Cytoskeleton Markers in the Spinal Cord and Mechanoreceptors of Thick-Toed Geckos after Prolonged Space Flights
Abstract
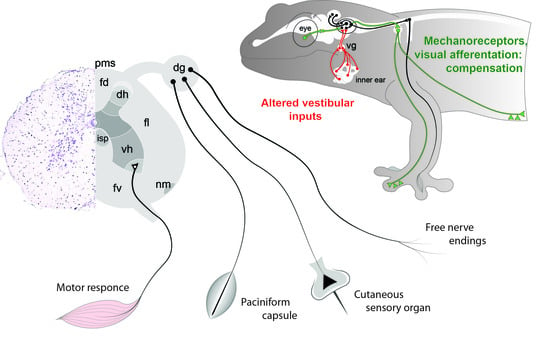
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethical Statement
2.2.1. ‘Foton-M3’
2.2.2. ‘Bion-M1’
2.3. Histology
2.4. Immunohistochemistry (IHC)
2.5. Microscopy and Morphometric Analysis
2.6. Statistical Analysis
3. Results
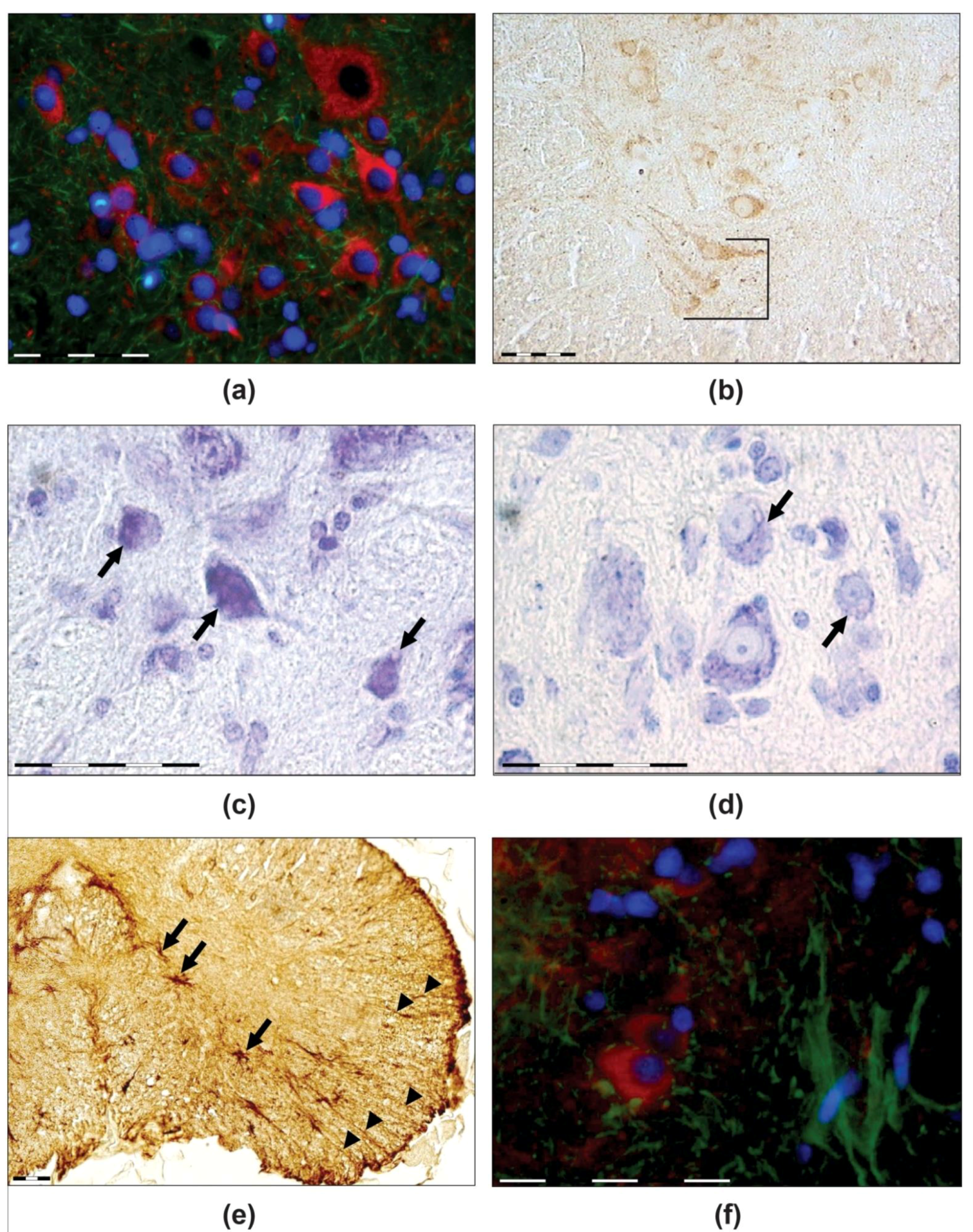
3.1. Mechanoreceptors
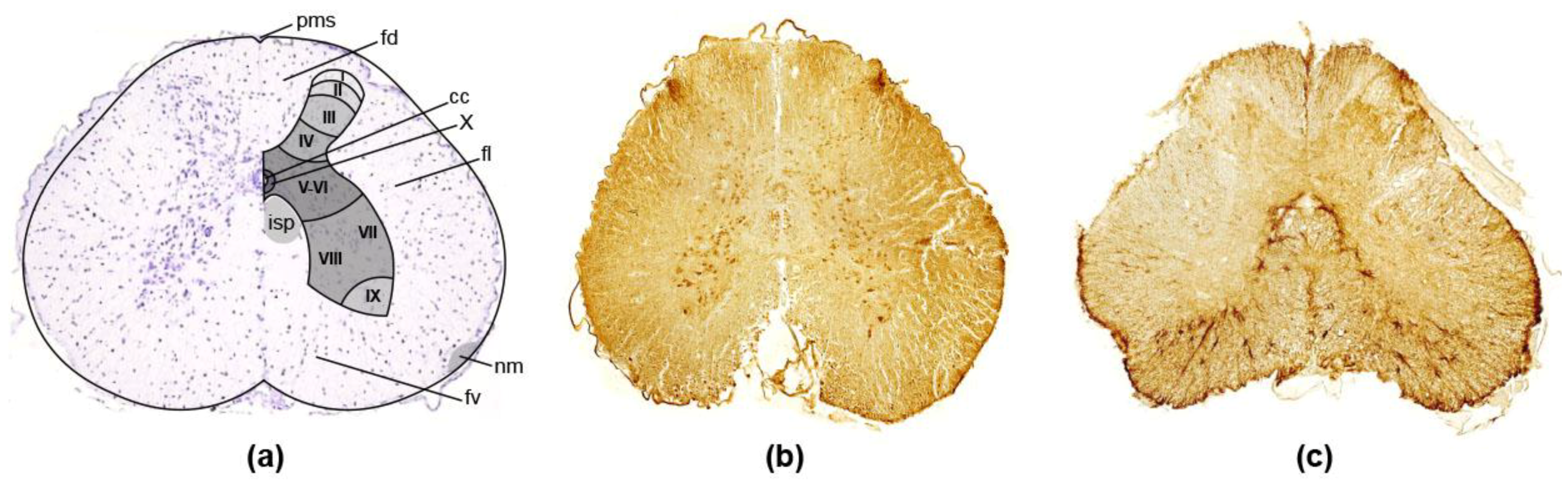
3.2. Spinal Cord
3.2.1. Dorsal Horn
3.2.2. Ventral Horn
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minor, L.B. Physiological principles of vestibular function on earth and in space. Otolaryngol. Head Neck Surg. 1998, 118, S5–S15. [Google Scholar] [CrossRef]
- Cohen, B.; Yakushin, S.B.; Holstein, G.R.; Dai, M.; Tomko, D.L.; Badakva, A.M.; Kozlovskaya, I.B. Vestibular experiments in space. Adv. Space Biol. Med. 2005, 10, 105–164. [Google Scholar] [CrossRef]
- Ortega, H.J.; Harm, D.L.; Reschke, M.F. Space and entry motion sickness. In Principles of Clinical Medicine for Space Flight; Barratt, M., Baker, E., Pool, S., Eds.; Springer: New York, NY, USA, 2019; pp. 221–222. [Google Scholar] [CrossRef]
- Hides, J.; Lambrecht, G.; Ramdharry, G.; Cusack, R.; Bloomberg, J.; Stokes, M. Parallels between astronauts and terrestrial patients—Taking physiotherapy rehabilitation “To infinity and beyond”. Musculoskelet. Sci. Pract. 2017, 27, S32–S37. [Google Scholar] [CrossRef][Green Version]
- Kozlovskaya, I.B.; Kreidich, Y.V.; Oganov, V.S.; Koserenko, O.P. Pathophysiology of motor functions in prolonged manned space flights. Acta Astronaut. 1981, 8, 1059–1072. [Google Scholar] [CrossRef]
- Grigoriev, A.I.; Kozlovskaya, I.B. Physiological reactions to muscle loading under conditions of long term hypogravity. Physiologist 1991, 30, 76–77. [Google Scholar]
- Shenkman, B.S.; Grigoriev, A.I.; Kozlovskaya, I.B. Gravitacionnyie mekhanizmy v tonicheskoi dvigatel’noi sisteme. Neirofiziologicheskie i myshechnie aspekty (Gravity mechanisms in tonic motor system. Neurophysiological and muscle aspects). Hum. Physiol. 2017, 43, 104–117. [Google Scholar]
