Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration
Abstract
:1. Introduction
1.1. The Space Radiation Environment
1.2. Measuring the Biological Effects of Radiation
1.3. Exploration Class Mission Profiles and the Need for Countermeasures
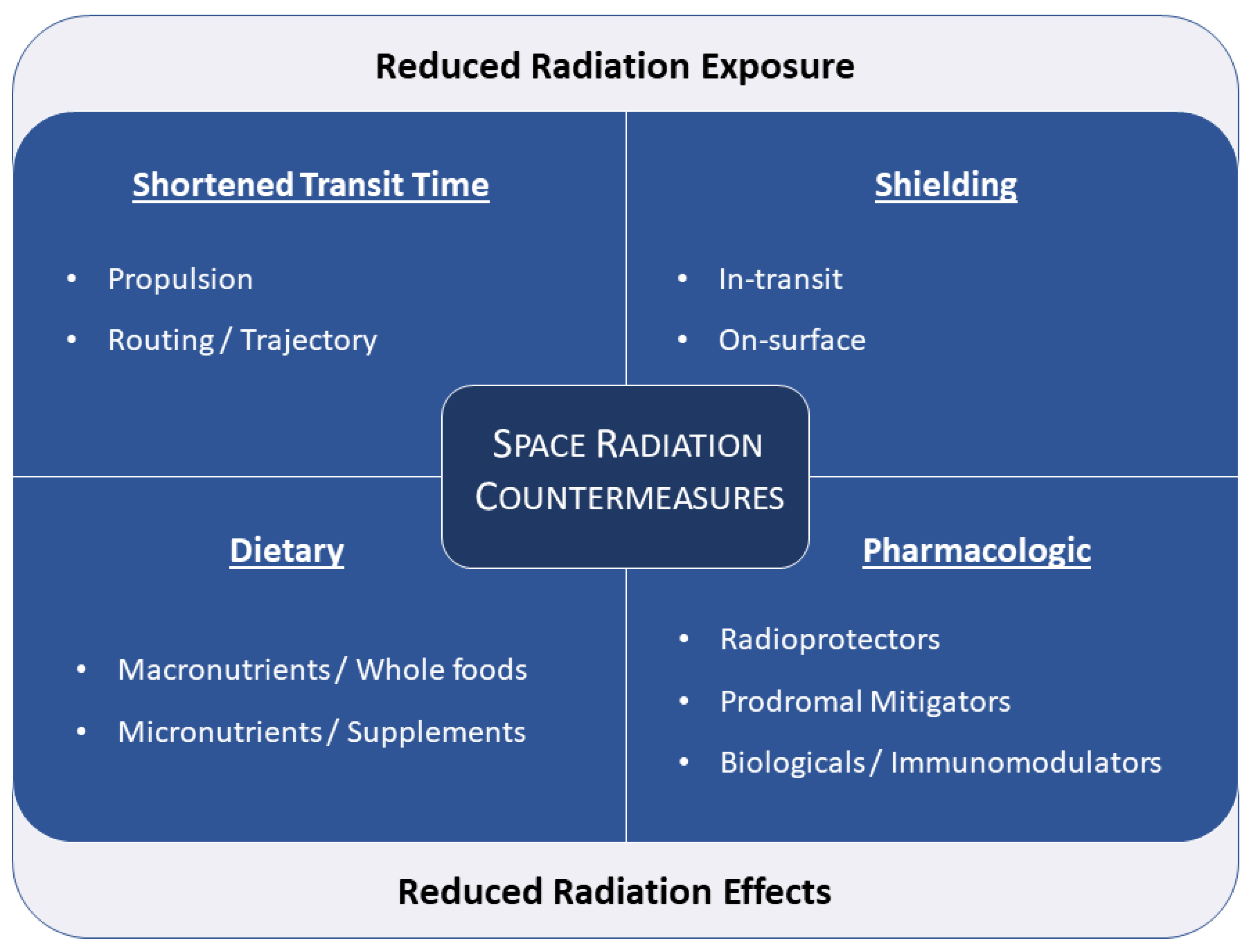
2. Countermeasures
2.1. Shielding
2.2. Transfer Time Reduction
2.3. Nutritional
2.3.1. Neuro-Cognitive Decline
2.3.2. Physical Endurance
2.3.3. Longevity and Reduction in Carcinogenesis
2.3.4. Epigenetics, Ophthalmic Change, and Impact on Visual Health
2.3.5. Safety and Tolerability
2.4. Pharmacologic
2.4.1. Pharmaceutical Radioprotectors
Strategy for Use of Radiation Protector Pharmacologic Agents
Multiple Pathways of Cell Death
Polypharmacy and Radiation Protection
2.4.2. Managing Higher Radiation Exposures
2.5. Immunomodulation
2.5.1. Antiradiation Antidote with Suppression Activity of Membrane Attack Complex (MAC)
2.5.2. Antiradiation DNA and RNA Vaccines
2.5.3. Immunomodulation of Activity of B-cells after Irradiation
2.5.4. Immunomodulation of Cytotoxic Activity of T-cells after Irradiation
2.5.5. Microgravity and the Immune System
3. Discussion
- Provide low weight, neutron-poor shielding in the transit vehicle and surface habitats, to include more heavily shielded storm shelters for the episodic SPE’s that employ easy-to-implement shielding strategies. Surface habitats can utilize naturally shielded areas e.g., lava tubes or regolith if an earth-moving capability is deployed.
- Have real-time active dosimetry and monitoring (including on EVA suits) which captures high-energy neutrons and includes real-time SPE alarm functions and automated/improved SPE forecasting
- Develop and fly minimally invasive radiation bioeffects monitoring equipment which may include biomarkers, tissue antioxidant levels, and cytogenetics
- Validate a radiation countermeasures nutritive and pharmacologic program. This will include an array of field-tested radioprotective molecules and chemoprevention agents, which are shown to be nontoxic, easily administered, of high bioavailability that could be given alone or in combination to ameliorate or prevent radiation cellular and tissue damage. The pharmacokinetics, pharmacodynamics, tissue distribution, safety, and efficacy of such agents need to be thoroughly characterized in both Earth-based simulated conditions and in microgravity with concomitant space radiation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. Immunomodulatory Background: Impact of Space Radiation Exposure on the Immune System
- Reduce activation of acute immunogenic, autoimmune reactions, and prevent mass destruction of irradiated cells by immune cells and active proteins, such as MAC, and granzyme.
- Gradually increase numbers of dendritic cells or radiation-specific T cells for slow elimination of irradiated cells.
- Radiation-induced Antibody-Dependent Cellular Cytotoxicity (RADCC).
- Radiation-induced Complement-Dependent Cytotoxicity (RCDC).
