Monitoring Air Quality with Transplanted Bryophytes in a Neotropical Andean City
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design and Data Collection
2.3. Elemental Bioccumulation
2.4. Data Analysis
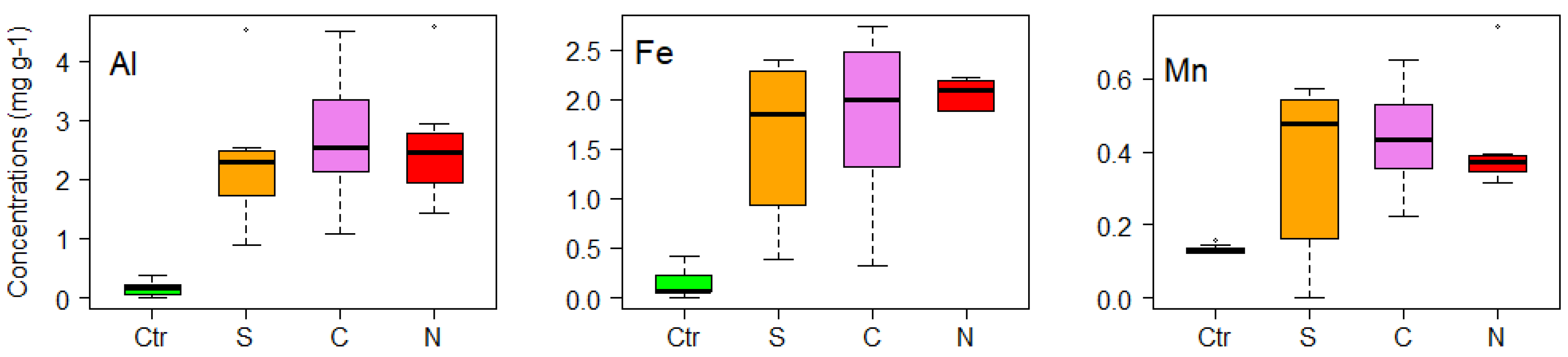
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Ojo, F.P.; Oluseye, O.C.; Abiola, O.G. Mosses as Biomonitors of Heavy Metal Deposition in the Atmosphere. Int. J. Environ. Sci. 2012, 1, 56–62. [Google Scholar]
- Mage, D.; Ozolins, G.; Peterson, P.; Webster, A.; Orthofer, R.; Vandeweerd, V.; Gwynne, M. Urban air pollution in megacities of the world. Atmos. Environ. 1996, 30, 681–686. [Google Scholar] [CrossRef]
- Gee, I.L.; Sollars, C.J. Ambient air levels of volatile organic compounds in Latin American and Asian cities. Chemosphere 1998, 36, 2497–2506. [Google Scholar] [CrossRef]
- Zhang, K.; Batterman, S. Air pollution and health risks due to vehicle traffic. Sci. Total Environ. 2013, 450, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amegah, A.K.; Jaakkola, J.J.K. Work as a street vendor, associated traffic-related air pollution exposures and risk of adverse pregnancy outcomes in Accra, Ghana. Int. J. Environ. Res. Public Health 2014, 217, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.; Urošević, M.A.; Goryainova, Z.; Pergal, M.; Škrivanj, S.; Samson, R.; Popović, A. Active moss biomonitoring for extensive screening of urban air pollution: Magnetic and chemical analyses. Sci. Total Environ. 2015, 521, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yan, Y.; Zhou, X.; Wang, Y.; Fang, Y. Monitoring Metal Contents with Sphagnum junghuhnianum Moss Bags in Relation to Traffic Volume in Wuxi, China. Int. J. Environ. Res. Public Health 2018, 15, 374. [Google Scholar] [CrossRef] [Green Version]
- Alexandrino, K.; Viteri, F.; Rybarczyk, Y.; Andino, J.E.G.; Zalakeviciute, R. Biomonitoring of metal levels in urban areas with different vehicular traffic intensity by using Araucaria heterophylla needles. Ecol. Indic. 2020, 117, 106701. [Google Scholar] [CrossRef]
- Capozzi, F.; Giordano, S.; Di Palma, A.; Spagnuolo, V.; De Nicola, F.; Adamo, P. Biomonitoring of atmospheric pollution by moss bags: Discriminating urban-rural structure in a fragmented landscape. Chemosphere 2016, 149, 211–218. [Google Scholar] [CrossRef]
- Payus, C.M.; Thevan, A.V.; Sentian, J. Impact of school traffic on outdoor carbon monoxide levels. City Environ. Interact. 2019, 4, 100032. [Google Scholar] [CrossRef]
- Charron, A.; Polo-Rehn, L.; Besombes, J.L.; Golly, B.; Buisson, C.; Chanut, H.; Jaffrezo, J.L. Identification and quantification of particulate tracers of exhaust and non-exhaust vehicle emissions. Atmos. Chem. Phys. 2019, 19, 5187–5207. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Martin, M.H.; Duncan, E.M.; Coughtrey, P.J. The distribution of metals in a contaminated woodland ecosystem. Environ. Pollut. B Chem. Phys. 1982, 3, 147–157. [Google Scholar] [CrossRef]