- Kuznetsov, M.S.; Lisukov, A.N.; Rizvanov, A.A.; Tyapkina, O.V.; Gusev, O.A.; Rezvyakov, P.N.; Kozlovskaya, I.B.; Tomilovskaya, E.S.; Nikolskiy, E.E.; Islamov, R.R. Bioinformatic study of transcriptome changes in the mice lumbar spinal cord after the 30-day spaceflight and subsequent 7-day readaptation on Earth: New insights into molecular mechanisms of the hypogravity motor syndrome. Front. Pharmacol. 2019, 10, 747. [Google Scholar] [CrossRef]
- Povysheva, T.V.; Rezvyakov, P.N.; Shaimardanova, G.F.; Nikolskii, E.E.; Islamov, R.R.; Chelyshev, Y.A.; Grygoryev, A.I. Myelinated fibers of the mouse spinal cord after a 30-day space flight. Dokl. Biol. Sci. 2016, 469, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S.; Tsaturyan, A.K.; Vikhlyantsev, I.M.; Kozlovskaya, I.B.; Grigoriev, A.I. Molecular Mechanisms of Muscle Tone Impairment under Conditions of Real and Simulated Space Flight. Acta Nat. 2021, 13, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Roy, R.R. Invited review: Gravitational biology of the neuromotor systems: A perspective to the next era. J. Appl. Physiol. 2000, 89, 1224–1231. [Google Scholar] [CrossRef][Green Version]
- Kuznetsov, M.S.; Rezvyakov, P.N.; Lisyukov, A.N.; Volkov, K.D.; Islamov, R.R.; Nikolskiy, E.E. Transkriptomnyi profil’ spinnogo mozga myshi posle 30-sutochnogo poleta na biosputnike “BION-M1” I posledujuschei 7-sutochnoi readaptacii na zemle (Transcriptomic profile of mice spinal cord after 30 day spaceflight on “BION-M1” biosatellite and subsequent 7 day readaptation). Aviakosm. Ekolog. Med. 2017, 51, 85–87. [Google Scholar] [CrossRef]
- Islamov, R.R.; Tiapkina, O.V.; Nikol’skiĭ, E.E.; Kozlovskaia, I.B.; Grigor’ev, A.I. Rol’ motoneironov spinnogo mozga v mekhanizmakh razvitiia gipogravitacionnogo dvigatel’nogo sindroma (The role of spinal motoneurons in the mechanisms of hypogravitational motor syndrome development). Ross. Fiziol. Zh. Im. IM Sechenova 2013, 99, 281–293. [Google Scholar]
- Krasnov, I.B. Gravitational neuromorphology. Adv. Space Biol. Med. 1994, 4, 85–110. [Google Scholar]