- Radiation-Occurring Antibody-Independent Cellular Cytotoxic (ROAICC) activity of circulating leucocytes toxicity.
- ATM-Controlled Radiation Toxicity (ATMCRT)
Appendix A.1. Radiation-Induced Antibody-Dependent Cellular Cytotoxicity (RADCC)
Appendix A.2. Radiation-Induced Complement-Dependent Cytotoxicity (RCDC)
Appendix A.3. Radiation-Occurring Antibody-Independent Cellular Cytotoxic Activity (ROAICC) of Circulating Leukocytes
Appendix A.4. TM Controlled Radiation Toxicity (ATMCRT)
References
- Borovsky, J.E.; Valdivia, J.A. The Earth’s Magnetosphere: A Systems Science Overview and Assessment. Surv. Geophys. 2018, 39, 817–859. [Google Scholar] [CrossRef] [Green Version]
- Council, N.R. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006; p. 422. [Google Scholar]
- Jones, J.; Karouia, F.; Pinsky, L.; Cristea, O. Radiation and Radiation Disorders. In Principles of Clinical Medicine for Space Flight; Barratt, M., Baker, E., Pool, S., Eds.; Springer: New York, NY, USA, 2019; pp. 39–108. [Google Scholar] [CrossRef]
- Simpson, J.A. Elemental and Isotopic Composition of the Galactic Cosmic Rays. Annu. Rev. Nucl. Part. Sci. 1983, 33, 323–382. [Google Scholar] [CrossRef]
- Walker, S.A.; Townsend, L.W.; Norbury, J.W. Heavy ion contributions to organ dose equivalent for the 1977 galactic cosmic ray spectrum. Adv. Space Res. 2013, 51, 1792–1799. [Google Scholar] [CrossRef]
- Jones, J.; Epperly, M.; Law, J.; Scheuring, R.; Montesinos, C.; Popov, D.; Maliev, V.; Prasad, K.; Greenberg, J. Space Radiation Hazards and Strategies for Astronaut/Cosmonaut Protection. Med. Radiol. Radiat. Saf. 2013, 58, 5–23. [Google Scholar]
- Baker, D.N.; Kanekal, S.G.; Hoxie, V.C.; Henderson, M.G.; Li, X.; Spence, H.E.; Elkington, S.R.; Friedel, R.H.; Goldstein, J.; Hudson, M.K.; et al. A long-lived relativistic electron storage ring embedded in Earth’s outer Van Allen belt. Science 2013, 340, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. NPJ Microgravity 2020, 6, 33. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [Green Version]
- Dziegielowski, J.; Goetz, W.; Baulch, J. Heavy ions, radioprotectors and genomic instability: Implications for human space exploration. Radiat. Environ. Biophys. 2010, 49, 303–316. [Google Scholar] [CrossRef]
- Jones, J.; Karouia, F.; Cristea, O.; Casey, R.; Popov, D.; Maliev, V. Ionizing Radiation as a Carcinogen; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–225. [Google Scholar]
- Fajardo, L.; Berthrong, M.; Anderson, R. (Eds.) Radiation Pathology; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Little, M.P.; Azizova, T.V.; Bazyka, D.; Bouffler, S.D.; Cardis, E.; Chekin, S.; Chumak, V.V.; Cucinotta, F.A.; de Vathaire, F.; Hall, P.; et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ. Health Perspect. 2012, 120, 1503–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerman, M.; Bracco Gartner, T.C.L.; Buikema, J.W.; Wu, S.M.; Siddiqi, S.; Bouten, C.V.C.; Grande-Allen, K.J.; Suyker, W.J.L.; Hjortnaes, J. Myocardial Disease and Long-Distance Space Travel: Solving the Radiation Problem. Front. Cardiovasc. Med. 2021, 8, 27. [Google Scholar] [CrossRef]
- Ainsbury, E.A.; Barnard, S.; Bright, S.; Dalke, C.; Jarrin, M.; Kunze, S.; Tanner, R.; Dynlacht, J.R.; Quinlan, R.A.; Graw, J.; et al. Ionizing radiation induced cataracts: Recent biological and mechanistic developments and perspectives for future research. Mutat. Res. 2016, 770, 238–261. [Google Scholar] [CrossRef] [PubMed]
- Bálentová, S.; Adamkov, M. Pathological changes in the central nervous system following exposure to ionizing radiation. Physiol. Res. 2020, 69, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, K.; Shimizu, Y.; Suyama, A.; Kasagi, F.; Soda, M.; Grant, E.J.; Sakata, R.; Sugiyama, H.; Kodama, K. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: An overview of cancer and noncancer diseases. Radiat. Res. 2012, 177, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weil, M.M.; Ray, F.A.; Genik, P.C.; Yu, Y.; McCarthy, M.; Fallgren, C.M.; Ullrich, R.L. Effects of 28Si ions, 56Fe ions, and protons on the induction of murine acute myeloid leukemia and hepatocellular carcinoma. PLoS ONE 2014, 9, e104819. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Auñón-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 2018, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [CrossRef]
- Cucinotta, F.A. Biophysics of NASA radiation quality factors. Radiat. Prot. Dosim. 2015, 166, 282–289. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Chappell, L.J. Updates to astronaut radiation limits: Radiation risks for never-smokers. Radiat. Res. 2011, 176, 102–114. [Google Scholar] [CrossRef]
- Cucinotta, F.A. Radiation Risk Acceptability and Limitations. In The Health Risks of Extraterrestrial Environments (THREE); Schimmerling, W., Ed.; NASA Human Research Program: Houston, TX, USA, 2010. Available online: https://three.jsc.nasa.gov/articles/AstronautRadLimitsFC.pdf (accessed on 1 June 2021).
- Badhwar, G.D.; Atwell, W.; Reitz, G.; Beaujean, R.; Heinrich, W. Radiation measurements on the Mir Orbital Station. Radiat. Meas. 2002, 35, 393–422. [Google Scholar] [CrossRef]
- Zhang, S.; Wimmer-Schweingruber, R.F.; Yu, J.; Wang, C.; Fu, Q.; Zou, Y.; Sun, Y.; Wang, C.; Hou, D.; Böttcher, S.I.; et al. First measurements of the radiation dose on the lunar surface. Sci. Adv. 2020, 6, eaaz1334. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [Green Version]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, B. Human Exploration of Mars Design Reference Architecture 5.0 Addendum; NASA: NASA Johnson Space Center: Houston, TX, USA, 2009.
