Exercise-Based Interventions in Middle-Aged and Older Adults after Myocardial Infarction: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Identification
2.2. Inclusion Criteria
2.3. Bias Risk Assessment
2.4. Data Extraction
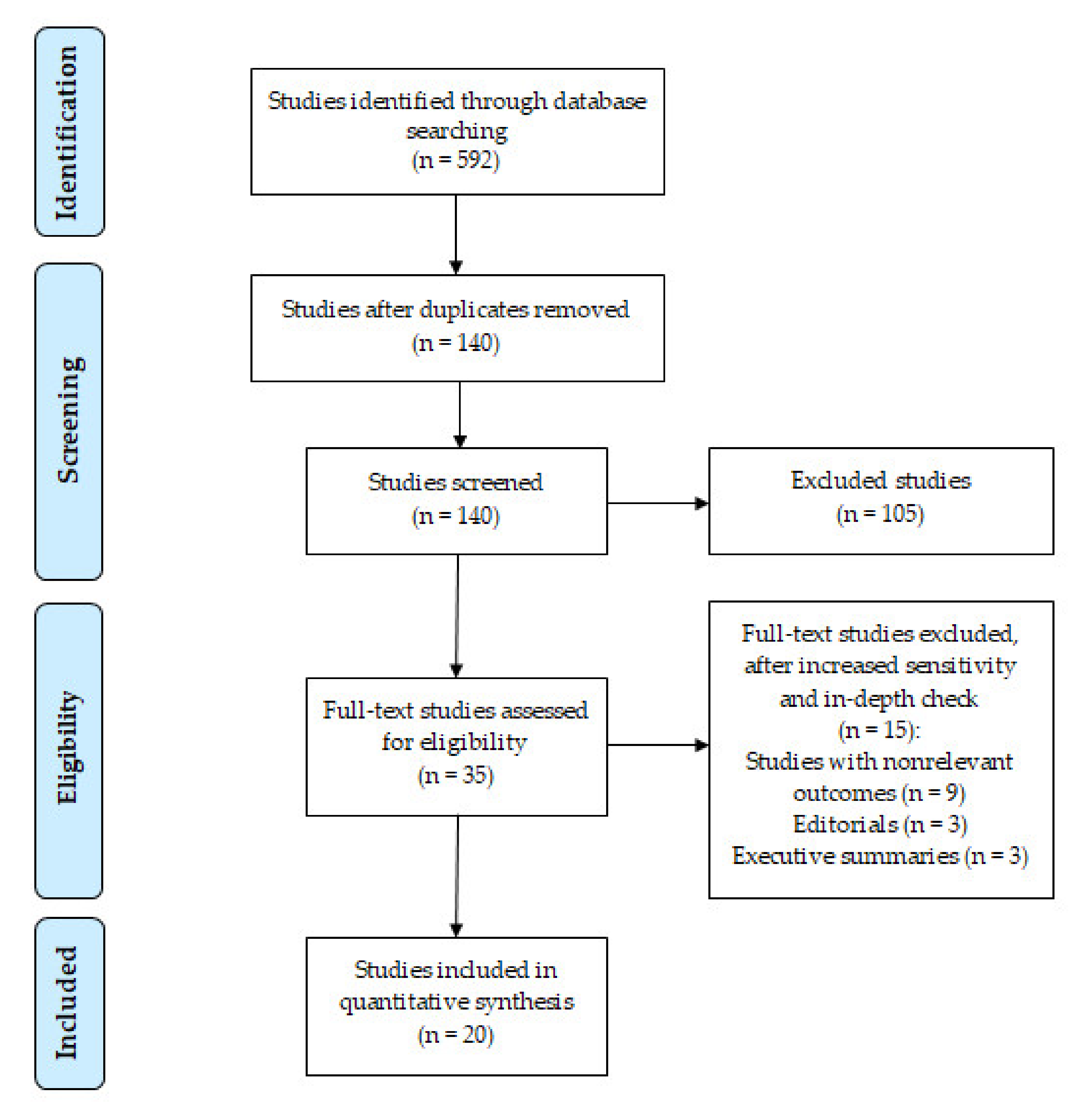
3. Results
3.1. Study Quality
3.2. Selection and Characteristics of Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosuge, M.; Kimura, K.; Ishikawa, T.; Ebina, T.; Hibi, K.; Tsukahara, K.; Kanna, M.; Iwahashi, N.; Okuda, J.; Nozawa, N.; et al. Differences Between Men and Women in Terms of Clinical Features of ST-Segment Elevation Acute Myocardial Infarction. Circ. J. 2006, 70, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Bonita, R. Epidemiology of stroke. Lancet 1992, 339, 342–344. [Google Scholar] [CrossRef]
- Hankey, G.J. Stroke: How large a public health problem, and how can the neurologist help? Arch. Neurol. 1999, 56, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Feitosa-Filho, G.S.; Baracioli, L.M.; Barbosa, C.J.D.G.; Franci, A.; Timerman, A.; Piegas, L.S.; Marin-Neto, J.A.; Nicolau, J. SBC Guidelines on Unstable Angina and Non-ST-Elevation Myocardial Infarction: Executive Summary. Arq. Bras. Cardiol. 2015, 105, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A. Cardiac Rehabilitation and Secondary Prevention of Coronary Heart Disease. N. Engl. J. Med. 2001, 345, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Dylewicz, P.; Przywarska, J.; Borowicz-Bienkowska, S. Selected problems of rehabilitation after myocardial infarction. In Acute Coronary Syndrome; Opolski, G., Filipiak, K.J., Polonski, L., Eds.; Urban & Partner: Wroclaw, Poland, 2002; pp. 465–471. [Google Scholar]
- Taylor, R.S.; Brown, A.; Ebrahim, S.; Jolliffe, J.; Noorani, H.; Rees, K.; Skidmore, B.; Stone, J.A.; Thompson, D.; Oldridge, N. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2004, 116, 682–692. [Google Scholar] [CrossRef]
- Schopfer, D.; Forman, D.E. Cardiac Rehabilitation in Older Adults. Can. J. Cardiol. 2016, 32, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J. Evaluating and Treating Frailty in Cardiac Rehabilitation. Clin. Geriatr. Med. 2019, 35, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.H.; Khachadurian, A.K.; Amorosa, L.F.; Clemow, L.; Ruderman, N. Ten-Year Experience with an Exercise-Based Outpatient Life-Style Modification Program in the Treatment of Diabetes Mellitus. Diabetes Care 1992, 15, 1800–1810. [Google Scholar] [CrossRef]
- Cauza, E.; Hanusch-Enserer, U.; Strasser, B.; Ludvik, B.; Metz-Schimmerl, S.; Pacini, G.; Wagner, O.; Georg, P.; Prager, R.; Kostner, K.; et al. The Relative Benefits of Endurance and Strength Training on the Metabolic Factors and Muscle Function of People with Type 2 Diabetes Mellitus. Arch. Phys. Med. Rehabil. 2005, 86, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Cononie, C.C.; Graves, J.E.; Pollock, M.L.; Phillips, M.I.; Sumners, C.; Hagberg, J.M. Effect of exercise training on blood pressure in 70- to 79-yr-old men and women. Med. Sci. Sports Exerc. 1991, 23, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.F.; Narayan, P.; Colleran, J.A.; Pittaras, A.; Notargiacomo, A.; Reda, D.; Papademetriou, V. Effects of Regular Exercise on Blood Pressure and Left Ventricular Hypertrophy in African-American Men with Severe Hypertension. N. Engl. J. Med. 1995, 333, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R. Physical exercise in the management of Hypertension. A consensus statement by the World Hypertension League. J. Hypertens. 1991, 9, 283–287. [Google Scholar]