- Day, J.R.; Frank, A.T.; O’Callaghan, J.P.; DeHart, B.W. Effects of microgravity and bone morphogenetic protein II on GFAP in rat brain. J. Appl. Physiol. 1998, 85, 716–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohn, F.P.M.; Ritzmann, R. Gravity and neuronal adaptation, in vitro and in vivo-from neuronal cells up to neuromuscular responses: A first model. Eur. Biophys. J. 2018, 47, 97–107. [Google Scholar] [CrossRef]
- Porseva, V.V.; Shilkin, V.V.; Strelkov, A.A.; Krasnov, I.B.; Masliukov, P.M. Changes in the neurochemical composition of motor neurons of the spinal cord in mice under conditions of space flight. Bull. Exp. Biol. Med. 2017, 162, 336–339. [Google Scholar] [CrossRef]
- Gorbunova, A.V.; Portugalov, V.V. O vliianii iskusstvennoĭ sily tiazhesti v kosmicheskom polete na soderzhanie vodorastvorimykh belkov v strukturakh nervnoĭ tkani (Effect of artificial gravitational force in space flight on the concentration of water soluble proteins in nerve tissue structures). Biull. Eksp. Biol. Med. 1978, 86, 421–424. [Google Scholar]
- Tyapkina, O.V.; Rezvyakov, P.N.; Nurullin, L.F.; Petrov, K.A.; Nilolskiy, E.E.; Islamov, R.R. Immunogistokhimicheskoe issledovanie motoneironov poiasnichnogo otdela spinnogo mozga myshei posle 30-sutochnogo kosmicheskogo poleta na biosputnike BION-M1 (Immunohistochemical study of motoneurons of the lumbar spinal cord of mice after the 30-days space flight on biosatellite BION-M1). Genes Cells 2014, 9, 263–266. [Google Scholar]
- Ilin, E.A.; Novikov, V.E. Stend dlia modelirovaniia fiziologicheskikh éffektov nevesomosti v laboratornykh éksperimentakh s krysami (Stand for modelling the physiological effects of weightlessness in laboratory experiments with rats). Kosm. Biol. Aviakosm. Med. 1980, 14, 79–80. [Google Scholar]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Islamov, R.R.; Mishagina, E.A.; Tyapkina, O.V.; Shajmardanova, G.F.; Eremeev, A.A.; Kozlovskaya, I.B.; Nikolskij, E.E.; Grigorjev, A.I. Mechanisms of spinal motoneurons survival in rats under simulated hypogravity on earth. Acta Astronaut. 2011, 68, 1469–1477. [Google Scholar] [CrossRef]
- Islamov, R.R.; Gusev, O.A.; Tanabe, A.; Terada, M.; Tyapkina, O.V.; Petrov, K.A.; Rizvanov, A.A.; Kozlovskaya, I.B.; Nikilsiy, E.E.; Grogorjev, A.I. Full-genome study of gene expression in lumbar spinal cord of mice after 30-day space flight on Bion-M1 biosatellite. Acta Astronaut. 2016, 122, 231–236. [Google Scholar] [CrossRef]