- Saiki, M.; Chaparro, C.G.; Vasconcellos, M.B.A.; Marcelli, M.P. Determination of trace elements in liquid fuels by instrumental neutron activation analysis. Anal. Lett. 1977, 217, 111–115. [Google Scholar] [CrossRef]
- Cucu-Man, S.; Mocanu, R.; Steinnes, E. Atmospheric heavy metal survey by means of mosses: A regional study (Iasi, Romania). Environ. Eng. Manag. J. 2002, 1, 533–540. [Google Scholar] [CrossRef]
- Besse, J.P.; Geffard, O.; y Coquery, M. Relevance and applicability of active biomonitoring in continental waters under the Water Framework Directive. TrAC-Trends Anal. Chem. 2012, 36, 113–127. [Google Scholar] [CrossRef]
- Govindapyari, H.; Leleeka, M.; Nivedita, M.; Uniyal, P.L. Bryophytes: Indicators and monitoring agents of pollution. NeBIO 2010, 1, 35–41. [Google Scholar] [CrossRef]
- Sahu, V.; Asthana, A.K.; Nath, V.; Yunus, M. Bryophytes: A useful tool in heavy metal monitoring. EnviroNews 2007, 13, 1–2. [Google Scholar]
- Kuik, P.; Wolterbeek, H.T. Factor analysis of atmospheric trace-element deposition data in the netherlands obtained by moss monitoring. Water Air Soil Pollut. 1995, 84, 323–346. [Google Scholar] [CrossRef]
- Falla, J.; Laval-Gilly, P.; Henryon, M.; Morlot, D.; Ferard, J.F. Biological air quality monitoring: A review. Environ. Monit. Assess. 2000, 64, 627–644. [Google Scholar] [CrossRef]
- Onianwa, P.C. Monitoring atmospheric metal pollution: A review of the use of mosses as indicators. Environ. Monit. Assess. 2001, 71, 13–50. [Google Scholar] [CrossRef] [PubMed]
- Fabure, J.; Meyer, C.; Denayer, F.; Gaudry, A.; Gilbert, D.; Bernard, N. Accumulation capacities of particulate matter in an acrocarpous and a pleurocarpous moss exposed at three differently polluted sites (industrial, urban and rural). Water Air Soil Pollut. 2010, 212, 205–217. [Google Scholar] [CrossRef]
- Cao, T.; Wang, M.; An, L.; Yu, Y.; Lou, Y.; Guo, S.; Zhu, Z. Air quality for metals and sulfur in Shanghai, China, determined with moss bags. Environ. Pollut. 2009, 157, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Biziuk, M. Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environ. Res. 2003, 93, 221–230. [Google Scholar] [CrossRef]
- Kosior, G.; Samecka-Cymerman, A.; Brudzińska-Kosior, A. Transplanted moss Hylocomium splendens as a bioaccumulator of trace elements from different categories of sampling sites in the Upper Silesia Area (SW Poland): Bulk and dry deposition impact. Bull. Environ. Contam. Toxicol. 2018, 101, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Adamo, P.; Bargagli, R.; Giordano, S.; Modenesi, P.; Monaci, F.; Pittao, E.; Tretiach, M. Natural and pre-treatments induced variability in the chemical composition and morphology of lichens and mosses selected for active monitoring of airborne elements. Environ. Pollut. 2008, 152, 11–19. [Google Scholar] [CrossRef]