- Kingwell, B.A.; Jennings, G.L. Effects of walking and other exercise programs upon blood pressure in normal subjects. Med. J. Aust. 1993, 158, 234–238. [Google Scholar] [CrossRef]
- Ornish, D.; Brown, S.E.; Billings, J.H.; Scherwitz, L.W.; Armstrong, W.T.; Ports, T.A.; McLanahan, S.M.; Kirkeeide, R.L.; Gould, K.L.; Brand, R.J. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990, 336, 129–133. [Google Scholar] [CrossRef]
- Tsoukas, A.; Andonakoudis, H.; Christakos, S. Short-term exercise training effect after myocardial infarction on myocardial oxygen consumption indices and ischemic threshold. Arch. Phys. Med. Rehabil. 1995, 76, 262–265. [Google Scholar] [CrossRef]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Windle, G.; Hughes, D.; Linck, P.; Russell, I.; Woods, R. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment. Heal. 2010, 14, 652–669. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.E.; Crespo, C.J.; Bartlett, S.J.; Bathon, J.M.; Fontaine, K.R. Relationship between Body Weight Gain and Significant Knee, Hip, and Back Pain in Older Americans. Obes. Res. 2003, 11, 1159–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slentz, C.A.; Houmard, J.A.; Johnson, J.L.; Bateman, L.A.; Tanner, C.J.; McCartney, J.S.; Duscha, B.D.; Kraus, W.E. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: A randomized, controlled study of exercise intensity and amount. J. Appl. Physiol. 2007, 103, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundby, C.; Montero, D. Did you know—why does maximal oxygen uptake increase in humans following endurance exercise training? Acta Physiol. 2019, 227, e13371. [Google Scholar] [CrossRef] [Green Version]
- Du, S.-F.; Wang, X.-L.; Ye, C.-L.; He, Z.-J.; Li, D.-X.; Du, B.-R.; Liu, Y.-J.; Zhu, X.-Y. Exercise training ameliorates bleomycin-induced epithelial mesenchymal transition and lung fibrosis through restoration of H2 S synthesis. Acta Physiol. 2019, 225, e13177. [Google Scholar] [CrossRef]
- Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; Neumann, J.T.; Lindahl, B.; Giannitsis, E.; Sörensen, N.A.; Badertscher, P.; Jann, J.E.; Wussler, D.; et al. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur. Heart J. 2018, 39, 3780–3794. [Google Scholar] [CrossRef] [Green Version]
- Khera, R.; Jain, S.; Pandey, A.; Agusala, V.; Kumbhani, D.J.; Das, S.R.; Berry, J.D.; de Lemos, J.A.; Girotra, S. Comparison of Readmission Rates after Acute Myocardial Infarction in 3 Patient Age Groups (18 to 44, 45 to 64, and ≥65 Years) in the United States. Am. J. Cardiol. 2017, 120, 1761–1767. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Hai, J.-J.; Siu, C.-W.; Ho, H.-H.; Li, S.-W.; Lee, S.; Tse, H.-F. Relationship between changes in heart rate recovery after cardiac rehabilitation on cardiovascular mortality in patients with myocardial infarction. Hearth Rhythm. 2010, 7, 929–936. [Google Scholar] [CrossRef]
- Kargarfard, M.; Rouzbehani, R.; Basati, F. Effects of Exercise Rehabilitation on Blood Pressure of Patients after Myocardial Infarction. Int. J. Prev. Med. 2010, 1, 124–130. [Google Scholar] [PubMed]
- Moholdt, T.; Aamot, I.L.; Granøien, I.; Gjerde, L.; Myklebust, G.; Walderhaug, L.; Brattbakk, L.; Hole, T.; Graven, T.; Stølen, T.O.; et al. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: A randomized controlled study. Clin. Rehabil. 2011, 26, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Alves, A.J.; Teixeira, M.; Miranda, F.; Azevedo, C.; Duarte, J.A.; Oliveira, J. Exercise training enhances autonomic function after acute myocardial infarction: A randomized controlled study. Rev. Port. Cardiol. 