- Sarkar, D.; Nagaya, T.; Koga, K.; Nomura, Y.; Gruener, R.; Seo, H. Culture in vector-averaged gravity under clinostat rotation results in apoptosis of osteoblastic ROS 17/2.8 cells. J. Bone Miner. Res. 2000, 15, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Uva, B.M.; Masini, M.A.; Sturla, M.; Prato, P.; Passalacqua, M.; Giuliani, M.; Tagliafierro, G.; Strollo, F. Clinorotation-induced weightlessness influences the cytoskeleton of glial cells in culture. Brain Res. 2002, 934, 132–139. [Google Scholar] [CrossRef]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2006, 50, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, J. Growth factor-induced signal transduction in adherent mammalian cells is sensitive to gravity. FASEB J. 1999, 13, S35–S42. [Google Scholar] [CrossRef]
- Infanger, M.; Ulbrich, C.; Baatout, S.; Wehland, M.; Kreutz, R.; Bauer, J.; Grosse, J.; Vadrucci, S.; Cogoli, A.; Derradji, H.; et al. Modeled gravitational unloading induced downregulation of endothelin-1 in human endothelial cells. J. Cell. Biochem. 2007, 101, 1439–1455. [Google Scholar] [CrossRef]
- Masini, M.A.; Strollo, F.; Ricci, F.; Prato, P.; Uva, B.M. Low gravity and integrins in cultured glial cells. Acta Astronaut. 2008, 63, 899–905. [Google Scholar] [CrossRef]
- Gershovich, Y.G.; Gershovich, P.M.; Andreeva, E.R.; Buravkova, L.B. Kletki stromal’nogo differona v usloviiakh real’noi i modeliruemoi mikrogravitacii. In Mekhanizmy Kletochoi Gravichuvstvitel’nosti; Buravkova, L.B., Ed.; GNC–IMBP RAN: Moscow, 2018; pp. 68–79. [Google Scholar]
- Pietsch, J.; Bauer, J.; Egli, M.; Infanger, M.; Wise, P.; Ulbrich, C.; Grimm, D. The effects of weightlessness on the human organism and mammalian cells. Curr. Mol. Med. 2011, 11, 350–364. [Google Scholar] [CrossRef]
- Barabanov, V.M.; Gulimova, V.I.; Berdiev, R.K.; Saveliev, S.V. Attachment of Turner’s thick-toed geckos (Chondrodactylus turneri GRAY 1864) during weightlessness and their responses to flotation. Life Sci. Space Res. 2018, 18, 21–28. [Google Scholar] [CrossRef]
