Bone-Marrow Stem Cells and Acellular Human Amniotic Membrane in a Rat Model of Heart Failure
Abstract
:1. Introduction
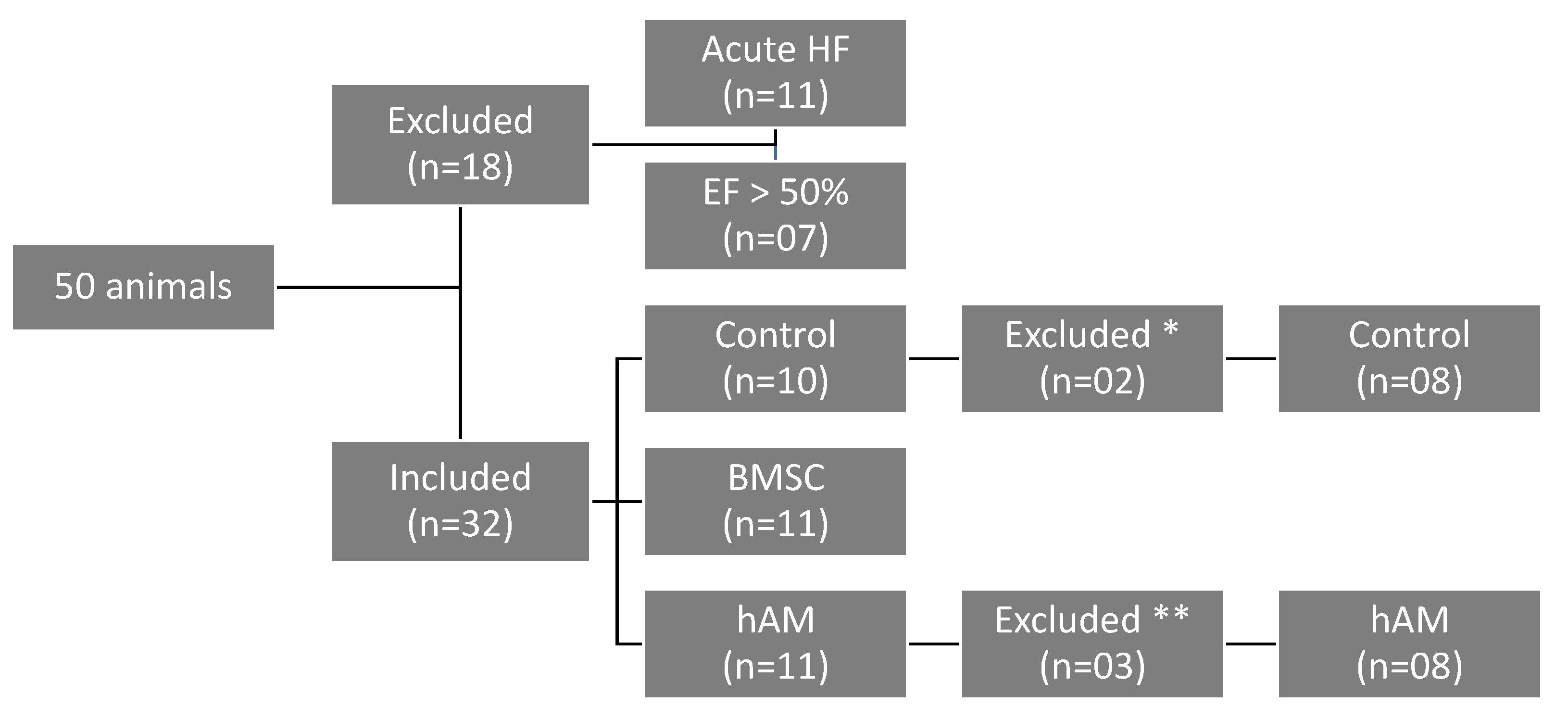
2. Materials and Methods
2.1. Animals
2.2. Myocardial Infarction Induction
2.3. Echocardiography (7th Day)
2.4. Preparation of Human Amniotic Membrane (hAM)
2.5. Isolation of Bone Marrow Stem Cells (BMSC)
2.6. BMSC Transplantation and hAM Implantation
2.7. Echocardiography (30th Day)
2.8. Anatomopathological Studies
2.8.1. Collagen Deposition Assessment
2.8.2. Immunohistochemical Analysis
2.9. Statistical Analysis
3. Results
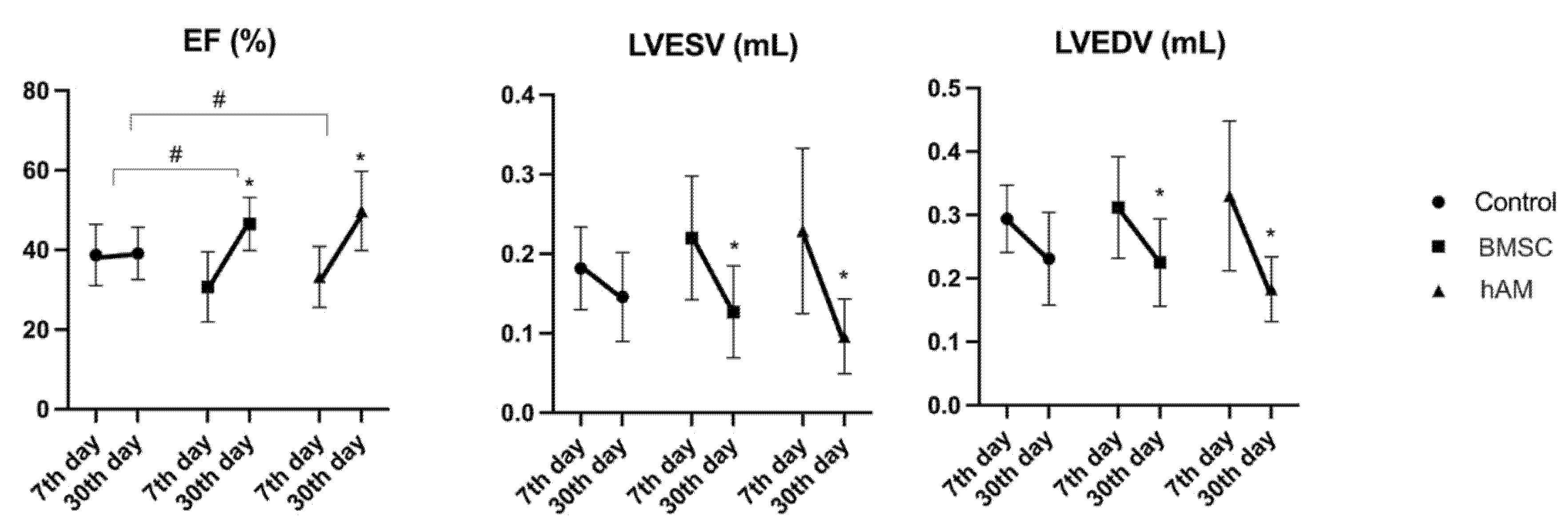
3.1. Echocardiographic Analysis
3.1.1. Ejection Fraction (EF)
3.1.2. Left Ventricular End-Systolic Volume (LVESV)
3.1.3. Left Ventricular End-Diastolic Volume (LVEDV)
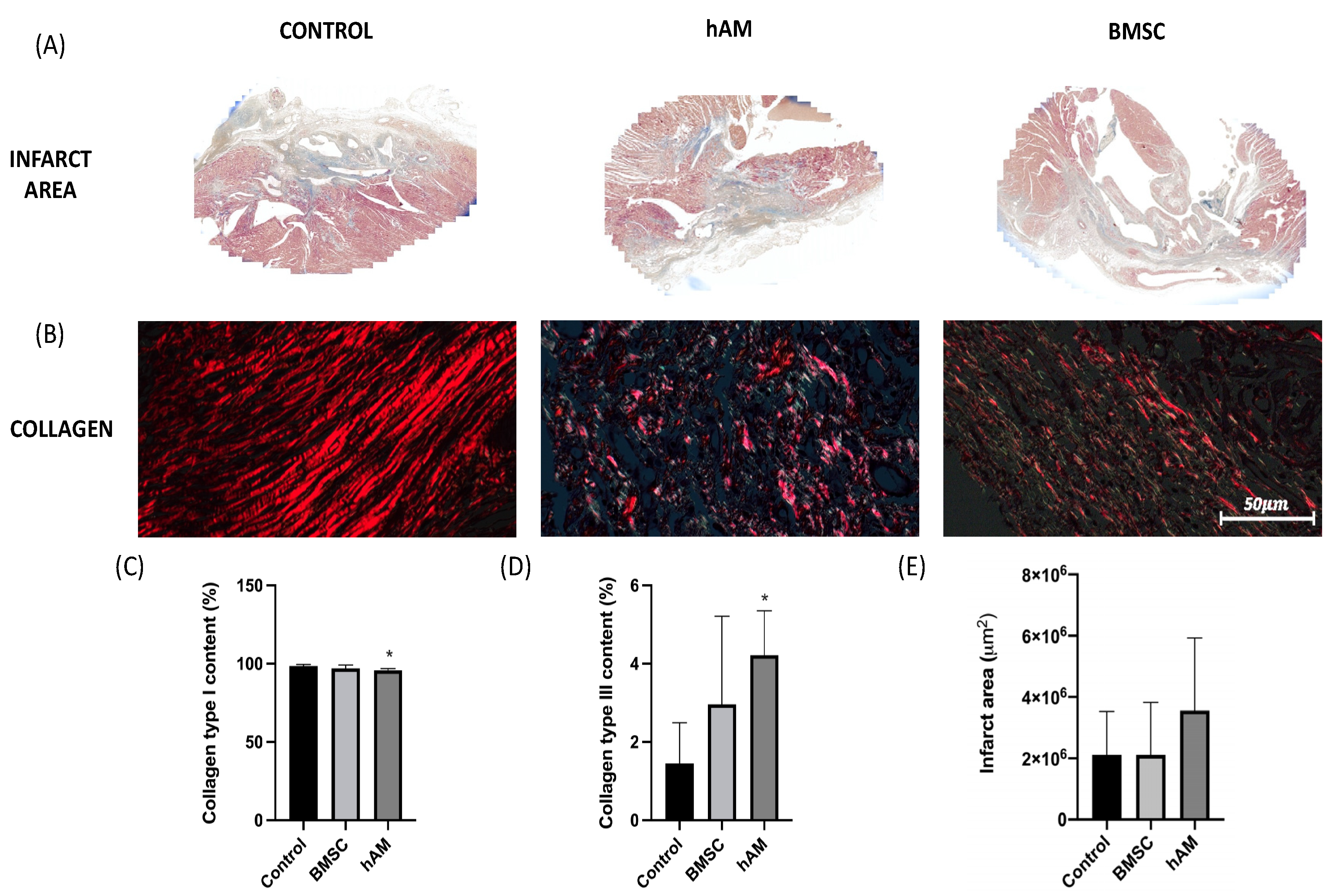
3.2. Analysis of the Infarct Area and Collagen
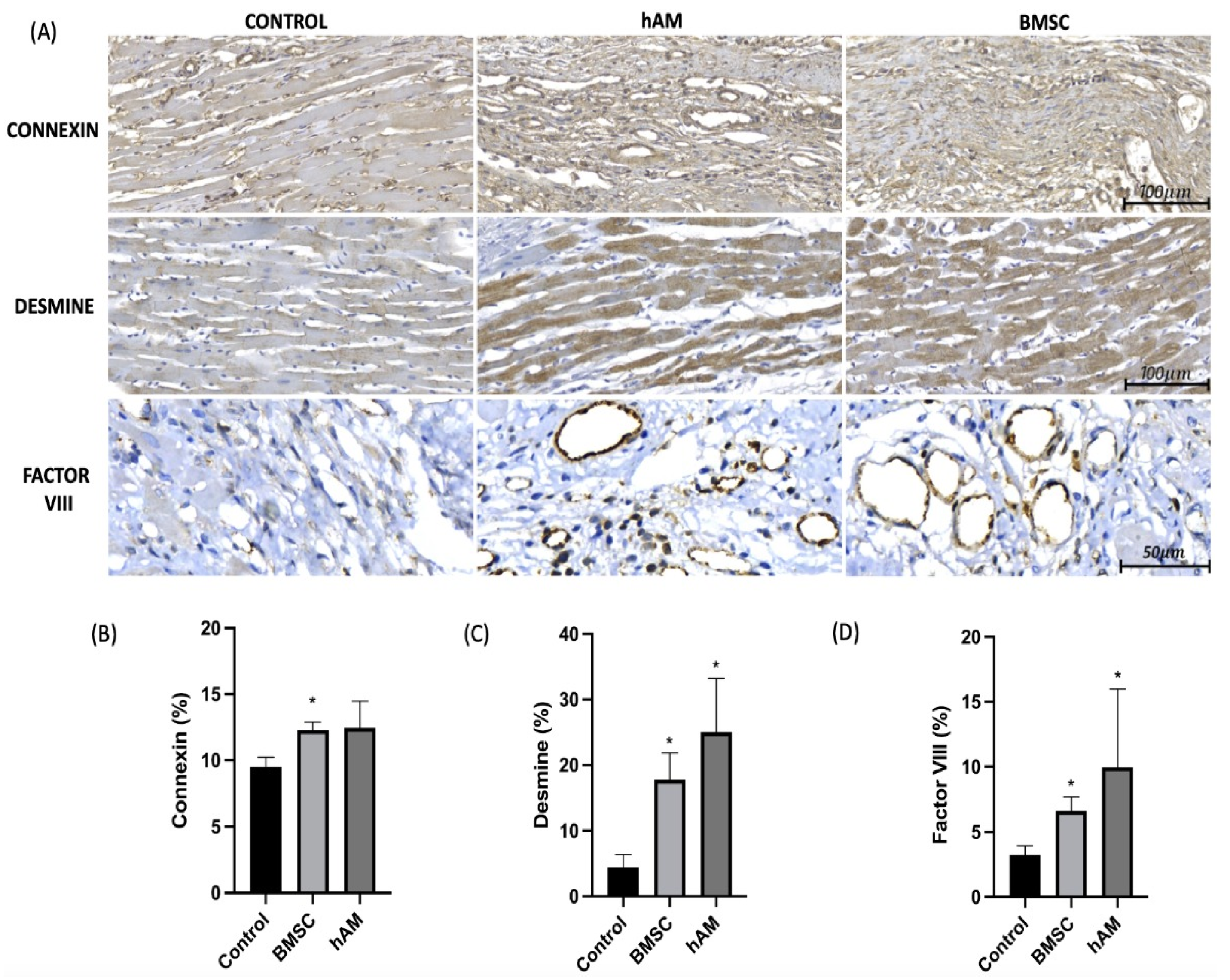
3.3. Factor VIII
3.4. Desmine
3.5. Connexin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Burchfield, S.J.; Hill, J.A. Pathological Ventricular Remodeling: Mechanisms: Part 1 of 2. Circulation 2014, 128, 388–400. [Google Scholar]
- Mythili, S.; Malathi, N. Diagnostic markers of acute myocardial infarction (Review). Biomed. Rep. 2015, 3, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasileira, S. Diretriz de Assistência Circulatória Mecânica da Sociedade Barsileira de Cardiologia. Arq. Bras. Cardiol. 2016, 107, 1–3. [Google Scholar]
- Albuquerque, D.C.D.; Souza, J.D.D.; Bacal, F.; Rohde, L.E.P.; Bernardez-Pereira, S.; Berwanger, O.; Almeida, D.R. I Brazilian Registry of Heart Failure—Clinical Aspects, Care Quality and Hospitalization Outcomes. Arq. Bras. Cardiol. 2015, 104, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24352519 (accessed on 18 December 2013). [CrossRef] [Green Version]
- Kim, I.C.; Youn, J.C.; Kobashigawa, J.A. The Past, Present and Future of Heart Transplantation. Korean Circ. J. 2018, 48, 565–590. [Google Scholar] [CrossRef]
- Machado-Junior, P.A.B.; Blume, G.G.; Francisco, J.C.; Guarita-Souza, L.C. Cell-Based Therapies for Myocardial Regeneration in Heart Failure: 20 Years of Debate. Braz. J. Cardiovasc. Surg. 2020, 35. [Google Scholar] [CrossRef]