2012, 31, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Balsam, P.; Główczyńska, R.; Zaczek, R.; Szmit, S.; Opolski, G.; Filipiak, K.J. The effect of cycle ergometer exercise training on improvement of exercise capacity in patients after myocardial infarction. Kardiol. Pol. 2013, 71, 1059–1064. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Ribeiro, F.; Teixeira, M.; Campos, L.; Alves, A.J.; Silva, G.; Oliveira, J. Effect of 8-week exercise-based cardiac rehabilitation on cardiac autonomic function: A randomized controlled trial in myocardial infarction patients. Am. Hearth J. 2014, 167, 753–761.e3. [Google Scholar] [CrossRef]
- Kim, C.; Choi, H.E.; Lim, M.H. Effect of High Interval Training in Acute Myocardial Infarction Patients with Drug-Eluting Stent. Am. J. Phys. Med. Rehabil. 2015, 94, 879–886. [Google Scholar] [CrossRef]
- McGregor, G.; Gaze, D.; Oxborough, D.; O’Driscoll, J.; Shave, R. Reverse left ventricular remodelling—Effect of cardiac rehabilitation exercise training in myocardial infarction patients with preserved ejection fraction. Eur. J. Phys. Rehabil. Med. 2015, 52, 370–378. [Google Scholar] [PubMed]
- Fontes-Carvalho, R.; Azevedo, A.I.; Sampaio, F.; Teixeira, M.; Bettencourt, N.; Campos, L.; Gonçalves, F.R.; Ribeiro, V.G.; Azevedo, A.; Leite-Moreira, A. The effect of exercise training on diastolic and systolic function after acute myocardial infarction: A randomized study. Medicine 2015, 94, e1450. [Google Scholar] [CrossRef]
- Lim, S.-K.; Han, J.-Y.; Choe, Y.-R. Comparison of the Effects of Cardiac Rehabilitation Between Obese and Non-obese Patients after Acute Myocardial Infarction. Ann. Rehabil. Med. 2016, 40, 924–932. [Google Scholar] [CrossRef] [Green Version]
- Matos-Garcia, B.C.; Rocco, I.S.; Maiorano, L.D.; Peixoto, T.C.; Moreira, R.S.L.; Carvalho, A.C.; Catai, A.M.; Arena, R.; Gomes, W.J.; Guizilini, S. A Home-Based Walking Program Improves Respiratory Endurance in Patients with Acute Myocardial Infarction: A Randomized Controlled Trial. Can. J. Cardiol. 2017, 33, 785–791. [Google Scholar] [CrossRef]
- Ribeiro, F.; Oliveira, N.; Silva, G.; Campos, L.; Miranda, F.; Teixeira, M.; Alves, A.; Oliveira, J. Exercise-based cardiac rehabilitation increases daily physical activity of patients following myocardial infarction: Subanalysis of two randomised controlled trials. Physiotherapy 2017, 103, 59–65. [Google Scholar] [CrossRef]
- Choe, Y.; Han, J.-Y.; Choi, I.-S.; Park, H.-K. Changes in Oxygen Consumption and Heart Rate after Acute Myocardial Infarction During 6-Month Follow-up. PM&R 2018, 10, 587–593. [Google Scholar] [CrossRef]
- Elshazly, A.; Khorshid, H.; Hanna, H.; Ali, A. Effect of exercise training on heart rate recovery in patients post anterior myocardial infarction. Egypt. Hearth J. 2018, 70, 283–285. [Google Scholar] [CrossRef]
- Santi, G.L.D.; Moreira, H.T.; Carvalho, E.E.V.D.; Crescêncio, J.C.; Schmidt, A.; Marin-Neto, J.A.; Gallo-Júnior, L. Influence of aerobic training on the mechanics of ventricular contraction after acute myocardial infarction: A pilot study. Arq. Bras. Cardiol. 2018, 110, 383–387. [Google Scholar] [CrossRef]
- Dun, Y.; Thomas, R.J.; Medina-Inojosa, J.R.; Squires, R.W.; Huang, H.; Smith, J.R.; Liu, S.; Olson, T.P. High-intensity interval training in cardiac rehabilitation: Impact on fat mass in patients with myocardial infarction. Mayo Clin. Proc. 2019, 94, 1718–1730. [Google Scholar] [CrossRef]
