Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. MTT Assay
2.3. Protein Extraction and Isobaric Labeling for Proteomics Analysis
2.4. LC-MS/MS Analysis
2.5. Proteomic Data Analysis
2.6. ROS Detection Cell-Based Assay
2.7. ATP Assay
2.8. Reverse-Transcription PCR and Quantitative PCR
ΔCtconrol = Cttarget gene − Ctinternal control gene
ΔΔCt = ΔCtsample − ΔCtconrol.
2.9. Statistical Analysis
3. Results
3.1. Effects of Hesperidin and Chlorogenic Acid on Breast Cancer Cells
3.2. Identification of Pathways Affected by Hesperidin and Chlorogenic Acid
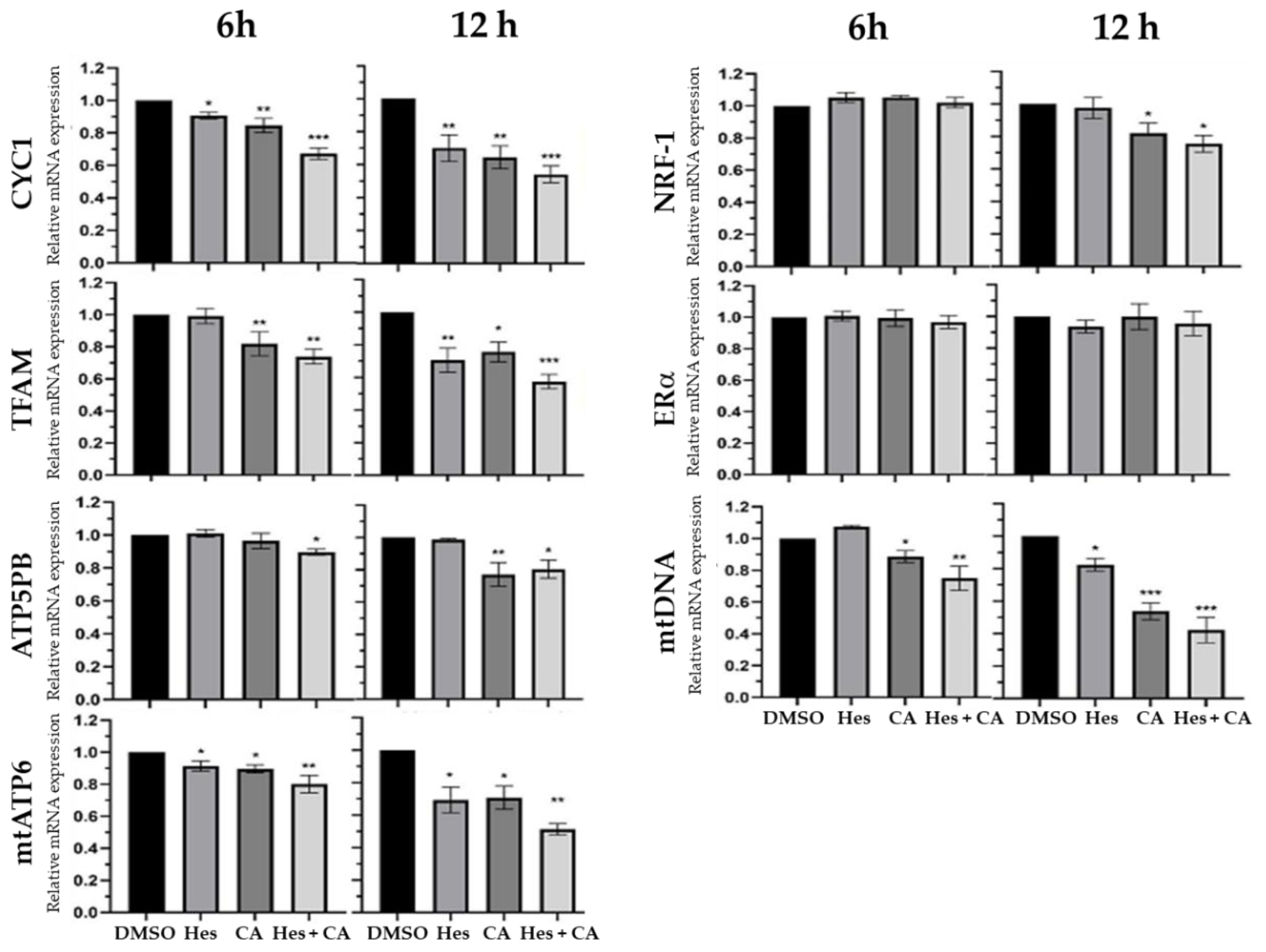
3.3. Estrogen-Receptor Pathways Were Modulated by Hesperidin and Chlorogenic Acid
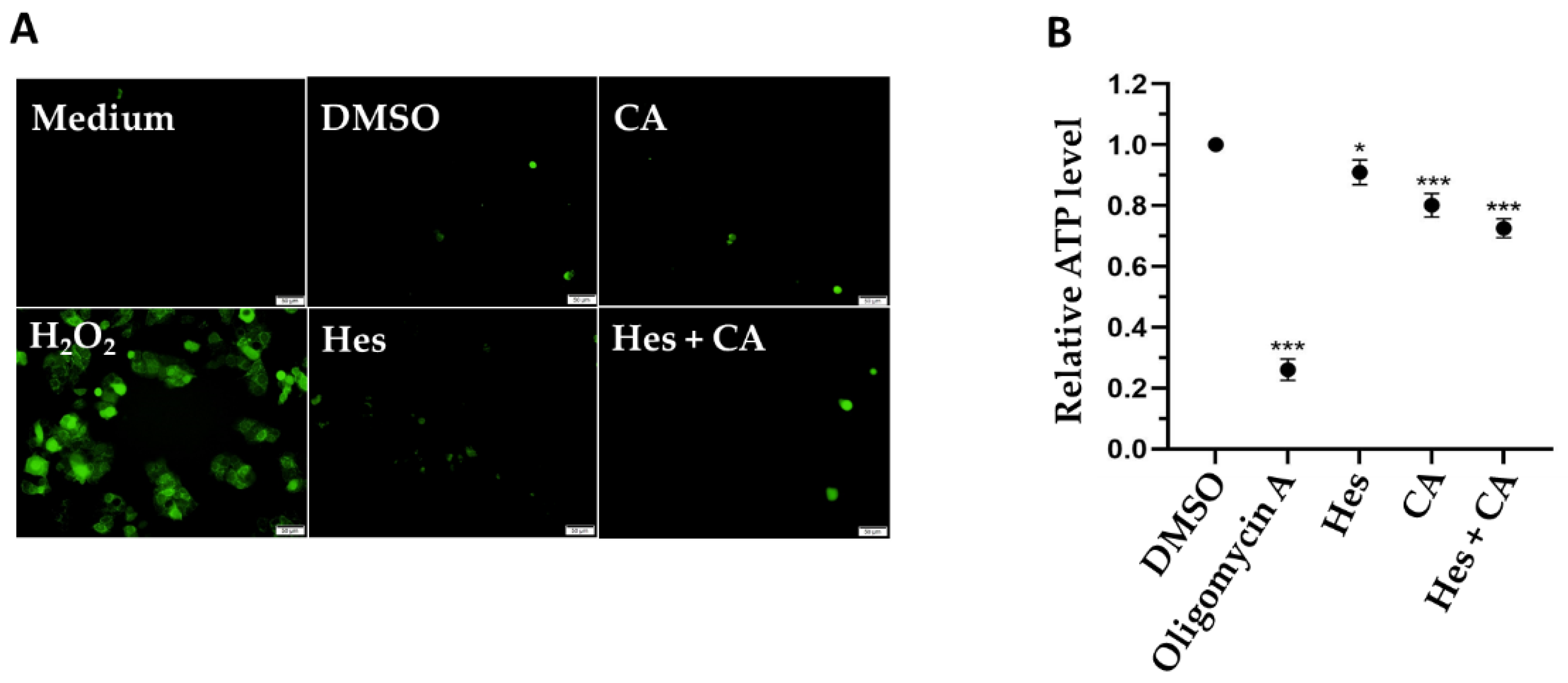
3.4. Combinational Treatments Reduced ATP Synthesis but Did Not Induce ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.-C.; Chen, Y.-H. Cancer incidence characteristic evolution based on the National Cancer Registry in Taiwan. J. Oncol. 2020, 2020, 1408793. [Google Scholar] [CrossRef]
- Kuo, C.-N.; Liao, Y.-M.; Kuo, L.-N.; Tsai, H.-J.; Chang, W.-C.; Yen, Y. Cancers in Taiwan: Practical insight from epidemiology, treatments, biomarkers, and cost. J. Formos. Med. Assoc. 2019, 119, 1731–1741. [Google Scholar] [CrossRef]
- Cheng, S.H.; Tsou, M.H.; Liu, M.C.; Jian, J.J.; Cheng, J.C.H.; Leu, S.Y.; Hsieh, C.-Y.; Huang, A.T. Unique features of breast cancer in Taiwan. Breast Cancer Res. Treat. 2000, 63, 213–223. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare, Taiwan (R.O.C.). 2020 Statistics of Causes of Death. 2018. Available online: https://dep.mohw.gov.tw/dos/lp-5069-113-xCat-y109.html (accessed on 30 August 2021).