- Tseng, C.C.S.; Ramjankhan, F.Z.; de Jonge, N.; Chamuleau, S.A.J. Advanced strategies for end-stage heart failure: Combining regenerative approaches with LVAD, a new horizon? Front. Surg. 2015, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jakus, A.E.; Laronda, M.M.; Rashedi, A.S.; Robinson, C.M.; Lee, C.; Jordan, S.W.; Orwig, K.E.; Woodruff, T.K.; Shah, R.N. “Tissue Papers” from Organ-Specific Decellularized Extracellular Matrices. Adv. Funct. Mater. 2017, 27, 1700992. [Google Scholar] [CrossRef]
- Guarita-souza, L.C.; Atahyde, K.; de Carvalho, T.; Rebelatto, C.; Hansen, P.; Furuta, M.; Miyague, N.; Francisco, J.C.; Olandoski, M.; Woitowicz, V.; et al. Comparison of mononuclear and mesenchymal stem cell transplantation in myocardium infarction. Braz. J. Cardiovasc. Surg. 2005, 20, 270–278. [Google Scholar] [CrossRef]
- Daniel, S.; Robert, M.; Viviana, L.C.; Tiziano, M.; Kaspar, R.; Sabrina, S.; Turchetto, L.; Radrizzani, M.; Astori, G.; Schwitter, J.; et al. Intracoronary Injection of Bone Marrow–Derived Mononuclear Cells Early or Late After Acute Myocardial Infarction. Circulation 2013, 127, 1968–1979. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Haase, T.; Ma, N.; Bader, A.; Troph, D.O.; Becker, M.; Choi, Y.-H.; Falk, V.; Stamm, C. Decellularized amniotic membrane attenuates postinfarct left ventricular remodeling. J. Surg. Res. 2016, 200, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Cargnoni, A.; Di Marcello, M.; Campagnol, M.; Nassuato, C.; Albertini, A.; Parolini, O. Amniotic Membrane Patching Promotes Ischemic Rat Heart Repair. Cell Transpl. 2009, 18, 1147–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, G.G.; Machado-Júnior, P.A.B.; Paludo Bertinato, G.; Simeoni, R.B.; Francisco, J.C.; Guarita-Souza, L.C. Tissue-engineered amniotic membrane in the treatment of myocardial infarction: A systematic review of experimental studies. Am. J. Cardiovasc. Dis. 2021, 11, 1–11. [Google Scholar]