- Dun, Y.; Thomas, R.J.; Smith, J.R.; Medina-Inojosa, J.R.; Squires, R.W.; Bonikowske, A.R.; Huang, H.; Liu, S.; Olson, T.P. High-intensity interval training improves metabolic syndrome and body composition in outpatient cardiac rehabilitation patients with myocardial infarction. Cardiovasc. Diabetol. 2019, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Jayo-Montoya, J.A.; Maldonado-Martín, S.; Aispuru, G.R.; Gorostegi-Anduaga, I.; Gallardo-Lobo, R.; Matajira-Chia, T.; Villar-Zabala, B.; Blanco-Guzmán, S. Low-volume high-intensity aerobic interval training is an efficient method to improve cardiorespiratory fitness after myocardial infarction: Pilot study from the interfarct project. J. Cardiopulm. Rehabil. Prev. 2020, 40, 48–54. [Google Scholar] [CrossRef]
- Eser, P.; Jaeger, E.; Marcin, T.; Herzig, D.; Trachsel, L.D.; Wilhelm, M. Acute and chronic effects of high-intensity interval and moderate-intensity continuous exercise on heart rate and its variability after recent myocardial infarction: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2020, 101444. [Google Scholar] [CrossRef]
- Lund, J.S.; Aksetøy, I.A.; Dalen, H.; Amundsen, B.H.; Støylen, A. Left ventricular diastolic function: Effects of high-intensity exercise after acute myocardial infarction. Echocardiography 2020, 37, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Tanaka, K.; Yabushita, N.; Sakai, T.; Shigematsu, R. Effects of exercise frequency on functional fitness in older adult women. Arch. Gerontol. Geriatr. 2007, 44, 163–173. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J.; ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Giallauria, F.; Lucci, R.; Pietrosante, M.; Gargiulo, G.; De Lorenzo, A.; D’Agostino, M.; Gerundo, G.; Abete, P.; Rengo, F.; Vigorito, C. Exercise-based cardiac rehabilitation improves heart rate recovery in elderly patients after acute myocardial infarction. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 713–717. [Google Scholar] [CrossRef] [Green Version]
- Munk, P.S.; Butt, N.; Larsen, A.I. High-intensity interval exercise training improves heart rate variability in patients following percutaneous coronary intervention for angina pectoris. Int. J. Cardiol. 2010, 145, 312–314. [Google Scholar] [CrossRef]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Menown, I.B.A.; Davies, S.; Gupta, S.; Kalra, P.R.; Lang, C.; Morley, C.; Padmanabhan, S. Resting Heart Rate and Outcomes in Patients with Cardiovascular Disease: Where Do We Currently Stand? Cardiovasc. Ther. 2013, 31, 215–223. [Google Scholar] [CrossRef]
- Ghardashi-Afousi, A.; Holisaz, M.T.; Shirvani, H.; Pishgoo, B. The effects of low-volume high-intensity interval versus moderate intensity continuous training on heart rate variability, and hemodynamic and echocardiography indices in men after coronary artery bypass grafting: A randomized clinical trial study. ARYA Atheroscler. 2018, 14, 260–271. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef]
- Ades, P.A.; Grunvald, M.H. Cardiopulmonary exercise testing before and after conditioning in older coronary patients. Am. Hearth J. 1990, 120, 585–589. [Google Scholar] [CrossRef]
- Hendriksen, I.J.M.; Zuiderveld, B.; Kemper, H.C.G.; Bezemer, P.D. Effect of commuter cycling on physical performance of male and female employees. Med. Sci. Sports Exerc. 2000, 32, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.E.; Rissel, C. Cycling and health: An opportunity for positive change? Med. J. Aust. 2009, 190, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.C.; Eng, J.J.; Dawson, A.S.; Gylfadóttir, S. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: A meta-analysis. Clin. Rehabil. 2006, 20, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Duru, F.; Candinas, R.; Dziekan, G.; Goebbels, U.; Myers, J.; Dubach, P. Effect of exercise training on heart rate variability in patients with new-onset left ventricular dysfunction after myocardial infarction. Am. Hearth J. 2000, 140, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Ståhle, A.; Nordlander, R.; Bergfeldt, L. Aerobic group training improves exercise capacity and heart rate variability in elderly patients with a recent coronary event. A randomized controlled study. Eur. Hearth J. 1999, 20, 1638–1646. [Google Scholar] [CrossRef]