- Trayes, K.P.; Cokenakes, S.E.H. Breast cancer treatment. Am. Fam. Phys. 2021, 104, 171–178. [Google Scholar]
- Fisusi, F.A.; Akala, E.O. Drug combinations in breast cancer therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Silvestri, M.; Cristaudo, A.; Morrone, A.; Messina, C.; Bennardo, L.; Nisticò, S.P.; Mariano, M.; Cameli, N. Emerging skin toxicities in patients with breast cancer treated with new cyclin-dependent kinase 4/6 inhibitors: A systematic review. Drug Saf. 2021, 44, 725–732. [Google Scholar] [CrossRef]
- Kim, W.; Lee, W.-B.; Lee, J.-W.; Min, B.-I.; Baek, S.K.; Lee, H.; Cho, S.-H. Traditional herbal medicine as adjunctive therapy for breast cancer: A systematic review. Complement. Ther. Med. 2015, 23, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Beckmann, K.; Franconi, G.; Meyer-Hamme, G.; Friedemann, T.; Greten, H.J.; Rostock, M.; Efferth, T. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemothera-py-induced peripheral neuropathy? Evid.-Based Complement Alternat. Med. 2013, 2013, 423713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C 2019, 98, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Katsumata, S.-I.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2009, 46, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food. Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferations of both Human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. 2009, 24, S15–S19. [Google Scholar] [CrossRef] [PubMed]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7-A comparative study. Cell Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activi-ties. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yu, H.G.; Sohn, J. The anti-angiogenic effect of chlorogenic acid on choroidal neovascularization. Korean J. Ophthalmol. 2010, 24, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Tsang, M.S.-M.; Jiao, D.; Chan, B.C.L.; Hon, K.-L.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Anti-inflammatory activities of pentaherbs formula, berberine, gallic acid and chlorogenic acid in atopic dermatitis-like skin inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef] [Green Version]
- Deka, S.J.; Gorai, S.; Manna, D.; Trivedi, V. Evidence of PKC binding and translocation to explain the anticancer mechanism of chlorogenic acid in breast cancer cells. Curr. Mol. Med. 2017, 17, 79–89. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kim, S.K.; Lee, N.Y.; Choi, Y.R.; Kim, H.S.; Gupta, H.; Youn, G.S.; Sung, H.; Shin, M.J.; Suk, K.T. Effect of Korean Red Ginseng on metabolic syndrome. J. Ginseng Res. 2020, 45, 380–389. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Cammarata, P.R.; Baines, C.P.; Yager, J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta BBA Bioenerg. 2009, 1793, 1540–1570. [Google Scholar] [CrossRef] [Green Version]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anti-Cancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef]
- Pandey, P.; Sayyed, U.; Tiwari, R.K.; Siddiqui, M.H.; Pathak, N.; Bajpai, P. Hesperidin induces ROS-mediated apoptosis along with cell cycle arrest at G2/M phase in human gall bladder carcinoma. Nutr. Cancer 2018, 71, 676–687. [Google Scholar] [CrossRef]
- Zuo, Z.; Huang, M.; Kanfer, I.; Chow, M.S.S.; Cho, W.C.S. Herb-drug interactions: Systematic review, mechanisms, and therapies. Evid.-Based Complement. Altern. Med. 2015, 2015, 239150. [Google Scholar] [CrossRef] [PubMed]