- Shavers, M.R.; Zapp, N.; Barber, R.E.; Wilson, J.W.; Qualls, G.; Toupes, L.; Ramsey, S.; Vinci, V.; Smith, G.; Cucinotta, F.A. Implementation of ALARA radiation protection on the ISS through polyethylene shielding augmentation of the Service Module Crew Quarters. Adv. Space Res. 2004, 34, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Durante, M. Space radiation protection: Destination Mars. Life Sci. Space Res. 2014, 1, 2–9. [Google Scholar] [CrossRef]
- Turner, N.D.; Braby, L.A.; Ford, J.; Lupton, J.R. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition 2002, 18, 904–912. [Google Scholar] [CrossRef]
- Vasin, M.V. Comments on the mechanisms of action of radiation protective agents: Basis components and their polyvalence. SpringerPlus 2014, 3, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef]
- Martin, C.J. Radiation shielding for diagnostic radiology. Radiat. Prot. Dosim. 2015, 165, 376–381. [Google Scholar] [CrossRef]
- Wilson, J.W.; Miller, J.; Konradi, A.; Cucinotta, F. Shielding Strategies for Human Space Exploration; NASA Conference Publication 3360; NASA Lyndon B. Johnson Space Center: Houston, TX, USA, 1997. Available online: https://ntrs.nasa.gov/api/citations/19980137598/downloads/19980137598.pdf (accessed on 1 June 2021).
- Hu, W.; Pei, H.; Li, H.; Ding, N.; He, J.; Wang, J.; Furusawa, Y.; Hirayama, R.; Matsumoto, Y.; Liu, C.; et al. Effects of shielding on the induction of 53BP1 foci and micronuclei after Fe ion exposures. J. Radiat. Res. 2014, 55, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Heilbronn, L.; Nakamura, T.; Iwata, Y.; Kurosawa, T.; Iwase, H.; Townsend, L.W. Overview of secondary neutron production relevant to shielding in space. Radiat. Prot. Dosim. 2005, 116, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Cucinotta, F.A.; Kim, M.-H.Y.; Ren, L. Evaluating shielding effectiveness for reducing space radiation cancer risks. Radiat. Meas. 2006, 41, 1173–1185. [Google Scholar] [CrossRef] [Green Version]
- Vana, N.; Hajek, M.; Berger, T.; Fugger, M.; Hofmann, P. Novel shielding materials for space and air travel. Radiat. Prot. Dosim. 2006, 120, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, S.; Tolochek, R.V.; Ambrozova, I.; Kawashima, H.; Yasuda, N.; Kurano, M.; Kitamura, H.; Uchihori, Y.; Kobayashi, I.; Hakamada, H.; et al. Verification of shielding effect by the water-filled materials for space radiation in the International Space Station using passive dosimeters. Adv. Space Res. 2014, 53, 1–7. [Google Scholar] [CrossRef]
- De Angelis, G.; Wilson, J.W.; Clowdsley, M.S.; Qualls, G.D.; Singleterry, R.C. Modeling of the Martian environment for radiation analysis. Radiat. Meas. 2006, 41, 1097–1102. [Google Scholar] [CrossRef]
- Simonsen, L.C.; Nealy, J.E.; Townsend, L.W.; Wilson, J.W. Martian regolith as space radiation shielding. J. Spacecr. Rocket 1991, 28, 7–8. [Google Scholar] [CrossRef]
- Naito, M.; Hasebe, N.; Shikishima, M.; Amano, Y.; Haruyama, J.; Matias-Lopes, J.A.; Kim, K.J.; Kodaira, S. Radiation dose and its protection in the Moon from galactic cosmic rays and solar energetic particles: At the lunar surface and in a lava tube. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2020, 40, 947–961. [Google Scholar] [CrossRef]
- Townsend, L.W.; Adams, J.H.; Blattnig, S.R.; Clowdsley, M.S.; Fry, D.J.; Jun, I.; McLeod, C.D.; Minow, J.I.; Moore, D.F.; Norbury, J.W.; et al. Solar particle event storm shelter requirements for missions beyond low Earth orbit. Life Sci. Space Res. 2018, 17, 32–39. [Google Scholar] [CrossRef]
- Carnell, L.S. Spaceflight medical countermeasures: A strategic approach for mitigating effects from solar particle events. Int. J. Radiat. Biol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, S.A.; Kang, J.H.; Sauti, G.; Park, C.; Fay, C.C.; King, G.C. Nanomaterials for radiation shielding. MRS Bull. 2015, 40, 836–841. [Google Scholar] [CrossRef]
- Cheraghi, E.; Chen, S.; Yeow, J.T.W. Boron Nitride-Based Nanomaterials for Radiation Shielding: A Review. IEEE Nanotechnol. Mag. 2021, 15, 8–17. [Google Scholar] [CrossRef]
- Spillantini, P.; Casolino, M.; Durante, M.; Mueller-Mellin, R.; Reitz, G.; Rossi, L.; Shurshakov, V.; Sorbi, M. Shielding from cosmic radiation for interplanetary missions: Active and passive methods. Radiat. Meas. 2007, 42, 14–23. [Google Scholar] [CrossRef]
- Ferrone, K. Active Magnetic Radiation Shielding for Long-Duration Human Spaceflight. Master’s Thesis, University of Texas MD Anderson Cancer Center, Houston, TX, USA, 2020. [Google Scholar]
- Washburn, S.A.; Blattnig, S.R.; Singleterry, R.C.; Westover, S.C. Active magnetic radiation shielding system analysis and key technologies. Life Sci. Space Res. 2015, 4, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.M.; Ehresmann, B.; Rafkin, S.C.R.; Guo, J.; Wimmer-Schweingruber, R.F.; Berger, T.; Matthiä, D. Measurements of radiation quality factor on Mars with the Mars Science Laboratory Radiation Assessment Detector. Life Sci. Space Res. 2019, 22, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mattfeld, B.; Stromgren, C.; Shyface, H.; Komar, D.; Cirillo, W.; Goodliff, K. Trades between Opposition and Conjunction Class Trajectories for Early Human Missions to Mars; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2015; Available online: https://ntrs.nasa.gov/api/citations/20150001240/downloads/20150001240.pdf (accessed on 1 June 2021).