- Tsai, M.-W.; Chie, W.-C.; Kuo, T.B.J.; Chen, M.-F.; Liu, J.-P.; Chen, T.T.H.H.; Wu, Y.-T. Effects of exercise training on heart rate variability after coronary angioplasty. Phys. Ther. 2006, 86, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Legramante, J.M.; Massaro, M.; Raimondi, G.; Galante, A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: A randomized, controlled study. J. Cardiopulm. Rehabil. 2001, 21, 116. [Google Scholar] [CrossRef]
- Gerlach, S.; Mermier, C.; Kravitz, L.; Degnan, J.; Dalleck, L.; Zuhl, M. Comparison of Treadmill and Cycle Ergometer Exercise During Cardiac Rehabilitation: A Meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 690–699. [Google Scholar] [CrossRef]
- Gademan, M.G.; Swenne, C.A.; Verwey, H.F.; van der Laarse, A.; Maan, A.C.; van de Vooren, H.; van Pelt, J.; van Exel, H.J.; Lucas, C.M.; Cleuren, G.V.; et al. Effect of Exercise Training on Autonomic Derangement and Neurohumoral Activation in Chronic Heart Failure. J. Card. Fail. 2007, 13, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Skali, H.; Anavekar, N.S.; Bourgoun, M.; Barvik, S.; Ghali, J.K.; Warnica, J.W.; Khrakovskaya, M.; Arnold, J.M.O.; Schwartz, Y.; et al. Changes in Ventricular Size and Function in Patients Treated with Valsartan, Captopril, or Both after Myocardial Infarction. Circulation 2005, 111, 3411–3419. [Google Scholar] [CrossRef]
- Dalal, H.M.; Zawada, A.; Jolly, K.; Moxham, T.; Taylor, R.S. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ 2010, 340, b5631. [Google Scholar] [CrossRef] [Green Version]
- Safiyari-Hafizi, H.; Taunton, J.; Ignaszewski, A.; Warburton, D.E. The Health Benefits of a 12-Week Home-Based Interval Training Cardiac Rehabilitation Program in Patients with Heart Failure. Can. J. Cardiol. 2016, 32, 561–567. [Google Scholar] [CrossRef]
- Peixoto, T.C.; Begot, I.; Bolzan, D.W.; Machado, L.; Reis, M.; Papa, V.; Carvalho, A.C.; Arena, R.; Gomes, W.J.; Guizilini, S. Early Exercise-Based Rehabilitation Improves Health-Related Quality of Life and Functional Capacity after Acute Myocardial Infarction: A Randomized Controlled Trial. Can. J. Cardiol. 2015, 31, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Cortopassi, F.; Castro, A.; Porto, E.F.; Colucci, M.; Fonseca, G.; Torre-Bouscoulet, L.; Iamonti, V.; Jardim, J. Comprehensive exercise training improves ventilatory muscle function and reduces dyspnea perception in patients with COPD. Monaldi Arch. Chest Dis. 2009, 71, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.N.; Hardman, A.E. Walking to health. Sport Med. 1997, 23, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Miyashita, M.; Takahashi, M.; Kawanishi, N.; Hayashida, H.; Kim, H.; Suzuki, K.; Nakamura, Y. Low-Volume Walking Program Improves Cardiovascular-Related Health in Older Adults. J. Sports Sci. Med. 2014, 13, 624–631. [Google Scholar] [PubMed]
| Criterion | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ∑ |
| Hai et al. [30] | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Kargarfard et al. [31] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Moholdt et al. [32] | Y | Y | Y | Y | Y | N | N | N | Y | Y | Y | 7 |
| Ribeiro et al. [33] | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 9 |
| Balsam et al. [34] | Y | N | Y | N | N | N | N | Y | Y | Y | Y | 5 |