- Riau, A.K.; Beuerman, R.W.; Lim, L.S.; Mehta, J.S. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010, 31, 216–225. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Zhang, M. Characterization of Ex Vivo Expanded Oral Mucosal Epithelium Cells on Acellular Porcine Corneal Stroma for Ocular Surface Reconstruction. J. Ophthalmol. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Jorge, L.F.; Francisco, J.C.; Bergonse, N.; Baena, C.; Carvalho, K.A.T.; Abdelwahid, E.; Neto, J.R.F.; Moreira, L.F.P.; Guarita–Souza, L.C. Tracheal repair with acellular human amniotic membrane in a rabbit model. J. Tissue Eng. Regen. Med. 2018, 12, e1525–e1530. [Google Scholar] [CrossRef]
- Uemura, L. Matrizes Amnióticas Descelularizadas Recobertas Com Nanoparticulas de 15,12,14 Prostaglandina J2 (15d-Pgj2) Melhoram a Função Ventricular Em Modelo Animal Com Disfunção Ventricular Pós-Infarto. Dissertação (Mestrado em Ciências da Saúde), Pontifícia Universidade Católica do Paraná, Curitiba, Brazil, 2017. [Google Scholar]
- Carvalho, K.A.T.; Guarita-Souza, L.C.; Hansen, P.; Rebelatto, C.L.K.; Senegaglia, A.C.; Miyague, N.; Olandoski, M.; Francisco, J.C.; Furuta, M.; Gremski, W. Cell Transplantation After The Coculture of Skeletal Myoblasts and Mesenchymal Stem Cells in the Regeneration of the Myocardium Scar: An Experimental Study in Rats. Transplant. Proc. 2006, 38, 1596–1602. [Google Scholar] [CrossRef]
- Hopper, R.A.; Woodhouse, K.; Semple, J.L. Acellularization of human placenta with preservation of the basement membrane: A potencial matrix for tissue engineering. Ann. Plast. Surg. 2003, 51, 598–602. [Google Scholar] [CrossRef]
- Francisco, J.C.; Correa Cunha, R.; Cardoso, M.A.; Baggio Simeoni, R.; Mogharbel, B.F.; Picharski, G.L.; Silva Moreira Dziedzic, D.; Guarita-Souza, L.C.; Carvalho, K.A. Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant. Proc. 2016, 48, 2845–2849. [Google Scholar] [CrossRef]
- Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar]