- Durante, M.; Bruno, C. Impact of rocket propulsion technology on the radiation risk in missions to Mars. Eur. Phys. J. D 2010, 60, 215–218. [Google Scholar] [CrossRef]
- Dujarric, C.; Santovincenzo, A.; Summerer, L. The nuclear thermal electric rocket: A proposed innovative propulsion concept for manned interplanetary missions. Prog. Propuls. Phys. 2013, 4, 293–312. [Google Scholar]
- Reynolds, C.B.; Joyner, C.R.; Kokan, T.S.; Levack, D.J.; Muzek, B.J. Mars Opposition Missions Using Nuclear Thermal Propulsion. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Reston, VA, USA, 24–28 August 2020; p. 3850. [Google Scholar]
- Borowski, S.K.; McCurdy, D.R.; Packard, T.W. Nuclear Thermal Propulsion (NTP): A proven growth technology for human NEO/Mars exploration missions. In Proceedings of the 2012 IEEE Aerospace Conference, Big Sky, MT, USA, 3–10 March 2012; pp. 1–20. [Google Scholar]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Stein, T.P.; Leskiw, M.J. Oxidant damage during and after spaceflight. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E375–E382. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Davis-Street, J.E.; Rice, B.L.; Nillen, J.L.; Gillman, P.L.; Block, G. Nutritional status assessment in semiclosed environments: Ground-based and space flight studies in humans. J. Nutr. 2001, 131, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine; Committee on the Longitudinal Study of Astronaut Health; Longnecker, D.E.; Manning, F.J.; Worth, M.H., Jr. (Eds.) Review of NASA’s Longitudinal Study of Astronaut Health; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Travassoli, M. Anemia of spaceflight. Blood 1982, 60, 1059–1067. [Google Scholar] [CrossRef]
- Rai1, B.; Kaur, J. Human Factor Studies on a Mars Analogue During Crew 100b International Lunar Exploration Working Group EuroMoonMars Crew: Proposed New Approaches for Future Human Space and Interplanetary Missions. N. Am. J. Med. Sci. 2012, 4, 548–557. [Google Scholar] [CrossRef]
- Johnston, R.S.; Dietlein, L.F. (Eds.) Biomedical Results from Skylab; NASA Headquarters: Washington, DC, USA, 1977.
- Smith, S.M.; Davis-Street, J.E.; Fesperman, J.V.; Smith, M.D.; Rice, B.L.; Zwart, S.R. Nutritional assessment during a 14-d saturation dive: The NASA Extreme Environment Mission Operations V Project. J. Nutr. 2004, 134, 1765–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pross, H.D.; Casares, A.; Kiefer, J. Induction and repair of DNA double-strand breaks under irradiation and microgravity. Radiat. Res. 2000, 153 Pt 1, 521–525. [Google Scholar] [CrossRef]
- Kiefer, J.; Pross, H.D. Space radiation effects and microgravity. Mutat. Res. 1999, 430, 299–305. [Google Scholar] [CrossRef]
- Hollander, J.; Gore, M.; Fiebig, R.; Mazzeo, R.; Ohishi, S.; Ohno, H.; Ji, L.L. Spaceflight downregulates antioxidant defense systems in rat liver. Free. Radic. Biol. Med. 1998, 24, 385–390. [Google Scholar] [CrossRef]
- McKenzie, R.C.; Beckett, G.J.; Arthur, J.R. Effects of selenium on immunity and aging. In Selenium: Its Molecular Biology and Role in Human Health, 2nd ed.; Hatfield, D.L., Berry, M.J., Gladyshev, V.N., Eds.; Springer: New York, NY, USA, 2006; pp. 311–323. [Google Scholar]
- Roy, M.; Kiremidjian-Schumacher, L.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol. Trace Elem. Res. 1994, 41, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol. Trace Elem. Res. 1994, 41, 115–127. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Glickman, R.; Schneider, K.; Rothstein, S.; Cooper, J.; Hochster, H.; Kim, M.; Newman, R. Selenium and immunocompetence in patients with head and neck cancer. Biol. Trace Elem. Res. 2000, 73, 97–111. [Google Scholar] [CrossRef]
- Baum, M.K.; Miguez-Burbano, M.J.; Campa, A.; Shor-Posner, G. Selenium and interleukins in persons infected with human immunodeficiency virus type 1. J. Infect. Dis. 2000, 182 (Suppl. 1), S69–S73. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [CrossRef]
- Sharma, S.; Stutzman, J.D.; Kelloff, G.J.; Steele, V.E. Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res. 1994, 54, 5848–5855. [Google Scholar]
- Lemon, J.A.; Rollo, C.D.; Boreham, D.R. A complex dietary supplement extends longevity of mice. J. Gerontol. Biol. Sci. 2005, 60, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Bump, E.A.; Malaker, K. (Eds.) Radioprotectors: Chemical, Biological and Clinical Perspectives; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Winter, L.; Fröhlich, K.; Jentsch, S.; Dawczynski, J.; Jahreis, G.; Böhm, V. Macular Xanthophylls and ω-3 Long-Chain Polyunsaturated Fatty Acids in Age-Related Macular Degeneration: A Randomized Trial. JAMA Ophthalmol. 2013, 131, 564–572. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Rendón-Ramírez, A.L.; Maldonado-Vega, M.; Quintanar-Escorza, M.A.; Hernández, M.; Arévalo-Rivas, B.I.; Zentella-Dehesa, A.; Calderón-Salinas, J.V. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ. Toxicol. Pharm. 2014, 37, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Lindblad, B.E.; Morgenstern, R.; Wolk, A. Total Antioxidant Capacity of the Diet and Risk of Age-Related Cataract: A Population-Based Prospective Cohort of Women. JAMA Ophthalmol. 2013, 132, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pemp, B.; Polska, E.; Karl, K.; Lasta, M.; Minichmayr, A.; Garhofer, G.; Wolzt, M.; Schmetterer, L. Effects of antioxidants (AREDS medication) on ocular blood flow and endothelial function in an endotoxin-induced model of oxidative stress in humans. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAnulty, L.S.; Miller, L.E.; Hosick, P.A.; Utter, A.C.; Quindry, J.C.; McAnulty, S.R. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kosaka, N.; Nakagawa, S. Alpha-Tocopherol protects PC12 cells from hyperoxia-induced apoptosis. J. Neurosci. Res. 1998, 52, 184–191. [Google Scholar] [CrossRef]
- Jung, J.H.; Jung, J.; Kim, S.K.; Woo, S.H.; Kang, K.M.; Jeong, B.K.; Kim, J.H.; Hahm, J.R. Alpha Lipoic Acid Attenuates Radiation-Induced Thyroid Injury in Rats. PLoS ONE 2014, 9, e112253. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, D.P.; Chang, L.S.; Huang, T.P.; Liu, H.J.; Luk, C.P.; Lo, Y.L.; Chang, H.H. Dietary α-lipoic acid prevents UVB-induced corneal and conjunctival degeneration through multiple effects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6757–6766. [Google Scholar] [CrossRef] [Green Version]
- McAnulty, S.R.; Nieman, D.C.; McAnulty, L.S.; Lynch, W.S.; Jin, F.; Henson, D.A. Effect of mixed flavonoids, n-3 fatty acids, and vitamin C on oxidative stress and antioxidant capacity before and after intense cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 328–337. [Google Scholar] [CrossRef]
- Abengózar-Vela, A.; Calonge, M.; Stern, M.E.; González-García, M.J.; Enríquez-De-Salamanca, A. Quercetin and Resveratrol Decrease the Inflammatory and Oxidative Responses in Human Ocular Surface Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2709–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.; Frye, C. Anti-anxiety, cognitive, and steroid biosynthetic effects of an isoflavone-based dietary supplement are gonad and sex-dependent in rats. Brain Res. 2011, 1379, 164–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. French Maritime Pine Bark Extract (Pycnogenol®) Effects on Human Skin: Clinical and Molecular Evidence. Ski. Pharm. Physiol. 2015, 29, 13–17. [Google Scholar] [CrossRef]
- Heer, M.; Baecker, N.; Smith, S.M.; Zwart, S.R. Nutritional Countermeasures for Spaceflight-Related Stress. In Stress Challenges and Immunity in Space; Choukèr; Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Smith, S.M. Nutrition for Space Exploration. Abstract & Presentation; Universities Space Research Association (USRA): Columbia, MD, USA, 2005. [Google Scholar]
- Smith, S.M.; Zwart, S.R.; Heer, M. Human Adaptation to Spaceflight: The Role of Nutrition; NASA Lyndon B. Johnson Space Center: Houston, TX, USA, 2014.
