FDG-PET/CT and Auricular Cartilage Biopsy Are Useful for Diagnosing with Relapsing Polychondritis in Patients without Auricular Symptoms
Abstract
:1. Introduction
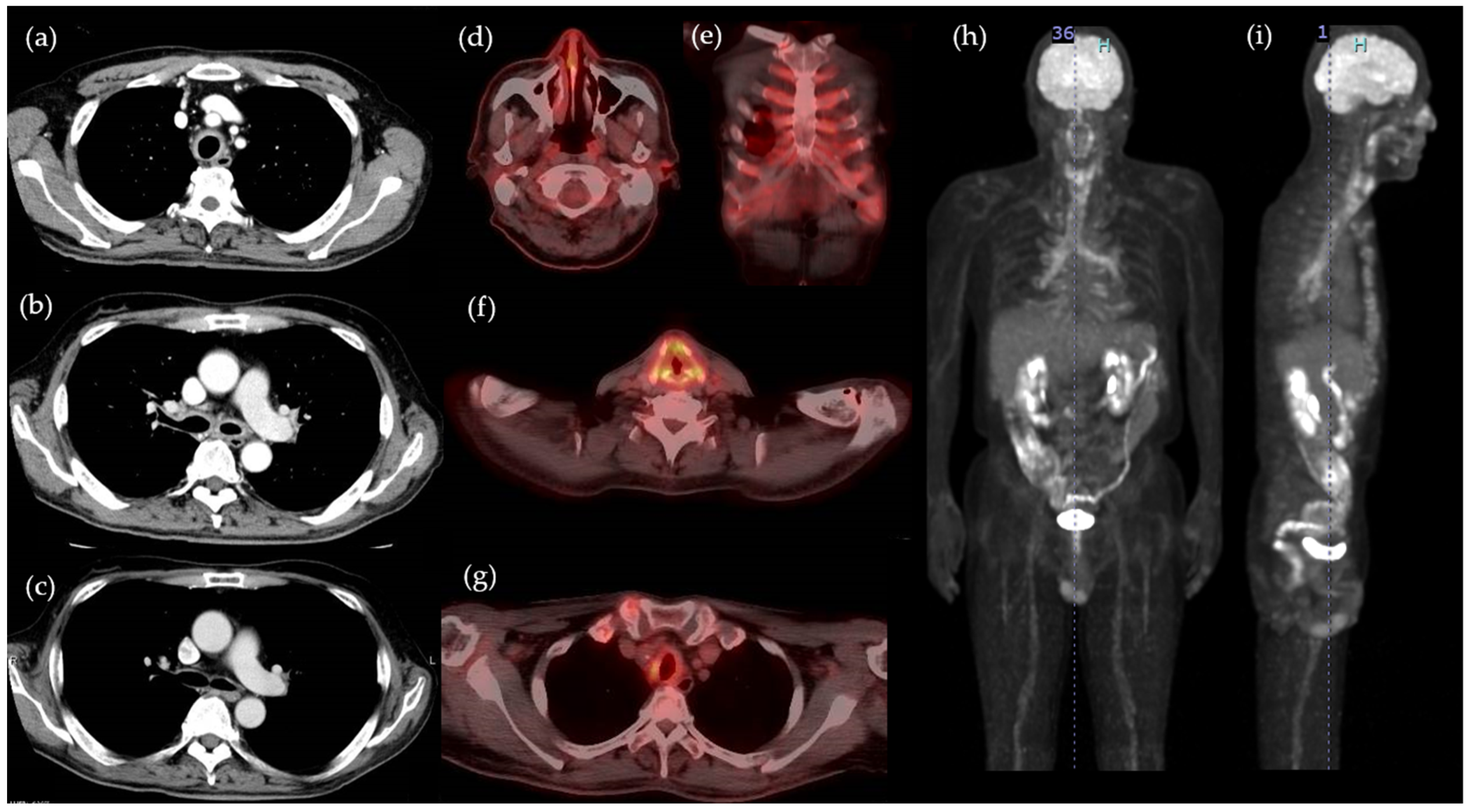
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McAdam, L.P.; O’Hanlan, M.A.; Bluestone, R.; Pearson, C.M. Relapsing polychondritis: Prospective study of 23 patients and a review of the literature. Medicine 1976, 55, 193–215. [Google Scholar] [CrossRef]
- Trentham, D.; Le, C. Trentham DE, Le CH. Relapsing polychondritis. Ann. Intern. Med. 1998, 129, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Giuffrida, R.; Guarneri, F.; Cannav, S.P. Relapsing polychondritis: An updated review. Biomedicines 2018, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Kingdon, J.; Roscamp, J.; Sangle, S.; D’Cruz, D. Relapsing polychondritis: A clinical review for rheumatologists. Rheumatology 2018, 57, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- de Montmollin, N.; Dusser, D.; Lorut, C.; Dion, J.; Costedoat-Chalumeau, N.; Mouthon, L.; Chassagnon, G.; Revel, M.P.; Puéchal, X. Tracheobronchial involvement of relapsing polychondritis. Autoimmun. Rev. 2019, 18, 102353. [Google Scholar] [CrossRef]
- Hazra, N.; Dregan, A.; Charlton, J.; Gulliford, M.C.; D’Cruz, D.P. Incidence and mortality of relapsing polychondritis in the UK: A population-based cohort study. Rheumatology 2015, 54, 2181–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, H.; Takahashi, H.; Kubota, K.; Ueda, Y.; Ozaki, T.; Yorifuji, H.; Bannai, E.; Minamimoto, R.; Morooka, M.; Miyata, Y.; et al. Utility of fluorodeoxyglucose positron emission tomography/computed tomography for early diagnosis and evaluation of disease activity of relapsing polychondritis: A case series and literature review. Rheumatology 2014, 53, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Sota, J.; Rigante, D.; Lopalco, G.; Molinaro, F.; Messina, M.; Iannone, F.; Cantarini, L. Relapsing Polychondritis: An Update on Pathogenesis, Clinical Features, Diagnostic Tools, and Therapeutic Perspectives. Curr. Rheumatol. Rep. 2016, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Papo, T.; Piette, J.C.; Du, L.T.H.; Godeau, P.; Meyer, O.; Kahn, M.F.; Bourgeois, P. Antineutrophil cytoplasmic antibodies in polychondritis. Ann. Rheum. Dis. 1993, 52, 384–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, J.M.; Levine, H.L. Relapsing polychondritis—Report of ten cases. Laryngoscope 1979, 89, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Michet, C.J.; McKenna, C.H.; Luthra, H.S.; O’Fallon, W.M. Relapsing polychondritis: Survival and predictive role of early disease manifestations. Ann. Intern. Med. 1986, 104, 74–78. [Google Scholar] [CrossRef]