- Mogharbel, B.F.; Abdelwahid, E.; Irioda, A.C.; Francisco, J.C.; Simeoni, R.B.; de Souza, D.; de Souza, C.M.C.O.; Beltrame, M.P.; Ferreira, R.J.; Guarita-Souza, L.C.; et al. Bone Marrow-Derived Stem Cell Populations Are Differentially Regulated by Thyroid or/and Ovarian Hormone Loss. Int. J. Mol. Sci. 2017, 18, 2139. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell. Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Wollert, K.C.; Meyer, G.P.; Lotz, J.; Lichtenberg, S.R.; Lippolt, P.; Breidenbach, C.; Fichtner, S.; Hertenstein, B. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet 2004, 364. [Google Scholar] [CrossRef]
- Mills, J.S.; Rao, S.V. REPAIR-AMI: Stem cells for acute myocardial infarction. Future Cardiol. 2007, 3, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Nijveldt, R.; van der Vleuten, P.A.; Biemond, B.J.; Doevendans, P.A.; van Rossum, A.C.; Tijssen, J.G.; Zijlstra, F.; Piek, J.J.; On Behalf of the HEBE Investigators. Intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells after primary per- cutaneous coronary intervention: Rationale and design of the HEBE trial—A prospective, multicenter, randomized trial. Am. Heart J. 2006, 152, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Henry, T.D.; Ellis, S.G.; Zhao, D.X.; Ellis, S.G.; Forder, J.R.; Perin, E.C.; Penn, M.S.; et al. Cardiovascular Cell Therapy Research Network (CCTRN). Effect of stem cell delivery following ST-elevation Myocardial Infarction on the Recovery of global and regional left ventricular function the TIME randomized trial. JAMA 2012, 308, 2380–2389. [Google Scholar] [CrossRef] [Green Version]
- Tendera, M.; Wojakowski, W.; Rużyłło, W.; Chojnowska, L.; Kępka, C.; Tracz, W.; Musiałek, P.; Piwowarska, W.; Nessler, J.; Buszman, P.; et al. Intracoronary infusion of bone marrow-derived selected CD34+ CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur. Heart J. 2009, 30, 1313–1321. [Google Scholar]
- Guarita-Souza, L.C.; Carvalho, K.A.; Woitowicz, V.; Rebelatto, C.; Senegaglia, A.; Hansen, P. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation 2006, 114, I120–I124. [Google Scholar] [CrossRef] [Green Version]