- Fernández, J.A.; Aboal, J.R.; Carballeira, A. Use of native and transplanted mosses as complementary techniques for biomonitoring mercury around an industrial facility. Sci. Total Environ. 2000, 256, 151–161. [Google Scholar] [CrossRef]
- Ares, A.; Fernández, J.A.; Carballeira, A.; Aboal, J.R. Towards the methodological optimization of the moss bag technique in terms of contaminants concentrations and replicability values. Atmos. Environ. 2014, 94, 496–507. [Google Scholar] [CrossRef]
- Aničić, M.; Tomašević, M.; Tasić, M.; Rajšić, S.; Popović, A.; Frontasyeva, M.V.; Steinnes, E. Monitoring of trace element atmospheric deposition using dry and wet moss bags: Accumulation capacity versus exposure time. J. Hazard. Mat. 2009, 171, 182–188. [Google Scholar] [CrossRef]
- Rodríguez-Quiel, E.; Arrocha, C.; Salazar-Allen, N. Utilization of Thuidium delicatulum (Hedw) Mitt. as bioindicator for environmental zinc, copper and lead contamination in Boquete, Province of Chiriquí, Panamá. Trop. Bryol. 2010, 32, 14–18. [Google Scholar]
- Munar, M.P.; Oreiro, R.R.B.; Hipol, R.M. Ectohydric moss, Thuidium tamariscellum, monitors atmospheric Lead (Pb) pollution in Baguio City, Philippines. Trop. Plant Res. 2014, 1, 4–7. [Google Scholar]
- Ellis, J.B.; Revitt, D.M. Incidence of heavy metals in street surface sediments: Solubility and grain size studies. Water Air Soil Pollut. 1982, 1, 87–100. [Google Scholar]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- Noriega, P.; Medici, A.; Murillo, A.; Bedón, J.; Haro, F.; Galecio, G. Estudio de la concentración de cadmio y plomo en el aire de la ciudad de Quito, empleando briofitas como biomonitores. La Granja 2008, 8, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Jiménez, D.; Cueva-Agila, A.; Prieto, M.; Aragón, G.; Benítez, Á. Cambios en la composición de líquenes epífitos relacionados con la calidad del aire en la ciudad de Loja (Ecuador). Caldasia 2015, 37, 333–343. [Google Scholar] [CrossRef]
- Benitez, Á.; Medina, J.; Vázquez, C.; Loaiza, T.; Luzuriaga, Y.; Calva, J. Lichens and Bromeliads as Bioindicators of Metal Deposition in Ecuador. Diversity 2019, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Ares, A.; Aboal, J.R.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernández, J.A. Moss bag biomonitoring: A methodological review. Sci. Total Environ. 2012, 432, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, C.; Calva, J.; Morocho, R.; Donoso, D.A.; Benítez, Á. Bryophyte Communities along a Tropical Urban River Respond to Heavy Metal and Arsenic Pollution. Water 2019, 11, 813. [Google Scholar] [CrossRef] [Green Version]