- Gulimova, V.; Proshchina, A.; Kharlamova, A.; Krivova, Y.; Barabanov, V.; Berdiev, R.; Asadchikov, V.; Buzmakov, A.; Zolotov, D.; Saveliev, S. Reptiles in space missions: Results and perspectives. Int. J. Mol. Sci. 2019, 20, 3019. [Google Scholar] [CrossRef]
- Kharlamova, A.; Proshchina, A.; Gulimova, V.; Krivova, Y.; Soldatov, P.; Saveliev, S. Cerebellar morphology and behavioural correlations of the vestibular function alterations in weightlessness. Neurosci. Biobehav. Rev. 2021, 126, 314–328. [Google Scholar] [CrossRef]
- Barabanov, V.; Gulimova, V.; Berdiev, R.; Saveliev, S. Object play in thick-toed geckos during a space experiment. J. Ethol. 2015, 33, 109–115. [Google Scholar] [CrossRef]
- Barabanov, V.M.; Gulimova, V.I.; Berdiev, R.K.; Saveliev, S.V. Individual features of play behavior in thick-toed geckos in weightlessness and normal gravity conditions. Life Sci. Space Res. 2019, 22, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Khvatov, I.A.; Gulimova, V.I.; Barabanov, V.M.; Sokolov, A.Y.; Saveliev, S.V.; Kharitonov, A.N. Osobennosti adaptivnogo povedeniia khriaschepalikh gekkonov v orbital’nom eksperimente (Peculiar features of the adaptive behavior of thick-toed geckos in the orbital spaceflight experiment). Exp. Psychol. 2014, 7, 44–56. [Google Scholar]
- Wassersug, R.J. Vertebrate biology in microgravity: Basic questions about how complex organisms respond to space flight and microgravity can only be answered by long-term study. Am. Sci. 2001, 89, 46–53. [Google Scholar] [CrossRef]
- Wassersug, R.J.; Roberts, L.; Gimian, J.; Hughes, E.; Saunders, R.; Devison, D.; Woodbury, J.; O’Reilly, J.C. The behavioral responses of amphibians and reptiles to microgravity on parabolic flights. Zoology 2005, 108, 107–120. [Google Scholar] [CrossRef] [PubMed]
- AVMA Panel on Euthanasia. American Veterinary Medical Association. 2000 Report of the AVMA Panel on Euthanasia. J. Am. Vet. Med. Assoc. 2001, 218, 669–696. [Google Scholar] [CrossRef]
- Rexed, B. The cytoarchitectonic organization of the spinal cord in the cat. J. Comp. Neurol. 1952, 96, 414–495. [Google Scholar] [CrossRef]
- Rexed, B. A cytoarchitectonic atlas of the spinal cord in the cat. J. Comp. Neurol. 1954, 100, 297–379. [Google Scholar] [CrossRef]