| Oliviera et al. [35] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Kim et al. [36] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| McGregor et al. [37] | Y | N | Y | Y | N | N | N | Y | Y | Y | Y | 6 |
| Fontes-Carvalho et al. [38] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Lim et al. [39] | Y | N | Y | N | N | Y | Y | Y | Y | Y | Y | 7 |
| Matos-Garcia et al. [40] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | 9 |
| Ribeiro et al. [41] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Choe et al. [42] | Y | N | Y | Y | Y | N | N | Y | Y | Y | Y | 7 |
| Elshazly et al. [43] | Y | N | Y | Y | Y | N | N | Y | Y | Y | Y | 7 |
| Santi et al. [44] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Dun et al. [45] | Y | N | Y | Y | N | N | N | Y | Y | Y | Y | 6 |
| Dun et al. [46] | Y | N | Y | Y | N | N | N | Y | Y | Y | Y | 6 |
| Jayo-Montoya et al. [47] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Eser et al. [48] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Lund et al. [49] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Study | Aim | Sample of Participants | Exercise Program | Parameters | Results | |
|---|---|---|---|---|---|---|
| Number and Groups | Age (Years) | |||||
| Hai et al. [30] | Evaluation of the effects in changes in heart rate recovery after exercise training in subjects after MI | N—386 E—334 C—52 | E—63.8 ± 11.2 K—65 ± 10.2 | 2× a week 8 weeks 45 min Individual assessment of intensity Treadmill | HR HRpeak HRR METs HRpeak-HR | E ** HRpeak (121.7 ± 22.8 to 125.4 ± 23.3 bpm) + 3% HRR ** (17.5 ± 10 to 19 ± 12.3 bpm) + 8.6% METs ** (5.4 ± 3.2 to 7.2 ± 3.4) + 33.3% HRpeak-HR ** (50.5 ± 20.8 to 53.4 ± 22) + 5.74% C ** METs (4.3 ± 2.7 to 5.1 ± 3.1) + 18.6% |
| Kargarfard et al. [31] | Assessment of BP’s response to aerobic exercise during exercise and at rest in subjects after MI | N—72 E—35 C—37 | E—57.7 ± 4.9 K—56.3 ± 5.9 | 3× a week 8 weeks 45–60 min 60–70% HRmax-HR Treadmill | HR HRpeak METs SBP DBP | E ** HR (79.83 ± 11.63 to 74.17 ± 10.11 bpm) − 7% * (p ≤ 0.001) HRpeak ** (130.93 ± 4.65 to 142 ± 3.14 bpm) + 8.4% * (p ≤ 0.001) METs ** (8.23 ± 1.15 to 9.42 ± 1.19) + 14.6% * (p ≤ 0.01) SBP ** (129.60 ± 10.97 to 123.54 ± 6.82 mmHg) − 4.7% * (p ≤ 0.01) DBP ** (81.43 ± 8.44 to 78.8 ± 4.34 mmHg) − 3.2% * (p ≤ 0.05) |
| Moholdt et al. [32] | Comparison of the effects of routine care and aerobic interval exercise on VO2peak in subjects after MI | N—89 E—30 C—59 | 57.4 ± 9.5 | 2× a week 12 weeks 60 min 85–95% HRmax Treadmill | VO2peak HR HRpeak HRR | E ** VO2peak (31.6 ± 5.8 to 36 ± 8.6 mL kg−1 min−1) + 13.9% * (p < 0.005) C ** HR (60.4 ± 9.3 to 56.8 ± 7.8 bpm) − 6% |
| Ribeiro et al. [33] | Evaluation of the effects of physical activities on the autonomic function of arterial diseases of the subjects after MI | N—38 E—20 C—18 | E—54.3 ± 10.8 K—57 ± 7.6 | 3× a week 8 weeks 55 min 65–75% HRmax Treadmill | VO2peak HR HRpeak SBP DBP HRR | E ** VO2peak (30.8 ± 7.8 to 33.9 ± 8.3) + 10% * (p < 0.05) HR ** (68 ± 9.2 to 62.6 ± 8.7 bpm) − 7.9% HRR ** (20 ± 6.4 to 24 ± 4.7 bpm) + 20% * (p < 0.05) |
| Balsam et al. [34] | Evaluation of the effects of physical activity on the basic parameters of the cardiopulmonary exercise test in subjects after MI | N—52 | 54.1 ± 7.1 | 3–5× a week 12 + 12 workouts 45 min 55-65 rpm cycle ergometer | VO2peak HRpeak VO2AT VE PEF METs | VO2peak ** (32.3 ± 7 to 39.3 ± 12.27 mL kg−1 min−1) + 21.7% HRpeak ** (122.9 ± 16 to 138.4 ± 21.48) + 12.6% VO2AT ** (18.3 ± 4.4 to 24.7 ± 7.56 mL kg−1 min−1) + 35% VE ** (60.5 ± 18 to 81.9 ± 25.1 L min−1) + 35.4% METs ** (9.39 to 11.79) + 25.6% |