- Benítez, Á.; Torres, S.; Morocho, R.; Carrillo, W.; Donoso, D.A.; Calva, J. Platyhypnidium aquaticum as Bioindicator of Metal and Metalloid Contamination of River Water in a Neotropical Mountain City. Plants 2020, 9, 974. [Google Scholar] [CrossRef]
- Frahm, J.P. Revision of the genus Rhacocarpus Lindb.(Musci). Cryptogam. Bryol. Lichenol. 1996, 17, 39–66. [Google Scholar]
- Robinson, H. Scanning electron microscope studies on moss leaves and peristomes. Bryologist 1971, 74, 473–483. [Google Scholar] [CrossRef]
- Valarezo, E.; Vidal, V.; Calva, J.; Jaramillo, S.P.; Febres, J.; Benítez, Á. Essential Oil Constituents of Mosses Species from Ecuador. TEOP 2018, 1, 189–197. [Google Scholar] [CrossRef]
- Pakarinen, P.; Tolonen, K. Regional Survey of Heavy Metals in Peat Mosses (Sphagnum). Ambio 1976, 5, 38–40. [Google Scholar]
- Arafat, N.M.; Glooschenko, W.A. The use of bog vegetation as an indicator of atmospheric deposition of arsenic in Northern Ontario. Environ. Pollut. B 1982, 4, 85–90. [Google Scholar] [CrossRef]
- Percy, K. Heavy metal and sulphur concentrations in Sphagnum magellanicum Brid. in the maritime provinces, Canada. Water Air Soil Pollut. 1983, 19, 341–349. [Google Scholar]
- Giordano, S.; Adamo, P.; Sorbo, S.; Vingiani, S. Atmospheric trace metal pollution in the Naples urban area based on results from moss and lichen bags. Environ. Pollut. 2005, 136, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.S.; Lehman, M.E. Bioindication of atmospheric heavy metal deposition in the Southeastern US using the moss Thuidium delicatulum. Atmos. Environ. 2002, 36, 1611–1618. [Google Scholar] [CrossRef]
- Dragovic, S.; Mihailovic, N.; Gajic, B. Quantification of transfer of (238) U, (226) Ra, (232) Th, (40) K and (137) Cs in mosses of a semi-natural ecosystem. J. Environ. Rad. 2010, 101, 159–164. [Google Scholar] [CrossRef]
- Käffer, M.I.; De Azevedo Martins, S.M.; Alves, C.; Pereira, V.C.; Fachel, J.; Vargas, V.M.F. Corticolous lichens as environmental indicators in urban areas in southern Brazil. Ecol. Indic. 2011, 11, 1319–1332. [Google Scholar] [CrossRef]
- Huang, L.; Bell, R.W.; Dell, B.; Woodward, J. Rapid nitric acid digestion of plant material with an open-vessel microwave system. Commun. Soil Sci. Plant Anal. 2004, 35, 427–440. [Google Scholar] [CrossRef]
- Debén, S.; Aboal, J.; Carballeira, A.; Cesa, M.; Real, C.; Fernández, J. Inland water quality monitoring with native bryophytes a methodological review. Ecol. Indic. 2015, 53, 115–124. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio Team: Boston, MA, USA, 2016. [Google Scholar]
- Tretiach, M.; Pittao, E.; Crisafulli, P.; Adamo, P. Influence of exposure sites on trace element enrichment in moss-bags and characterization of particles deposited on the biomonitor surface. Sci. Total Environ. 2011, 409, 822–830. [Google Scholar] [CrossRef]