- Khamis, A.; Ali, E.M.; El-Moneim, M.A.A.; Abd-Alhaseeb, M.; Abu El-Magd, M.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018, 105, 1335–1343. [Google Scholar] [CrossRef]
- El-Sisi, A.E.; Sokkar, S.S.; Ibrahim, H.A.; Hamed, M.F.; Abu-Risha, S.E. Targeting MDR-1 gene expression, BAX/BCL2, caspase-3, and Ki-67 by nanoencapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundam. Clin. Pharmacol. 2020, 34, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Febriansah, R.; Dyaningtyas, D.P.; Sarmoko; Nurulita, N.A.; Meiyanto, E.; Nugroho, A.E. Hesperidin as a preventive resistance agent in MCF–7 breast cancer cells line resistance to doxorubicin. Asian Pac. J. Trop. Biomed. 2014, 4, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Al-Rikabi, R.; Al-Shmgani, H.; Dewir, Y.H.; El-Hendawy, S. In vivo and in vitro evaluation of the protective effects of hesperidin in lipopolysaccharide-induced inflammation and cytotoxicity of cell. Molecules 2020, 25, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, X.; Cuiping, C.; Pu, R.; Weihua, Y. Study on the anticancer effect of an astragaloside- and chlorogenic acid-containing herbal medicine (RLT-03) In breast cancer. Evid.-Based Complement. Altern. Med. 2020, 2020, 1515081. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Perks, C.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.; Jernström, H. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef] [Green Version]
- Cincin, Z.; Kiran, B.; Baran, Y.; Cakmakoglu, B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed. Pharmacother. 2018, 103, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhou, C.-Y.; Qiu, C.-H.; Lu, X.-M.; Wang, Y.-T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostoker, R.; Abelson, S.; Bitton-Worms, K.; Genkin, I.; Ben-Shmuel, S.; Dakwar, M.; Orr, Z.S.; Caspi, A.; Tzukerman, M.; LeRoith, D. Highly specific role of the insulin receptor in breast cancer progression. Endocr. Relat. Cancer 2015, 22, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Rose, D.P.; Vona-Davis, L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr.-Relat. Cancer 2012, 19, R225–R241. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Zheng, Y.; Kim, H.-S.; Xu, X.; Cao, L.; Lahusen, T.; Lee, M.-H.; Xiao, C.; Vassilopoulos, A.; Chen, W.; et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-Associated Tumorigenesis. Mol. Cell 2008, 32, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.-G.; Huang, J.-D.; Zhou, Z.; et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef] [Green Version]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front. Pharmacol. 2019, 10, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battisti, N.M.L.; Tong, D.; Ring, A.; Smith, I. Long-term outcome with targeted therapy in advanced/metastatic HER2-positive breast cancer: The Royal Marsden experience. Breast Cancer Res. Treat. 2019, 178, 401–408. [Google Scholar] [CrossRef]
- Masoud, V.; Pagès, G. Targeted therapies in breast cancer: New challenges to fight against resistance. World J. Clin. Oncol. 2017, 8, 120–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta BBA Bioenerg. 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid nanoparticles: A promising approach for cancer therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Chen, Z.; Qi, X.; Wu, X.; Di, G.; Fan, J.; Guo, C. Improved bioavailability and anticancer efficacy of Hesperetin on breast cancer via a self-assembled rebaudi-oside A nanomicelles system. Toxicol. Appl. Pharmacol. 