- Popov, D.; Jones, J.; Maliev, V. Radiation Toxins—Effects of Radiation Toxicity, Molecular Mechanisms of Action, Radiomimetic Properties and Possible Countermeasures for Radiation Injury. In Current Topics in Ionizing Radiation Research; Mitsuru, N., Ed.; InTech: New York, NY, USA, 2012; pp. 215–242. [Google Scholar]
- Jones, J.; Karouia, F.; Epperly, M.; Montesinos, C.; Petrosino, J.; Cristea, O.; Greenberger, J. Intestinal Microbiome: Considerations of Radiation Exposure and Health Effects for Exploration Class Space Flight; Abstract and Presentation; International Academy of Astronautics, Humans in Space Symposium (HIS): Prague, Czech Republic, 2015. [Google Scholar]
- Lemon, J.A.; Rollo, C.D.; Boreham, D.R. A dietary supplement abolishes age-related cognitive decline in transgenic mice expressing elevated free radical processes. Exp. Biol. Med. 2003, 228, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Lemon, J.A.; Boreham, D.R.; Rollo, C.D. A dietary supplement abolishes massive brain cell loss in mice expressing elevated free radical processes. Exp. Biol. Med. 2003, 228, 800–810. [Google Scholar] [CrossRef]
- Matuszczak, Y.; Farid, M.; Jones, J.; Lansdowne, S.; Smith, M.A.; Taylor, A.A.; Reid, M.B. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve 2005, 32, 633–638. [Google Scholar] [CrossRef]
- Aksenov, V.; Boreham, D.; Rollo, C.D. Impact of a complex nutraceutical supplement on primary tumour formation and metastasis in Trp53+/- cancer-prone mice. Mutagenesis 2014, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Epperly, M.W.; Wang, H.; Jones, J.A.; Dixon, T.; Montesinos, C.A.; Greenberger, J.S. Antioxidant-Chemoprevention Diet Ameliorates Late Effects of Total-Body Irradiation and Supplements Radioprotection by MnSOD-Plasmid Liposome Administration. Radiat. Res. 2011, 175, 759–765. [Google Scholar] [CrossRef]
- Shahmirzadi, A.A.; Edgar, D.; Liao, C.Y.; Riley, R.R.; Kennedy, B.K.; Lithgow, G.L. Alpha-Ketoglutarate, an endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456. [Google Scholar] [CrossRef]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Filip, R.S.; Pierzynowski, S.G.; Lindegard, B.; Wernerman, J.; Haratym-Maj, A.; Podgurniak, M. Alpha-ketoglutarate decreases serum levels of C-terminal cross-linking telopeptide of type I collagen (CTX) in postmenopausal women with osteopenia: Six-month study. Int. J. Vitam. Nutr. Res. 2007, 77, 89–97. [Google Scholar] [CrossRef]
- Dellinger, R.W.; Santos, S.R.; Morris, M.; Evans, M.; Alminana, D.; Guarente, L.; Marcotulli, E. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: A randomized, double-blind, placebo-controlled study. NPJ Aging Mech. Dis. 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minois, N. Molecular Basis of the “Anti-Aging” Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int. J. Mol. Sci. 2019, 20, 5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef] [Green Version]
- Cucinotta, F.A.; Manuel, F.K.; Jones, J.; Iszard, G.; Murrey, J.; Djojonegro, B.; Wear, M. Space radiation and cataracts in astronauts. Radiat. Res. 2001, 156 Pt 1, 460–466. [Google Scholar] [CrossRef]
- Huang, A.S.; Stenger, M.B.; Macias, B.R. Gravitational Influence on Intraocular Pressure: Implications for Spaceflight and Disease. J. Glaucoma 2019, 28, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Rice, B.L.; Dlouhy, H.; Zwart, S.R. Assessment of nutritional intake during space flight and space flight analogs. Procedia Food Sci. 2013, 2, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Zwart, S.R.; Gibson, C.R.; Mader, T.H.; Ericson, K.; Ploutz-Snyder, R.; Heer, M.; Smith, S.M. Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J. Nutr. 2012, 142, 427–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesinos, C.A.; Jones, J.A.; Sabbagh, M.N.; Burkart, J.A. Toxycity and safety evaluation of an omega-3 fatty acid and a multivitamin + antioxidant-chemopreention preparation (AS10). U. S. Nat. Library Med. 2021, Manuscript, unpublished. [Google Scholar]
- Greenberger, J.S. Radioprotection. Vivo 2009, 23, 323–336. [Google Scholar]
- Greenberger, J.S.; Epperly, M.W.; Gretton, J.; Jefferson, M.; Nie, S.; Bernarding, M.; Kagan, V.; Guo, H.L. Radioprotective gene therapy. Curr. Gene Ther. 2003, 3, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, J.S.; Epperly, M.W. Radioprotective antioxidant gene therapy: Potential mechanisms of action. Gene Ther. Mol. Biol. 2003, 3, 183–195. [Google Scholar]
- Greenberger, J.S. Gene therapy approaches for stem cell protection. Gene Ther. 2008, 15, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinman, J.; Epperly, M.; Hou, W.; Willis, J.; Wang, H.; Fisher, R.; Liu, B.; Bahar, I.; McCaw, T.; Kagan, V.; et al. Improved total-body irradiation survival by delivery of two radiation mitigators that target distinct cell death pathways. Radiat. Res. 2018, 189, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Thermozier, S.; Hou, W.; Zhang, X.; Shields, D.; Fisher, R.; Bayir, H.; Kagan, V.; Yu, J.; Liu, B.; Bahar, I.; et al. Anti-ferroptosis drug, Baicalein, enhances total body irradiation mitigation by two other drugs that target apoptosis and necroptosis. Radiat. Res. 2020, 193, 435–450. [Google Scholar] [CrossRef]
- Zhang, X.; Fisher, R.; Hou, W.; Shields, D.; Epperly, M.W.; Wang, H.; Yu, J.; van Pijkeren, J.P.; Watkins, S.W.; Greenberger, J.S. Second generation probiotics producing IL-22 increase survival of mice after total body irradiation. In Vivo 2020, 34, 39–50. [Google Scholar] [CrossRef]
- Barratt, M.R.; Ellen, S.B.; Pool, S.L. Principles of Clinical Medicine for Space Flight; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; KWansley, E.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Drutman, S.B.; Trombetta, E.S. Dendritic cells continue to capture and present antigens after maturation in vivo. J. Immunol. 2010, 185, 2140–2146. [Google Scholar] [CrossRef] [Green Version]
- Creative Biolabs. Available online: https://www.creative-biolabs.com/complement-therapeutics/serine-protease-inhibitor-development.htm (accessed on 1 June 2021).