- Monaci, F.; Moni, F.; Lanciotti, E.; Grechi, D.; Bargagli, R. Biomonitoring of airborne metals in urban environments: New tracers of vehicle emission, in place of lead. Environ. Pollut. 2000, 107, 321–327. [Google Scholar] [CrossRef]
- Aničić, M.; Frontasyeva, M.V.; Tomašević, M.; Popović, A. Assessment of Atmospheric Deposition of Metals and Other Elements in Belgrade Using the Moss Biomonitoring Technique and Neutron Activation Analysis. Environ. Monit. Assess. 2007, 129, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, H.G.; Hohenwallner, D.; Riss, A.; Hanus-Illar, A. Estimation of element deposition derived from road traffic sources by using mosses. Environ. Pollut. 2005, 138, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Kuhlbusch, T.A.J.; Querol, X.; Alastuey, A.; Harrison, R.M.; Hopke, P.K.; Hitzenberger, R. Source apportionment of particulate matter in Europe: A review of methods and results. Aerosol Sci. 2008, 39, 827–849. [Google Scholar] [CrossRef]
- Adamo, P.; Giordano, S.; Sforza, A.; Bargagli, R. Implementation of airborne trace element monitoring with devitalised transplants of Hypnum cupressiforme Hedw: Assessment of temporal trends and element contribution by vehicular traffic in Naples city. Environ. Pollut. 2011, 159, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.; Anicić, M.; Razumenic, I.; Kuzmanoski, M.; Pergal, M.; Skrivanj, S.; Popović, A. Air quality in urban parking garages (PM 10, major and trace elements, PAHs): Instrumental measurements vs. active moss biomonitoring. Atmos. Environ. 2014, 85, 31–40. [Google Scholar] [CrossRef]
- Mohanraj, R.; Azeez, P.A.; Priscilla, T. Heavy metals in airborne particulate matter of urban Coimbatore. Arch. Environ. Cont. Toxicol. 2004, 47, 162–167. [Google Scholar] [CrossRef]
- Edelmann, H.G.; Neinhuis, C.; Jarvis, M.; Evans, B.; Fischer, E.; Barthlott, W. Ultrastructure and chemistry of the cell wall of the moss Rhacocarpus purpurascens (Rhacocarpaceae): A puzzling architecture among plants. Planta 1998, 49, 315–321. [Google Scholar] [CrossRef]
- González, A.G.; Pokrovsky, O.S. Metal adsorption on mosses: Towards a universal adsorption model. J. Colloid Interface Sci. 2013, 1, 39. [Google Scholar] [CrossRef] [Green Version]
- Saitanis, C.J.; Frontasyeva, M.V.; Steinnes, E.; Palmer, M.W.; Ostrovnaya, T.M.; Gundorina, S.F. Spatiotemporal distribution of airborne elements monitored with the moss bags technique in the Greater Thriasion Plain, Attica, Greece. Environ. Monit. Assess. 2013, 185, 955–968. [Google Scholar] [CrossRef]
- Ross, H.B. On the use of mosses (Hylocomium splendens and Pleurozium schreberi) for estimating atmospheric trace metal deposition. Water Air Soil Pollut. 1990, 50, 63–76. [Google Scholar] [CrossRef]