- Souza, L.C.; Carvalho, K.A.; Rebelatto, C.; Senegaglia, A.; Furuta, M.; Miyague, N.; Hansen, P.; Francisco, J.C.; Olandowski, M.; Brofman, P.R. Combined transplantation of skeletal myoblasts and mesenchymal cells (co-cultivation) in ventricular dysfunction after myocardial infarction. Arq. Bras. de Cardiol. 2004, 83, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Duelen, R.; Sampaolesi, M. Stem cell technology in cardiac regeneration: A pluripotent stem cell promise. EBioMedicine 2017, 16, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Sheng, C.C.; Zhou, L.; Hao, J. Current stem cell delivery methods for myocardial repair. BioMed. Res. Int. 2013, 2013, 547902. [Google Scholar] [CrossRef] [Green Version]
- Muller-Ehmsen, J.; Whittaker, P.; Kloner, R.A.; Dow, J.S.; Sakoda, T.; Long, T.I.; Laird, P.W.; Kedes, L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell. Cardiol. 2002, 34, 107–116. [Google Scholar] [CrossRef]
- Nakamuta, J.S.; Danoviz, M.E.; Marques, F.L.N.; Santos, L.D.; Becker, C.; Gonçalves, G.A.; Vassallo, P.F.; Schettert, I.T.; Tucci, P.J.F.; Krieger, J.E. Cell therapy attenuates cardiac dysfunction post myocardial infarction: Effect of timing, routes of injection and a fibrin scaffold. PLoS ONE 2009, 4, e6005. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.J.D.; Delrosario, L.; Fang, J.; Wong, S.Y.; Fang, Q.; Sievers, R.; Kotha, S.; Wang, A.; Farmer, D.; Janaswamy, P.; et al. Development of in jectable amniotic membrane matrix for post-myocardial infarction tissue repair. Adv. Healthc. Mater. 2020, 9, e1900544. [Google Scholar] [CrossRef]
- Kim, S.W.; Zhang, H.Z.; Kim, C.E.; Kim, J.M.; Kim, M.H. Amniotic mesenchymal stem cells with ro bust chemotactic properties are effective in the treatment of a myocardial infarction model. Int. J. Cardiol. 2013, 168, 1062–1069. [Google Scholar] [CrossRef]
- Fang, C.H.; Jin, J.; Joe, J.H.; Song, Y.S.; So, B.I.; Lim, S.M.; Cheon, G.J.; Woo, S.K.; Ra, J.C.; Lee, Y.Y.; et al. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: Comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2012, 21, 1687–1696. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Left ventricular rupture threshold during the healing phase after myocardial infarction in the dog. Can. J. Physiol. Pharmacol. 1987, 65, 307–316. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Han, W.; Li, J.; Xiang, Y.; Liu, F.; Ma, X.; Zhang, J.; Fu, Z.; Su, Y.-D.; et al. Age-related differences in postinfarct left ventricular rupture and remodeling. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1815–H1822. [Google Scholar] [CrossRef] [PubMed]