- Pierce, S.K.; Morris, J.F.; Grusby, M.J.; Kaumaya, P.; van Buskirk, A.; Srinivasan, M.; Crump, B.; Smolenski, L.A. Antigen-presenting function of B lymphocytes. Immunol. Rev. 1988, 106, 149–180. [Google Scholar] [CrossRef]
- Popov, D.; Jones, J. Radiation Protection with Anti-Radiation Vaccine and Anti-Radiation Antidote in Reducing the Biological Impact of High Dose and Dose-Rate, Low-Linear Energy Transfer Radiation Exposure. In Proceedings of the 43rd COSPAR Scientific Assembly, Sydney, Australia, 28 January–4 February 2021. [Google Scholar] [CrossRef]
- Lanzavecchia, A. Antigen presentation by B lymphocytes: A critical step in T-B collaboration. Curr. Top Microbiol. Immunol. 1986, 130, 65–78. [Google Scholar] [CrossRef]
- Maliev, V.; Popov, D.; Casey, R.C.; Jones, J.A. Mechanisms of action for an anti-radiation vaccine in reducing the biological impact of high dose and dose-rate, low-linear energy transfer radiation exposure. Radiats. Biol. Radioecol. 2007, 47, 286–291. [Google Scholar] [PubMed]
- Pandita, T.K.; Lieberman, H.B.; Lim, D.S.; Dhar, S.; Zheng, W.; Taya, Y.; Kastan, M.B. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene 2000, 19, 1386–1391. [Google Scholar] [CrossRef] [Green Version]
- Canman, C.E.; Lim, D.S.; Cimprich, K.A.; Taya, Y.; Tamai, K.; Sakaguchi, K.; Appella, E.; Kastan, M.B.; Siliciano, J.D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998, 281, 1677–1679. [Google Scholar] [CrossRef]
- Khanna, K.K.; Lavin, M.F. Ionizing radiation and UV induction of p53 protein by different pathways in ataxia-telangiectasia cells. Oncogene 1993, 8, 3307–3312. [Google Scholar] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Momčilović, O.; Choi, S.; Varum, S.; Bakkenist, C.; Schatten, G.; Navara, C. Ionizing radiation induces ATM dependent checkpoint signaling and G2 but not G1 cell cycle arrest in pluripotent human embryonic stem cells. Stem Cells 2009, 27, 1822–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Koike-Soko, C.; Sugimoto, J.; Yoshida, T.; Okabe, M.; Nikaido, T. Human amnion-derived stem cells have immunosuppressive properties on NK cells and monocytes. Cell Transpl. 2015, 24, 2065–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. 2009, 9, 15–27. [Google Scholar] [CrossRef]
- Maliev, V.; Popov, D.; Jones, J.A.; Casey, R. Mechanism of action for anti-radiation vaccine in reducing the biological impact of high-dose gamma irradiation. Adv. Space Res. 2007. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.C.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef] [Green Version]
- Saito, E.; Fujimoto, M.; Hasegawa, M.; Komura, K.; Hamaguchi, Y.; Kaburagi, Y.; Nagaoka, T.; Takehara, K.; Tedder, T.F.; Sato, S. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J. Clin. Investig. 2002, 109, 1453–1462. [Google Scholar] [CrossRef]
- Li, R.; Rezk, A.; Healy, L.M.; Muirhead, G.; Prat, A.; Gommerman, J.L.; Bar-Or, A. Cytokine-defined B cell responses as therapeutic targets in multiple sclerosis. Front. Immunol. 2015, 6, 626. [Google Scholar] [CrossRef] [Green Version]
- Magatti, M.; Masserdotti, A.; Bonassi Signoroni, P.; Vertua, E.; Stefani, F.R.; Silini, A.R.; Parolini, O. B Lymphocytes as Targets of the Immunomodulatory Properties of Human Amniotic Mesenchymal Stromal Cells. Front. Immunol. 2020, 11, 1156. [Google Scholar] [CrossRef]
- Kumari, A.; Simon, S.S.; Moody, T.D.; Garnett-Benson, C. Immunomodulatory effects of radiation: What is next for cancer therapy? Future Oncol. 2016, 12, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Crucian, B.E.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune system dysregulation following short- vs. long duration spaceflight. Aviat. Space Environ. Med. 2008, 79, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Meng, Y.; Zhang, M.; Guo, Z.; Liu, F.; Zhang, W.; Ke, X.; Su, Y.; Wang, M.; Yao, Y.; et al. ATM Expression Is Elevated in Established Radiation-Resistant Breast Cancer Cells and Improves DNA Repair Efficiency. Int. J. Biol. Sci. 2020, 16, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, J.K.; Wan, X.S.; Krigsfeld, G.S.; Wroe, A.J.; Gridley, D.S.; Kennedy, A.R. The Effects of Gamma and Proton Radiation Exposure on Hematopoietic Cell Counts in the Ferret Model. Gravit. Space Res. 2013, 1, 79–94. [Google Scholar] [PubMed]
- Singh, V.K.; Fatanmi, O.O.; Singh, P.K. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine 2012, 58, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Christensen, J.; Fatanmi, O.O.; Gille, D.; Ducey, E.J.; Wise, S.Y.; Karsunky, H.; Sedello, A.K. Myeloid progenitors: A radiation countermeasure that is effective when initiated days after irradiation. Radiat. Res. 2012, 177, 781–791. [Google Scholar] [CrossRef]
- Zaki-Dizaji, M.; Akrami, S.M.; Abolhassani, H.; Rezaei, N.; Aghamohammadi, A. Ataxia telangiectasia syndrome: Moonlighting ATM. Expert Rev. Clin. Immunol. 2017, 13, 1155–1172. [Google Scholar] [CrossRef]
- Gene Cards. The Human Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ATM (accessed on 1 June 2021).