- Zechmeister, H.G. Annual growth of four pleurocarpous moss species and their applicability for biomonitoring heavy metals. Environ. Monit. Assess. 1998, 52, 441–451. [Google Scholar] [CrossRef]
- Reimann, C.; Niskavaara, H.; Kashulina, G.; Filzmoser, P.; Boyd, R.; Volden, T.; Bogatyrev, I. Critical remarks on the use of terrestrial moss (Hylocomium splendens and Pleurozium schreberi) for monitoring of airborne pollution. Environ. Pollut. 2001, 113, 41–57. [Google Scholar] [CrossRef]
- Castello, M. A Comparison Between Two Moss Species Used as Transplants for Airborne Trace Element Biomonitoring in NE Italy. Environ. Monit. Assess. 2007, 133, 267–276. [Google Scholar] [CrossRef]
- Gjengedal, E.; Steinnes, E. Uptake of metal ions in moss from artificial precipitation. Environ. Monit. Assess. 1990, 14, 77–87. [Google Scholar] [CrossRef] [PubMed]






| Species | Metal | Control | South | Central | North | p Value | r | p Value |
|---|---|---|---|---|---|---|---|---|
| Rhacocarpus purpurascens | Al | 2.783 ± 0.623 | 5.090 ± 2.339 | 3.876 ± 2.066 | 3.948 ± 1.902 | 0.041 | 0.246 | 0.115 |
| Fe | 1.228 ± 0.372 | 4.170 ± 3.674 | 3.064 ± 1.366 | 3.331 ± 0.867 | 0.0004 | 0.398 | 0.009 | |
| Mn | 0.086 ± 0.010 | 0.173 ± 0.040 | 0.221 ± 0.030 | 0.223 ± 0.069 | <0.0001 | 0.801 | <0.0001 | |
| Pb | 0.006 ± 0.007 | 0.005 ± 0.003 | 0.005 ± 0.002 | 0.005 ± 0.002 | 0.696 | −0.160 | 0.309 | |
| Zn | 0.042 ± 0.078 | 0.086 ± 0.026 | 0.131 ± 0.044 | 0.107 ± 0.023 | 0.0050 | 0.5652 | <0.0001 | |
| Sphagnum sp. | Al | 2.771 ±0.570 | 3.718 ± 2.109 | 4.545 ± 1.335 | 4.429 ± 1.5342 | 0.0124 | 0.485 | 0.002 |
| Fe | 1.368 ± 0.411 | 6.197 ± 2.553 | 5.396 ± 1.596 | 4.281 ± 1.5420 | <0.0001 | 0.789 | <0.0001 | |
| Mn | 0.147 ± 0.023 | 0.186 ± 0.057 | 0.241 ± 0.168 | 0.188 ± 0.0527 | 0.012 | 0.343 | 0.041 | |
| Pb | 0.004 ± 0.003 | 0.006 ± 0.003 | 0.006 ± 0.003 | 0.006 ± 0.0026 | 0.44 | 0.278 | 0.099 | |
| Zn | 0.026 ± 0.010 | 0.110 ± 0.017 | 0.149 ± 0.047 | 0.130 ± 0.055 | <0.0001 | 0.831 | <0.0001 | |
| Thuidium delicatulum | Al | 0.151 ± 0.107 | 2.312 ± 1.048 | 2.750 ± 1.061 | 2.560 ± 1.027 | <0.0001 | 0.803 | <0.0001 |
| Fe | 0.309 ± 0.646 | 3.453 ± 5.558 | 2.238 ± 1.701 | 2.176 ± 1.11 | <0.0001 | 0.368 | 0.022 | |
| Mn | 0.132 ± 0.011 | 0.367 ± 0.239 | 0.441 ± 0.127 | 0.414 ± 0.148 | <0.0001 | 0.687 | <0.0001 | |
| Pb | 0.001 ± 0.002 | 0.001 ± 0.002 | 0.002 ± 0.002 | 0.002 ± 0.002 | 0.0035 | 0.271 | 0.039 | |
| Zn | 0.015 ± 0.018 | 0.075 ± 0.047 | 0.131 ± 0.035 | 0.105 ± 0.033 | <0.0001 | 0.768 | <0.0001 |
| Tukey Test | Al | Dunn Test | Fe | Mn | Pb | Zn | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Est | p-Value | Zone | Est | p-Value | Est | p-Value | Est | p-Value | Est | p-Value |
| N-C | 0.073 | 1.000 | N-C | −0.567 | 1 | 0.445 | 1 | 0.107 | 1 | 0.768 | 1 |
