 2017, 63, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Sun, M. Genetic diversity and relationships of sweetpotato and its wild relatives in Ipomoea series Batatas (Convolvulaceae) as revealed by inter-simple sequence repeat (ISSR) and restriction analysis of chloroplast DNA. Theor. Appl. Genet. 2000, 100, 1050–1060. [Google Scholar] [CrossRef]
- Bock, R.; Khan, M.S. Taming plastids for a green future. TRENDS Biotechnol. 2004, 22, 311–318. [Google Scholar] [CrossRef]
- Chen, H.; Deng, L.; Jiang, Y.; Lu, P.; Yu, J. RNA Editing Sites Exist in Protein-coding Genes in the Chloroplast Genome of Cycas taitungensis. J. Integr. Plant Biol. 2011, 53, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Park, S. Analysis of the complete chloroplast genome of a medicinal plant, Dianthus superbus var. longicalyncinus, from a comparative genomics perspective. PLoS ONE 2015, 10, e0141329. [Google Scholar] [CrossRef] [Green Version]
- Wakasugi, T.; Hirose, T.; Horihata, M.; Tsudzuki, T.; Kössel, H.; Sugiura, M. Creation of a novel protein-coding region at the RNA level in black pine chloroplasts: The pattern of RNA editing in the gymnosperm chloroplast is different from that in angiosperms. Proc. Natl. Acad. Sci. USA 1996, 93, 8766–8770. [Google Scholar] [CrossRef] [Green Version]
- Moreira, S.; Valach, M.; Aoulad-Aissa, M.; Otto, C.; Burger, G. Novel modes of RNA editing in mitochondria. Nucleic Acids Res. 2016, 44, 4907–4919. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-H.; Gojobori, T. Rapid evolution of goat and sheep globin genes following gene duplication. Mol. Biol. Evol. 1983, 1, 94–108. [Google Scholar] [PubMed] [Green Version]
- Hughes, A.L.; Nei, M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 1988, 335, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Liberles, D.A. A systematic search for positive selection in higher plants (Embryophytes). BMC Plant Biol. 2006, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pareek, A.; Mishra, D.; Rathi, D.; Verma, J.K.; Chakraborty, S.; Chakraborty, N. The small heat shock proteins, chaperonin 10, in plants: An evolutionary view and emerging functional diversity. Environ. Exp. Bot. 2021, 182, 104323. [Google Scholar] [CrossRef]
- Robba, L.; Russell, S.J.; Barker, G.L.; Brodie, J. Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am. J. Bot. 2006, 93, 1101–1108. [Google Scholar] [CrossRef]
- Song, H.; Chen, Y.; Gibson, K.; Liu, S.; Yu, Z.; Chen, N. High genetic diversity of the harmful algal bloom species Phaeocystis globosa revealed using the molecular marker COX1. Harmful Algae 2021, 107, 102065. [Google Scholar] [CrossRef]
- Sharp, P.M.; Cowe, E. Synonymous codon usage in Saccharomyces cerevisiae. Yeast 1991, 7, 657–678. [Google Scholar] [CrossRef]
- Bi, C.; Lu, N.; Xu, Y.; He, C.; Lu, Z. Characterization and Analysis of the Mitochondrial Genome of Common Bean (Phaseolus vulgaris) by Comparative Genomic Approaches. Int. J. Mol. Sci. 2020, 21, 3778. [Google Scholar] [CrossRef]
- Richardson, A.O.; Rice, D.W.; Young, G.J.; Alverson, A.J.; Palmer, J.D. The “fossilized” mitochondrial genome of Liriodendron tulipifera: Ancestral gene content and order, ancestral editing sites, and extraordinarily low mutation rate. BMC Biol. 2013, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Weihe, A.; Börner, T. The mitochondrial genome of higher plants. Proc. Plant Sci. 1984, 93, 305–316. [Google Scholar]