- Soufen, H.N.; Salemi, V.M.C.; Aneas, I.M.S.; Ramires, F.J.A.; Benício, A.M.D.; Benvenuti, L.A.; Krieger, J.E.; Mady, C. Collagen content, but not the ratios of collagen type III/I mRNAs, differs among hypertensive, alcoholic and idiopathic dilated cardiomyopathy. Braz. J. Med. Biol. Res. 2008, 41, 1098–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorjipour, F.; Hosseini-Gohari, L.; Alizadeh Ghavidel, A.; Hajimiresmaiel, S.J.; Naderi, N.; Darbandi Azar, A.; Pazoki-Toroudi, H. Mesenchymal stem cells from human amniotic membrane differentiate into cardiomyocytes and endothelial-like cells without improving cardiac func tion after surgical administration in rat model of chronic heart failure. J. Cardiovasc. Thorac. Res. 2019, 11, 35–42. [Google Scholar] [CrossRef]
- Song, Y.S.; Joo, H.W.; Park, I.H.; Shen, G.Y.; Lee, Y.; Shin, J.H.; Kim, H.; Shin, I.S.; Kim, K.S. Transplanted human amniotic epithelial cells secrete para crine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplant. 2015, 24, 2055–2064. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Kukucka, M.; Messroghli, D.; Kunkel, D.; Brodarac, A.; Klose, K.; Geißler, S.; Becher, P.M.; Kang, S.K.; Choi, Y.H.; et al. Epithelial-to-mesenchymal transition enhances the cardio protective capacity of human amniotic epithelial cells. Cell Transplant. 2015, 24, 985–1002. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Kameli, S.M.; Fendereski, K.; Daryabari, S.S.; Kajbafzadeh, A.M. Evaluating the efficacy of tissue-engineered human amniotic membrane in the treatment of myocardial infarction. Regen. Med. 2019, 14, 113–126. [Google Scholar] [CrossRef]