- Van Kaer, L.; Wu, L.; Joyce, S. Mechanisms and Consequences of Antigen Presentation by CD1. Trends Immunol. 2016, 738–754. [Google Scholar] [CrossRef] [Green Version]
- Den Haan, J.M.; Arens, R.; van Zelm, M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014, 162 Pt B, 103–112. [Google Scholar] [CrossRef]
- Spatz, J.M.; Fulford, M.H.; Tsai, A.; Gaudilliere, D.; Hedou, J.; Ganio, E.; Angst, M.; Aghaeepour, N.; Gaudilliere, B. Human immune system adaptations to simulated microgravity revealed by single-cell mass cytometry. Sci. Rep. 2021, 11872. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2021, 1489–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaignier, F.; Schenten, V.; De Carvalho Bittencourt, M.; Gauquelin-Koch, G.; Frippiat, J.P.; Legrand-Frossi, C. Three weeks of murine hindlimb unloading induces shifts from B to T and from th to tc splenic lymphocytes in absence of stress and differentially reduces cell-specific mitogenic responses. PLoS ONE 2014, 9, e92664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, C.L.; Stowe, R.P.; St John, L.; Sams, C.F.; Mehta, S.K.; Crucian, B.E.; Pierson, D.L.; Komanduri, K.V. Decreases in thymopoiesis of astronauts returning from space flight. JCI Insight 2016, e88787. [Google Scholar] [CrossRef] [PubMed]
- Cogoli, A. The effect of hypo-gravity and hyper-gravity on cells of the immune system. J. Leukoc. Biol. 1993, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Pross, H.D.; Kost, M.; Kiefer, J. Repair of radiation induced genetic damage under microgravity. Adv. Space Res. 1994, 125–130. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Aristizabal, B.; Gonzalez, A. Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013; ISBN 9789587383669. [Google Scholar]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Part V, The Immune System in Health and Disease; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Lemon, J.A.; Rollo, C.D.; McFarlane, N.M.; Boreham, D.R. Radiation-induced apoptosis in mouse lymphocytes is modified by a complex dietary supplement: The effect of genotype and gender. Mutagenesis 2008, 23, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.A.; Riggs, P.K.; Yang, T.C.; Pedemonte, C.H.; Clarke, M.S.; Feeback, D.L.; Au, W.W. Ionizing radiation-induced bioeffects in space and strategies to reduce cellular injury and carcinogenesis. Aviat. Space Environ. Med. 2007, 78, A67–A78. [Google Scholar] [PubMed]
- Richter, M.; Banerjee, D.; Sklar, S. The antibody-independent cytotoxic activity of normal circulating human leukocytes. Immunology 1981, 44, 109. [Google Scholar]
- Riboldi, G.M.; Samanta, D.; Frucht, S. Ataxia Telangiectasia; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Savitsky, K.; Bar-Shira, A.; Gilad, S.; Rotman, G.; Ziv, Y.; Vanagaite, L.; Tagle, D.A.; Smith, S.; Uziel, T.; Sfez, S.; et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 1995, 268, 1749–1753. [Google Scholar] [CrossRef]
- Hivroz, C.; Chemin, K.; Tourret, M.; Bohineust, A. Crosstalk between T lymphocytes and dendritic cells. Crit. Rev. Immunol. 2012, 32, 139–155. [Google Scholar] [CrossRef]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef]
- Lanzavecchia, A. Immunology. Licence to kill. Nature 1998, 393, 413–414. [Google Scholar] [CrossRef]
- Crispe, I.N. APC licensing and CD4 + T cell help in liver-stage malaria. Front. Microbiol. 2014, 5, 617. [Google Scholar] [CrossRef]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.P.; Hauer-Jensen, M.; Pollard, H.B. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev. Mol. Diagn. 2016, 1, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Fu, J.; Wang, J.; Ye, S.; Liu, W.; Shao, C. Role of ROS-mediated autophagy in radiation induced bystander effect of hepatoma cells. Int. J. Radiat. Biol. 2015, 91, 452–458. [Google Scholar] [CrossRef]
- Dalmasso, A.P. Complement, Membrane Attack Pathway. Encyclopedia of Immunology, 2nd ed. 1998. Available online: https://www.sciencedirect.com/topics/immunology-and-microbiology/complement-membrane-attack-complex-cells (accessed on 1 June 2021).
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M.; et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat Res. 2012, 751, 258–286. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.K.; Gatti, R.; Concannon, P.; Weemaes, C.; Hoekstra, M.F.; Lavin, M.; D’Andrea, A. Cellular responses to DNA damage and human chromosome instability syndromes. In DNA Damage and Repair; Nickloff, J.A., Hoekstra., M.F., Eds.; Humana Press: Totowa, NJ, USA, 1998; pp. 395–442. [Google Scholar]
- GeneCards. Available online: www.genecards.org/cgi-bin/carddisp.pl?gene=ATM&keywords=atm%2Cgene (accessed on 1 June 2021).