- Lei, W.; Zeng, D.X.; Chen, T.; Jiang, J.H.; Wang, C.G.; Zhu, Y.H.; Huang, J.A. FDG PET-CT combined with TBNA for the diagnosis of atypical relapsing polychondritis: Report of 2 cases and a literature review. J. Thorac. Dis. 2014, 6, 1285–1292. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Zeng, Y. 18F-FDG PET/CT is a valuable tool for relapsing polychondritis diagnose and therapeutic response monitoring. Ann. Nucl. Med. 2014, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, M.; Chen, S.; Lin, L.; Li, S.; He, J.; Wang, J. Is 18 F-FDG PET/CT useful for diagnosing relapsing polychondritis with airway involvement and monitoring response to steroid-based therapy? Arthritis Res. Ther. 2019, 21, 282. [Google Scholar] [CrossRef] [Green Version]
- Rafeq, S.; Trentham, D.; Ernst, A. Pulmonary Manifestations of Relapsing Polychondritis. Clin. Chest Med. 2010, 31, 513–518. [Google Scholar] [CrossRef]
- Sato, M.; Hiyama, T.; Abe, T.; Ito, Y.; Yamaguchi, S.; Uchiumi, K.; Hashimoto, I.K.T. F-18 FDG PET/CT in relapsing polychondritis. Ann. Nucl. Med. 2010, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Pu, C.; Chen, Y. 18F-FDG PET/CT is an ideal imaging modality for the early diagnosis of relapsing polychondritis: A case report. Medicine 2017, 96, e7503. [Google Scholar] [CrossRef]
- Lei, W.; Zeng, H.; Zeng, D.X.; Zhang, B.; Zhu, Y.H.; Jiang, J.H.; Huang, J.A. (18)F-FDG PET-CT: A powerful tool for the diagnosis and treatment of relapsing polychondritis. Br. J. Radiol. 2016, 89, 20150695. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Su, M.; Li, L. 18F-FDG PET/CT imaging of relapsing polychondritis: A case report. Medicine 2016, 95, e4496. [Google Scholar] [CrossRef] [PubMed]

| Complete Blood Counts: | Value | Reference Interval | Biochemistry | Value | Reference Interval |
|---|---|---|---|---|---|
| White blood cells | 8280/μL | 3300–8600 | Aspartate transaminase | 6 U/L | 13–30 |
| Neutrophils | 77.7% | 38–77 | Alanine aminotransferase | 7 U/L | 10–42 |
| Lymphocytes | 11.0% | 20.2–53.2 | Lactate dehydrogenase | 96 U/L | 124–222 |
| Monocytes | 9.1% | 2.7–9.3 | Creatinine kinase | 20 U/L | 59–248 |
| Eosinophils | 2.5% | 0.2–4.1 | HbA1c | 8.3% | 4.9–6.2 |