- Danieli, P.; Malpasso, G.; Ciuffreda, M.C.; Cervio, E.; Calvillo, L.; Copes, F.; Pisano, F.; Mura, M.; Kleijn, L.; de Boer, R.A.; et al. Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angio-genesis. Stem. Cells Transl. Med. 2015, 4, 448–458. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyoshi, S.; Ikegami, Y.; Hida, N.; Asada, H.; Togashi, I.; Suzuki, J.; Satake, M.; Nakamizo, H.; Tanaka, M.; et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and trans-differentiated into cardiomyocytes. Circ. Res. 2010, 106, 1613–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamede, A.; Carvalho, M.; Abrantes, A.; Laranjo, M.; Maia, C.; Botelho, M. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2010, 349, 447–458. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group | 7th Day | 30th Day | p (7th × 30th Day Echo) |
|---|---|---|---|---|
| EF (%) | Control (n = 8) | 38.73 ± 7.68 | 39.13 ± 6.54 | 0.896 |
| hAM (n = 8) | 33.21 ± 7.62 | 49.77 ± 9.91 | 0.001 | |
| BMSC (n = 11) | 30.67 ± 8.80 | 46.49 ± 6.68 | <0.001 | |
| LVESV (mL) | Control (n = 8) | 0.182 ± 0.052 | 0.146 ± 0.056 | 0.118 |
| hAM (n = 8) | 0.229 ± 0.104 | 0.096 ± 0.047 | 0.001 | |
| BMSC (n = 11) | 0.220 ± 0.078 | 0.127 ± 0.058 | 0.004 | |
| LVEDV (mL) | Control (n = 8) | 0.294 ± 0.053 | 0.231 ± 0.073 | 0.053 |
| hAM (n = 8) | 0.330 ± 0.118 | 0.183 ± 0.051 | 0.002 | |
| BMSC (n = 11) | 0.312 ± 0.080 | 0.225 ± 0.069 | 0.011 |
| Intergroup Comparison | 30th Day EF |
|---|---|
| p | |
| Control × hAM | 0.006 |
| Control × BMSC | 0.034 |
| hAM × BMSC | 0.326 |
| Variable | Group | Mean | Standard Deviation | p (Comparison of 3 Groups) |
|---|---|---|---|---|
| Infarct area (%) | Control (n = 8) | 2,111,209 | 1,419,393 | |
| hAM (n = 8) | 3,555,715 | 2,373,125 | ||
| BMSC (n = 11) | 2,809,873 | 1,713,152 | 0.383 | |
| Type I | Control (n = 8) | 98.6 | 1 | |
| Collagen (%) | hAM (n = 8) | 95.8 | 1.1 | 0.014 |
| BMSC (n = 11) | 97 | 2.2 | ||
| Type III | Control (n = 8) | 1.45 | 1.04 | |
| Collagen (%) | hAM (n = 8) | 4.22 | 1.13 | 0.014 |
| BMSC (n = 11) | 2.96 | 2.25 |
| Comparison Groups | p |
|---|---|
| Control × BMSC | 0.084 |
| Control × hAM | 0.002 |
| BMSC × hAM | 0.070 |
| Variable | Group | Mean | Median | Standard Deviation | p (Comparison of 3 Groups) |
|---|---|---|---|---|---|