- Rainey, M.D.; Charlton, M.E.; Stanton, R.V.; Kastan, M.B. Transient Inhibition of ATM Kinase Is Sufficient to Enhance Cellular Sensitivity to Ionizing Radiation. Cancer Res. 2008, 68, 7466–7474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, K.K.; Lavin, M.F.; Jackson, S.P.; Mulhern, T.D. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ. 2001, 8, 1052–1065. [Google Scholar] [CrossRef] [Green Version]
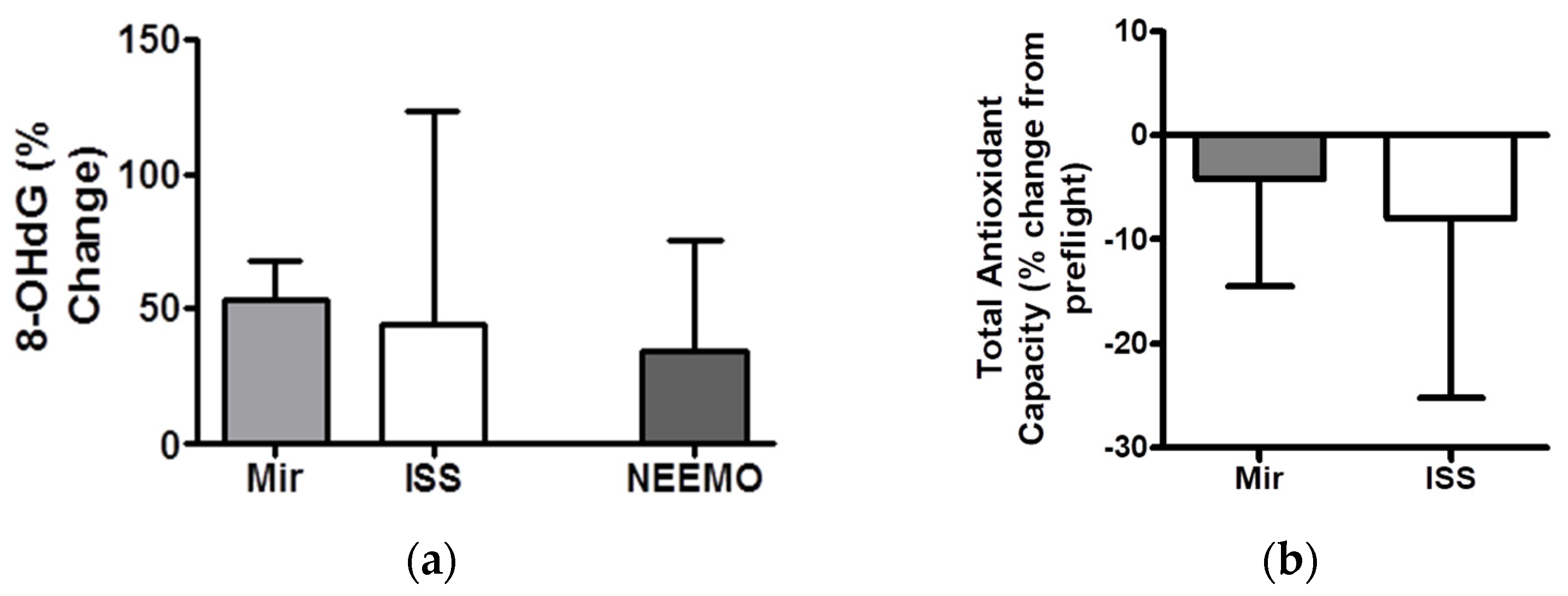






| Compound Analyzed. | Example Pre-Flight Value | Example Post-Flight Value | Normal Ranges Observed in-Flight | Maximal Changes Observed Post-Flight (Percentage Change from Pre-Flight) |
|---|---|---|---|---|
| Total Antioxidant Capacity | 1.54 | 1.47 | 1.29–1.83 | Decreased up to 30% |
| Superoxide Dismutase (SOD) | 1318 | 1172 | 1092–1817 | Decreased 10–30% |
| Glutathione Peroxidase | 51.5 | 50.8 | 27.5–73.6 | Decreased 5–15% |
| Malondialdehyde (MDA) | 0.8 | 0.6 | 0–2.00 | Increased 100–200% |
| 4-OH-alkenal | 0.45 | 0.45 | 0–2.00 | Increased 50–150% |
| Urinary 8OHdG | 3.2 | 3.7 | 0.49–7.29 | Increased 40–200% |
| Compounds | Sources |
|---|---|
| N-Acetyl L-Cysteine (NAC), Diallyl sulfide | Onions, garlic, chives, scallions |
| Sulphoranes, indoles, isothiocyanates | Cruciferous vegetables (e.g., broccoli, kale, cauliflower, cabbage) |
| Isoflavones and phytoestrogens | Soybeans (e.g., tofu, miso, soy milk) |
| Ascorbic acid, flavonoids [quercetin, rutin, isoquercetin], terpenes [limonene] | Citrus fruit (e.g., lemon, grapefruit), cherry, oregano, parsley, artichokes |
| Curcumins | Turmeric |
| Carotenoids [lutein, lycopene, astaxanthin] | Tomato, carrots, squash, algae, salmon |
| Polyphenols | Green and black tea, grape, blueberry |
| Component | Target |
|---|---|
| Vitamin B1 | Insulin sensitivity, anti-inflammatory |
| Vitamin B3 | Insulin sensitivity, anti-inflammatory |
| Vitamin B6 | Insulin sensitivity, anti-inflammatory, scavenges O2− |
| Vitamin B12 | Insulin sensitivity, anti-inflammatory |
| Vitamin E | Antioxidants in the lipid membrane, scavenges O2−, H2O2 |
| Astaxanthin | Suppresses NF-κB activation, lipid antioxidant |
| Bioflavonoids | O2−, metal chelator |
| Coenzyme Q10 | Mitochondrial support, antioxidant in mitochondria |
| Green Tea | Antioxidants in the cytosol, scavenges O2−, H2O2 |
| Glutathione | Enzymatic antioxidant support, antioxidants in the cytosol |
| N-Acetylcysteine | Mucolytic, hepatocyte support |
| Zinc | Neural support (zinc þ antioxidants), insulin sensitivity |
| Lutein | Retinal radioprotectant, ROS scavenger in the lens |
| Folic Acid | Antioxidant, maintains glutathione levels, endothelial support |
| Vitamin C | Antioxidants in the cytosol, scavenges O2−, H2O2 |
| Vitamin D | Antioxidants in the lipid membrane |
| Alpha Lipoic Acid | Mitochondrial support, antioxidant, insulin sensitivity |
| Chromium | Insulin sensitivity, scavenges H2O2 |
| Omega-3 FA | Anti-inflammatory, membrane fluidity |
| Vitamin A | Antioxidant in lipid membrane and retina |
| Magnesium | Insulin sensitivity, cellular support |
| Potassium | Insulin sensitivity, cellular support |
| Selenium | Enzymatic antioxidant support, insulin sensitivity, scavenges H2O2 |
| Resveratrol | Mitochondrial support, increase SOD activity, scavenges O2−, H2O2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montesinos, C.A.; Khalid, R.; Cristea, O.; Greenberger, J.S.; Epperly, M.W.; Lemon, J.A.; Boreham, D.R.; Popov, D.; Gorthi, G.; Ramkumar, N.; et al. Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration. Life 2021, 11, 829. https://doi.org/10.3390/life11080829
Montesinos CA, Khalid R, Cristea O, Greenberger JS, Epperly MW, Lemon JA, Boreham DR, Popov D, Gorthi G, Ramkumar N, et al. Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration. Life. 2021; 11(8):829. https://doi.org/10.3390/life11080829
Chicago/Turabian StyleMontesinos, Carlos A., Radina Khalid, Octav Cristea, Joel S. Greenberger, Michael W. Epperly, Jennifer A. Lemon, Douglas R. Boreham, Dmitri Popov, Gitika Gorthi, Nandita Ramkumar, and et al. 2021. "Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration" Life 11, no. 8: 829. https://doi.org/10.3390/life11080829
APA StyleMontesinos, C. A., Khalid, R., Cristea, O., Greenberger, J. S., Epperly, M. W., Lemon, J. A., Boreham, D. R., Popov, D., Gorthi, G., Ramkumar, N., & Jones, J. A. (2021). Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration. Life, 11(8), 829. https://doi.org/10.3390/life11080829