2021, 419, 115511. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Lazer, L.M.; Sadhasivam, B.; Palaniyandi, K.; Muthuswamy, T.; Ramachandran, I.; Balakrishnan, A.; Pathak, S.; Narayan, S.; Ramalingam, S. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int. J. Biol. Macromol. 2018, 107, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.K.; Hussein, M.Z.; Fakurazi, S.; Yusoff, K.; Masarudin, M.J. Increased ROS scavenging and antioxidant efficiency of chlorogenic acid compound delivered via a chitosan nanoparticulate system for efficient in vitro visualization and accumulation in human renal adenocarcinoma cells. Int. J. Mol. Sci. 2019, 20, 4667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.J.; Chang, L.; Chu, H.W.; Lin, H.J.; Chang, P.C.; Wang, R.Y.; Unnikrishnan, B.; Mao, J.-Y.; Chen, S.-Y.; Huang, C.-C. High amplification of the antiviral activity of curcumin through transformation into carbon quantum dots. Small 2019, 15, 1902641. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| CYC1 | 5′-CCAGATAGCCAAGGATGTGTGC-3′ | 5′-GACTGACCACTTGTGCCGCTTT-3′ |
| TFAM | 5′-AGCTCAGAACCCAGATGC-3′ | 5′-CCACTCCGCCCTATAAGC-3′ |
| ATP5PB | 5′-TCACAGGGACGCTAAGATTGC-3′ | 5′-CCTTGTTGCCTGCAATACCC-3′ |
| mtDNA | 5′-ACACCCTCCTAGCCTTACTAC-3′ | 5′-GATATAGGGTCGAAGCCGC-3′ |
| mt-ATP6 | 5′-GAAGCGCCACCCTAGCAATA-3′ | 5′-GCTTGGATTAAGGCGACAGC-3′ |
| NRF-1 | 5′-CCACGTTACAGGGAGGTGAG-3′ | 5′-TGTAGCTCCCTGCTGCATCT-3′ |
| ERα | 5′-ACTGCAGGATGAGCTGG-3′ | 5′-TGCACAGAGTCTGAATTGG-3′ |
| Combinational Treatment | |||
|---|---|---|---|
| Hes Dose (μM) | CA Dose (μM) | Cell Death (%) | CI Value |
| 100 | 350 | 68.22 | 0.76 |
| Ingenuity Canonical Pathways | −log(p-Value) | Ratio | Z-Score |
|---|---|---|---|
| Oxidative Phosphorylation | 35.1 | 0.284 | −5.568 |
| Sirtuin Signaling Pathway | 24.6 | 0.117 | 2.138 |
| Necroptosis Signaling Pathway | 10.9 | 0.102 | −2.5 |
| Induction of Apoptosis by HIV1 | 4.32 | 0.0984 | −2.449 |
| Estrogen Receptor Signaling | 3.64 | 0.0366 | −2.887 |
| Insulin Secretion Signaling Pathway | 2.9 | 0.037 | −1.667 |
| Systemic Lupus Erythematosus in B Cell Signaling Pathway | 0.47 | 0.0145 | 0 |
| Senescence Pathway | 0.47 | 0.0145 | −1 |
| Hepatic Fibrosis Signaling Pathway | 0.446 | 0.0136 | −0.447 |
| Cardiac Hypertrophy Signaling | 0.377 | 0.0123 | −0.447 |
| Protein Kinase A Signaling | 0.374 | 0.0125 | 0 |
| Synaptogenesis Signaling Pathway | 0.369 | 0.0128 | −1 |
| Insulin Secretion Signaling Pathway | 2.9 | 0.037 | −1.667 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-H.; Chen, W.-H.; Juan-Lu, C.; Hsieh, S.-C.; Lin, S.-C.; Mai, R.-T.; Chen, S.-Y. Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life 2021, 11, 950. https://doi.org/10.3390/life11090950
Hsu P-H, Chen W-H, Juan-Lu C, Hsieh S-C, Lin S-C, Mai R-T, Chen S-Y. Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life. 2021; 11(9):950. https://doi.org/10.3390/life11090950
Chicago/Turabian StyleHsu, Pang-Hung, Wei-Hsuan Chen, Chen Juan-Lu, Shu-Chen Hsieh, Shih-Chao Lin, Ru-Tsun Mai, and Shiow-Yi Chen. 2021. "Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway" Life 11, no. 9: 950. https://doi.org/10.3390/life11090950
APA StyleHsu, P.-H., Chen, W.-H., Juan-Lu, C., Hsieh, S.-C., Lin, S.-C., Mai, R.-T., & Chen, S.-Y. (2021). Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life, 11(9), 950. https://doi.org/10.3390/life11090950