| Group of Genes | Gene Names |
|---|---|
| Complex I (NADH dehydrogenase) | nad1, nad2 a,c, nad3, nad4 a, nad4l, nad5 a, nad6, nad7 a,d, nad9 |
| Complex II (succinate dehydrogenase) | sdh3 |
| Complex III (ubiquinol cytochrome c reductase) | cob |
| Complex IV (cytochrome c oxidase) | cox1 a, cox2 b(2) |
| Complex V (ATP synthase) | atp1, atp4, atp6, atp8, atp9 b(2) |
| Cytochrome c biogenesis | ccmc, ccmfca |
| Ribosomal proteins (SSU) | rps3 a, rps4 b(2), rps12,rps13, rps14,rps19 |
| Ribosomal proteins (LSU) | rpl5, rpl10 b(2), rpl16 |
| Maturases | matr |
| Transport membrane protein | mttB |
| Ribosomal RNAs | rrn26 b(2), rrnS b(3), rrnLb |
| Transfer RNAs | trnC a, trnG a, trnH b(4), trnL b(3) trnM b(8), trnN, trnP, trnR b(2), trnS, trnV b(2), trnW a, trnY |
| Species Name | Genome Size | Total Gene Content | No of PCGs | rRNA | tRNA | AT% | GC% | AT Skew | GC Skew |
|---|---|---|---|---|---|---|---|---|---|
| E. alsinoides | 344184 | 67 | 35 | 6 | 26 | 56.46 | 43.54 | 0.0016 | 0.0018 |
| I. nil | 265768 | 53 | 30 | 3 | 20 | 55.55 | 44.45 | 0.0075 | −0.0012 |
| N. tabaccum | 430597 | 62 | 35 | 4 | 23 | 55.04 | 44.96 | 0.0072 | 0.0057 |
| N. attenuata | 394341 | 68 | 40 | 4 | 24 | 55.01 | 45.05 | 0.0020 | 0.0086 |
| N. sylverstris | 430597 | 64 | 37 | 4 | 23 | 55.04 | 44.96 | 0.0072 | 0.0057 |
| S. lycopersicum | 446257 | 41 | 39 | 5 | 27 | 54.93 | 45.07 | 0.0025 | −0.0005 |
| S.pennellii | 423596 | 70 | 41 | 5 | 24 | 55.01 | 44.99 | −0.0059 | −0.0050 |
| C. annum | 511530 | 61 | 33 | 3 | 25 | 55.48 | 44.52 | 0.0069 | 0.0019 |
| H. niger | 501401 | 70 | 38 | 4 | 28 | 54.82 | 45.18 | −0.0015 | 0.0003 |
| P. orientalis | 684857 | 65 | 37 | 4 | 24 | 55.19 | 44.81 | −0.0001 | −0.8216 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shidhi, P.R.; Biju, V.C.; Anu, S.; Vipin, C.L.; Deelip, K.R.; Achuthsankar, S.N. Genome Characterization, Comparison and Phylogenetic Analysis of Complete Mitochondrial Genome of Evolvulus alsinoides Reveals Highly Rearranged Gene Order in Solanales. Life 2021, 11, 769. https://doi.org/10.3390/life11080769
Shidhi PR, Biju VC, Anu S, Vipin CL, Deelip KR, Achuthsankar SN. Genome Characterization, Comparison and Phylogenetic Analysis of Complete Mitochondrial Genome of Evolvulus alsinoides Reveals Highly Rearranged Gene Order in Solanales. Life. 2021; 11(8):769. https://doi.org/10.3390/life11080769
Chicago/Turabian StyleShidhi, Pattayampadam Ramakrishnan, Vadakkemukadiyil Chellappan Biju, Sasi Anu, Chandrasekharan Laila Vipin, Kumar Raveendran Deelip, and Sukumaran Nair Achuthsankar. 2021. "Genome Characterization, Comparison and Phylogenetic Analysis of Complete Mitochondrial Genome of Evolvulus alsinoides Reveals Highly Rearranged Gene Order in Solanales" Life 11, no. 8: 769. https://doi.org/10.3390/life11080769
APA StyleShidhi, P. R., Biju, V. C., Anu, S., Vipin, C. L., Deelip, K. R., & Achuthsankar, S. N. (2021). Genome Characterization, Comparison and Phylogenetic Analysis of Complete Mitochondrial Genome of Evolvulus alsinoides Reveals Highly Rearranged Gene Order in Solanales. Life, 11(8), 769. https://doi.org/10.3390/life11080769