| S-C | 1.214 | 0.422 | S-C | 0.010 | 1 | 1.741 | 0.2452 | 0.248 | 1 | 1.537 | 0.371 |
| Ctr-C | −1.092 | 0.379 | T-C | 3.391 | 0.0021 | 5.137 | < 0.0001 | 1.113 | 0.797 | 3.490 | 0.0015 |
| S-N | 1.142 | 0.593 | S-N | 0.523 | 1 | 1.108 | 0.8034 | 0.1181 | 1 | 0.639 | 1 |
| Ctr-N | −1.165 | 0.484 | T-N | 3.396 | 0.0021 | 3.823 | 0.0004 | 0.8176 | 1 | 2.126 | 0.101 |
| Ctr-S | −2.307 | 0.025 | T-S | 2.936 | 0.0100 | 2.700 | 0.0208 | 0.7160 | 1 | 1.413 | 0.421 |
| Tukey Test | Al | Fe | Pb | Dunn Test | Mn | Zn | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Est | p-Value | Est | p-Value | Est | p-Value | Zone | Est | p-Value | Est | p-Value |
| N-C | −0.117 | 1 | −1.115 | 0.9650 | −4.413 | 0.990 | N-C | −0.273 | 1 | 0.807 | 1 |
| S-C | −0.827 | 0.654 | 0.8008 | 0.3849 | −4.689 | 0.990 | S-C | 0.483 | 1 | 1.348 | 0.5335 |
| Ctr-C | −1.774 | 0.023 | −4.028 | <0.0001 | −1.917 | 0.462 | T-C | 2.539 | 0.033 | 4.284 | 0.0001 |
| S-N | −0.711 | 0.751 | 1.9125 | 0.1913 | −2.767 | 0.999 | S-N | 0.736 | 1 | 0.601 | 1 |
| Ctr-N | −1.657 | 0.034 | −2.912 | <0.0001 | −1.475 | 0.670 | T-N | 2.847 | 0.013 | 3.373 | 0.0022 |
| Ctr-S | −0.947 | 0.464 | −4.829 | 0.0130 | −1.448 | 0.742 | T-S | 1.772 | 0.229 | 2.399 | 0.049 |
| Dunn Test | Al | Fe | Mn | Pb | Tukey Test | Zn | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Est | p-Value | Est | p-Value | Est | p-Value | Est | p-Value | Zone | Est | p-Value |
| N-C | 0.251 | 1 | −0.183 | 1 | 0.554 | 1 | −0.715 | 1 | N-C | −0.026 | 0.395 |
| S-C | 0.801 | 1 | 0.238 | 1 | 0.735 | 1 | 1.603 | 0.3441 | S-C | −0.056 | 0.005 |
| Ctr-C | 4.449 | <0.0001 | 3.512 | 0.0013 | 3.890 | 0.0003 | 2.847 | 0.0145 | T-C | −0.115 | <0.0001 |
| S-N | 0.484 | 1 | 0.385 | 1 | 0.143 | 1 | 2.107 | 0.1136 | S-N | −0.030 | 0.317 |
| Ctr-N | 3.649 | 0.0005 | 3.273 | 0.0032 | 2.852 | 0.0130 | 3.225 | 0.0043 | T-N | −0.089 | <0.0001 |
| Ctr-S | 3.253 | 0.0034 | 2.974 | 0.0088 | 2.809 | 0.0149 | 0.972 | 0.9956 | T-S | −0.059 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, Á.; Armijos, L.; Calva, J. Monitoring Air Quality with Transplanted Bryophytes in a Neotropical Andean City. Life 2021, 11, 821. https://doi.org/10.3390/life11080821
Benítez Á, Armijos L, Calva J. Monitoring Air Quality with Transplanted Bryophytes in a Neotropical Andean City. Life. 2021; 11(8):821. https://doi.org/10.3390/life11080821
Chicago/Turabian StyleBenítez, Ángel, Lizbeth Armijos, and James Calva. 2021. "Monitoring Air Quality with Transplanted Bryophytes in a Neotropical Andean City" Life 11, no. 8: 821. https://doi.org/10.3390/life11080821
APA StyleBenítez, Á., Armijos, L., & Calva, J. (2021). Monitoring Air Quality with Transplanted Bryophytes in a Neotropical Andean City. Life, 11(8), 821. https://doi.org/10.3390/life11080821