| Connexin (%) | Control (n = 4) | 9.51 | 9.39 | 0.73 | |
| hAM (n = 4) | 12.44 | 12.04 | 2.03 | 0.034 | |
| BMSC (n = 4) | 12.27 | 12.28 | 0.62 | ||
| Desmine (%) | Control (n = 4) | 4.41 | 3.61 | 1.98 | |
| hAM (n = 4) | 25.01 | 26.60 | 8.19 | 0.015 | |
| BMSC (n = 4) | 17.74 | 18.41 | 4.12 | ||
| Factor VIII (%) | Control (n = 4) | 3.23 | 3.47 | 0.71 | |
| hAM (n = 4) | 9.95 | 7.23 | 6.04 | 0.018 | |
| BMSC (n = 4) | 6.63 | 6.48 | 1.04 |
| Comparison Groups | p | ||
|---|---|---|---|
| Connexin | Desmine | Factor VIII | |
| Control × BMSC | 0.018 | 0.022 | 0.024 |
| Control × hAM | 0.058 | 0.002 | 0.003 |
| BMSC × hAM | 1.000 | 0.313 | 0.622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blume, G.G.; Machado-Junior, P.A.B.; Simeoni, R.B.; Bertinato, G.P.; Tonial, M.S.; Nagashima, S.; Pinho, R.A.; de Noronha, L.; Olandoski, M.; de Carvalho, K.A.T.; et al. Bone-Marrow Stem Cells and Acellular Human Amniotic Membrane in a Rat Model of Heart Failure. Life 2021, 11, 958. https://doi.org/10.3390/life11090958
Blume GG, Machado-Junior PAB, Simeoni RB, Bertinato GP, Tonial MS, Nagashima S, Pinho RA, de Noronha L, Olandoski M, de Carvalho KAT, et al. Bone-Marrow Stem Cells and Acellular Human Amniotic Membrane in a Rat Model of Heart Failure. Life. 2021; 11(9):958. https://doi.org/10.3390/life11090958
Chicago/Turabian StyleBlume, Gustavo Gavazzoni, Paulo André Bispo Machado-Junior, Rossana Baggio Simeoni, Giovana Paludo Bertinato, Murilo Sgarbossa Tonial, Seigo Nagashima, Ricardo Aurino Pinho, Lucia de Noronha, Marcia Olandoski, Katherine Athayde Teixeira de Carvalho, and et al. 2021. "Bone-Marrow Stem Cells and Acellular Human Amniotic Membrane in a Rat Model of Heart Failure" Life 11, no. 9: 958. https://doi.org/10.3390/life11090958
APA StyleBlume, G. G., Machado-Junior, P. A. B., Simeoni, R. B., Bertinato, G. P., Tonial, M. S., Nagashima, S., Pinho, R. A., de Noronha, L., Olandoski, M., de Carvalho, K. A. T., Francisco, J. C., & Guarita-Souza, L. C. (2021). Bone-Marrow Stem Cells and Acellular Human Amniotic Membrane in a Rat Model of Heart Failure. Life, 11(9), 958. https://doi.org/10.3390/life11090958