| Basophils | 0.3% | 0.2–1.3 | TSH | 1.15 μIU/mL | 0.5–5 |
| Red blood cells | 314 × 104/μL | 435 × 104–555 × 104 | Free thyroxine | 1.4 ng/dL | 0.9–1.7 |
| Hemoglobin | 8.4 g/dL | 13.7–16.8 | Immunology: | ||
| Hematocrit | 27.20% | 40.7–50.1 | Rheumatoid factor | Negative | 0–15 |
| Platelets | 37.3 × 104/μL | 15.8–34.8 | Antinuclear antibody | Negative | Negative |
| Urinalysis: | Anti-ds-DNA antibody | Negative | Negative | ||
| Protein | 2+ | – | Anti-centromere antibodies | 20.4 Index | <10 |
| Creatinine | 1.14 g/day | PR3-ANCA | 3.2 U/mL | 0–3.4 | |
| Estimated urine protein | 0.22 g/day | <0.15 | MPO-ANCA | Negative | 0–3.4 |
| Biochemistry: | CH50 | 71.6 U/mL | 11–31 | ||
| CRP | 11.2 mg/dL | 0–0.14 | IgG | 1627 mg/dL | 861–1747 |
| Blood urea nitrogen | 22 mg/dL | 8–20 | IgA | 408 mg/dL | 93–393 |
| Creatinine | 0.58 mg/dL | 0.65–1.07 | IgM | 152 mg/dL | 33–183 |
| Total protein | 7.0 g/dL | 6.6–8.1 | IgE | 149 IU/mL | 0–232 |
| Albumin | 2.4 g/dL | 4.1–5.1 | Tumor markers: | ||
| Total bilirubin | 0.2 mg/dL | 0.4–1.5 | CEA | 2.6 ng/mL | 0–5 |
| γ-glutamyltransferase | 14 U/L | 13–64 | CA19-9 | 4 ng/mL | 0–37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuda, S.; Hirooka, Y.; Itami, T.; Nozaki, Y.; Sugiyama, M.; Kinoshita, K.; Funauchi, M.; Matsumura, I. FDG-PET/CT and Auricular Cartilage Biopsy Are Useful for Diagnosing with Relapsing Polychondritis in Patients without Auricular Symptoms. Life 2021, 11, 956. https://doi.org/10.3390/life11090956
Okuda S, Hirooka Y, Itami T, Nozaki Y, Sugiyama M, Kinoshita K, Funauchi M, Matsumura I. FDG-PET/CT and Auricular Cartilage Biopsy Are Useful for Diagnosing with Relapsing Polychondritis in Patients without Auricular Symptoms. Life. 2021; 11(9):956. https://doi.org/10.3390/life11090956
Chicago/Turabian StyleOkuda, Saki, Yasuaki Hirooka, Tetsu Itami, Yuji Nozaki, Masafumi Sugiyama, Koji Kinoshita, Masanori Funauchi, and Itaru Matsumura. 2021. "FDG-PET/CT and Auricular Cartilage Biopsy Are Useful for Diagnosing with Relapsing Polychondritis in Patients without Auricular Symptoms" Life 11, no. 9: 956. https://doi.org/10.3390/life11090956
APA StyleOkuda, S., Hirooka, Y., Itami, T., Nozaki, Y., Sugiyama, M., Kinoshita, K., Funauchi, M., & Matsumura, I. (2021). FDG-PET/CT and Auricular Cartilage Biopsy Are Useful for Diagnosing with Relapsing Polychondritis in Patients without Auricular Symptoms. Life, 11(9), 956. https://doi.org/10.3390/life11090956