| Oliveira et al. [35] | Assessing the effects of cardiac rehabilitation exercise programs on HRV, assessing the effects of physical activity and dietary intake | N—92 E—47 C—45 | 56 ± 10 | 3× a week 4 weeks 50 min 70–85% HRmax Treadmill or cycle ergometer | SBP DBP HR HRV VO2peak | VO2peak ** (27.6 ± 7.3 to 29.7 ± 8.8) + 7.6% * (p = 0.024) |
| Kim et al. [36] | Assessment of the effects of HIT and MCT on VO2peak, as well as assessment of HIT safety in subjects after MI with an implanted stent | N—28 E1—14 E2—14 | E1—57 ± 11.5 E2—60.2 ± 13.6 | 3× a week 6 weeks E1—45 min 85–95% HRR E2—45 min 70–85% HRR Treadmill | VO2max VO2peak HR HRpeak HRR1min | E1 VO2peak ** (29.15 ± 5.46 to 35.6 ± 7.71 mL kg−1 min−1) + 22.3%E2 VO2peak ** (27.1 ± 8.19 to 29.59 ± 8.65 mL kg−1 min−1) + 9.19% * (p = 0.021) E2 HRR1min ** (18 ± 7.4 to 23.7 ± 7.98) + 31.7% |
| McGregor et al. [37] | Evaluation of the effect of cardiac exercise on left ventricular structure and heart function in subjects after MI | M—50 E—33 C—17 | E—55.8 ± 9.2 K—56.2 ± 10.8 | 2× a week 10 weeks 25–40 min 60–80% VO2peak Treadmill, cycle ergometer, rowing machine, cross trainers | HR BP VO2peak VT utv | E VO2peak** (24 ± 4.1 to 27.5 ± 4.6 mL kg−1 min−1) + 14.6% * (p < 0.05) VT ** (12.5 ± 2.8 to 14.6 ± 3.5) + 16.8% utv ** (8.6 ± 1 to 9.9 ± 1.2) + 15.1% |
| Fontes-Carvalho et al. [38] | Assessment of the effects of exercise on diastolic and systolic function at rest in subjects after MI | N—175 E—89 C—86 | E—55.4 ± 10.3 K—55.9 ± 10.8 | 3× a week 8 weeks 70 min 70–85% HRmax Treadmill and cycle ergometer | VO2peak utv VO2AT BP | E VO2peak ** (29.1 ± 7.6 to 31 ± 9.5 mL kg−1 min−1) + 6.53% * (p < 0.01) utv ** (553 ± 136.1 to 625.7 ± 147.5 s) + 13.2% * (p < 0.01) |
| Lim et al. [39] | Evaluation of the effects of cardiac rehabilitation of aerobic type in both obese and non-obese subjects after MI | N—359 E1—170 E2—189 | E1—54.3 ± 9.9 E2—59.1 ± 11.5 | 3× a week 6 weeks 50 min Individual assesment of intensity based on target HR Treadmill | HR HRmax METs VO2max utv | E1 and E2 HR ** (E1: 74 ± 1 to 70.17 ± 0.9 bpm) − 5.2% (E2: 77.23 ± 0.9 to 72.7 ± 0.87 bpm) − 5.9% * (p < 0.046) |
| Matos-Garcia et al. [40] | Assessment of the effects of a home-based walking program on respiratory strength and endurance in subjects after MI | N—72 E1—23 E2—31 C—18 | E1—55.8 ± 7.5 E2—55.9 ± 14.6 K—48.3 ± 8.4 | 4× a week 60 days 35–60 min 11–12 RPE Home program | SFK | E2 SFK ** (43.1 ± 17.44 to 48.76 ± 13.92 cmH2O) + 13.3% * (p < 0.05) |
| Ribeiro et al. [41] | Assessment of the effects of aerobic exercise in cardiac rehabilitation on the level of daily physical activity in subjects after MI | N—50 E—25 C—25 | E—54 ± 9 K—58 ± 9 | 3× a week 8 weeks 50 min 70–85% HRmax Treadmill and cycle ergometer | utv BP HR VO2peak | E utv ** (437 ± 204 to 502 ± 226 min) + 14.9% VO2peak ** (30 ± 8.1 to 32.8 ± 9.1 mL kg−1 min−1) + 9.3% |
| Choe et al. [42] | Relationship between %HRmax and %VO2max, as well as changes in the correlation of the same parameters in subjects after MI | N—66 M—54 F—12 | 56.7 ± 9.48 | 3× a week 4 weeks 50 min 60–80% VO2max Treadmill | HR METs utv VO2max HRmax | HR ** (76.3 ± 14.5 to 70.4 ± 12.1 bpm) − 7.7% METs ** (7.1 ± 1.4 to 8 ± 1.8) + 12.7% utv ** (782.3 ± 119 to 842.2 ± 146 s) + 7.7% VO2max ** (25.48 ± 6 to 27.86 ± 6.24 mL kg−1 min−1) + 9.3% |
| Elshazly et al. [43] | Assessment of the impact of physical activity on heart rate recovery in subjects after MI | N—50 M—44 F—6 | 51.50 ± 7.46 | 3× a week 12 weeks 40–60% 30min HRmax-HR Treadmill | HR HRmax HRmax-HR HRR1min HRR2min METs BP BPpeak | HR ** (76.20 ± 14.21 to 68.16 ± 8.39 bpm) − 10.6% HRmax-HR ** (58.08 ± 20.50 to 65.68 ± 16.38 bpm) + 13.1% HRR1 ** (18 ± 8.47 to 24.7 ± 7.57 bpm) + 37.2% HRR2 ** (30.52 ± 8.62 to 24.7 ± 7.57 bpm) − 19.1% METs ** (7.16 ± 1.13 to 7.92 ± 0.78) + 10.6% |
| Santi et al. [44] | Assessment of the impact of aerobic training at two different intensities on physical capacity and mechanical contractions of the left ventricle in subjects after MI | N—30 E1—10 E2—10 C—10 | 55.1 ± 8.9 | 3× a week 12 weeks 40 min E1—60–70% HRpeak E2—85–85% HRpeak Treadmill | VO2peakVEHRpeakHR | E1 and E2 ** VO2peak (E1: 19.2 ± 5.1 to 21.9 ± 5.6 mL kg−1 min−1) + 14.1% (E2: 18.8 ± 3.7 to 21.6 ± 4.5 mL kg−1 min−1) + 14.9% |
| Dun et al. [45] | Assessment of the effect of HIIT on TK and adipose tissue distribution in subjects after MI | N—120 E1—90 E2—30 | E1—67 ± 12 E2—67 ± 16 | 3× a week 12 weeks 20–45 min E1—15–17 RPE E2—12–14 RPE Treadmill and cycle ergometer | TK HR | E2 TK ** BF%: 38.9 ± 6.1 to −1.9 ± 2, * (p < 0.001) BFM: 32.7 ± 9.2 to −1.7 ± 1.9, * (p < 0.001) AF%: 47.5 ± 8.1 to −2.6 ± 3.3, * (p < 0.001) LBM: 51 ± 10.7 to change of 1.1 ± 1.6 * (p < 0.01) HR ** (−4 ± 12 bpm) * (p < 0.003) |
| Dun et al. [46] | Assessment of the effect of HIIT on METs and TK in subjects after MI | N—56 E1—14 E2—42 | E1—69 ± 14 E2—68 ± 10 | 3× a week 12 weeks 20-45 min E1—15–17 RPE E2—12–14 RPE Treadmill and cycle ergometer | TK VO2peak METs | E2 TK ** BFM: 30.9 ± 7.6 to −1.7 ± 1.8 * (p = 0.002) BF%: 38.8 ± 7.5 to −1.8 ± 1.7 * (p = 0.002) AF%: 48.1 ± 7.4 to −2.4 ± 2.4* (p = 0.004) LBM: 48.5 ± 8.5 to change of 1.2 ± 1.4 * (p = 0.01) |
| Jayo-Montoya et al. [47] | Assessment of changes in crf and TK using low- and high-volume HIIT programs with a Mediterranean diet in subjects after MI | N—70 E1—28 E2—28 C—14 | E1—58.9 ± 9.6 E2—58.9 ± 8 | 2× a week 12 weeks E1—20–40 min 90% VO2peak E2—20 min 70% VO2peak Treadmill and cycle ergometer | VO2peak VT METpeak RERpeak utv | E1 and E2 VO2peak ** (E1: 23.1 ± 8 to 26.6 ± 7.1 mL kg−1 min−1) + 15.6% (E2: 23.2 ± 5.2 to 28.2 ± 7.7 mL kg−1 min−1) + 21.6% * (p = 0.03) METpeak ** (E1: 6.6 ± 2.3 to 7.6 ± 2) + 15.6% (E2: 6.7 ± 1.4 to 8 ± 2.3) + 19.4% * (p = 0.038) utv ** (E1: 14.3 ± 4.2 to 16.7 ± 4.7) + 16.8% (E2: 6.7 ± 1.4 to 8 ± 2.3 min) + 19.4% * (p = 0.002) |
| Eser et al. [48] | Assessment of acute and chronic effects of HIIT versus MICE on HR and HRV in subjects after acute MI elevation | N—69 E1—34 E2—3 | E1—49–66 E2—52–62 | 3× a week 9 weeks 90 min E1—13–14 RPE E2—15–16 RPE Cycle ergometer | VO2peak HRmax HRR DBP | E1 DBP ** (75 to 80 mmHg) + 6.7% |
| Lund et al. [49] | Evaluation of the effect of HIIT on diastolic pressure in subjects after MI | N—28 | 56 ± 8 | 2× a week 12 weeks 4 × 4min 85–95% HRpeak Treadmill and cycle ergometer | VO2peak HR SBP DBP HRpeak | VO2peak ** (35.2 ± 7.5 vs 38.9 ± 7.4 mL kg−1 min−1) + 10.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trajković, N.; Đorđević, D.; Stanković, M.; Petrušič, T.; Bogataj, Š.; Peršič, V. Exercise-Based Interventions in Middle-Aged and Older Adults after Myocardial Infarction: A Systematic Review. Life 2021, 11, 928. https://doi.org/10.3390/life11090928
Trajković N, Đorđević D, Stanković M, Petrušič T, Bogataj Š, Peršič V. Exercise-Based Interventions in Middle-Aged and Older Adults after Myocardial Infarction: A Systematic Review. Life. 2021; 11(9):928. https://doi.org/10.3390/life11090928
Chicago/Turabian StyleTrajković, Nebojša, Dušan Đorđević, Mima Stanković, Tanja Petrušič, Špela Bogataj, and Vanja Peršič. 2021. "Exercise-Based Interventions in Middle-Aged and Older Adults after Myocardial Infarction: A Systematic Review" Life 11, no. 9: 928. https://doi.org/10.3390/life11090928