- Kusuma, A. The organization of the Spinal Cord in Reptiles with Different Locomotor Patterns. Ph.D. Thesis, University of Nijmegen, Nijmegen, The Netherlands, 1979; 154p. [Google Scholar]
- ten Donkelaar, H.J. Reptiles. In The Central Nervous System of Vertebrates; Nieuwenhuys, R., ten Donkelaar, H.J., Nicholson, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1315–1524. [Google Scholar]
- Vega, J.A.; García-Suárez, O.; Montaño, J.A.; Pardo, B.; Cobo, J.M. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc. Res. Tech. 2009, 72, 299–309. [Google Scholar] [CrossRef]
- Russell, A.P. Descriptive and functional anatomy of the digital vascular system of the tokay, Gekko gecko. J. Morphol. 1981, 169, 293–323. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Franceschini, V. Glial fibrillary acidic protein and vimentin immunoreactivity of astroglial cells in the central nervous system of adult Podarcis sicula (Squamata, Lacertidae). J. Anat. 2001, 198, 67–75. [Google Scholar] [CrossRef]
- Lazzari, M.; Franceschini, V. Astroglial cells in the central nervous system of the adult brown anole lizard, Anolis sagrei, revealed by intermediate filament immunohistochemistry. J. Morphol. 2005, 265, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.R.; Higham, T.E. Attachment beyond the adhesive system: The contribution of claws to gecko clinging and locomotion. Integr. Comp. Biol. 2019, 59, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Peattie, A.M. Mechanisms of adhesion in geckos. Integr. Comp. Biol. 2002, 42, 1081–1090. [Google Scholar] [CrossRef]
- Russell, A.P. The morphological basis of weight-bearing in the scansors of the tokay gecko (Repitilia: Sautia). Can. J. Zool. 1986, 64, 948–955. [Google Scholar] [CrossRef]
- Russell, A.P. Integrative functional morphology of the gekkotan adhesive system (reptilia: Gekkota). Integr. Comp. Biol. 2002, 42, 1154–1163. [Google Scholar] [CrossRef]
- Johnson, M.K.; Russell, A.P. Configuration of the setal fields of Rhoptropus (Gekkota: Gekkonidae): Functional, evolutionary, ecological and phylogenetic implications of observed pattern. J. Anat. 2009, 214, 937–955. [Google Scholar] [CrossRef]
- Rizzo, N.W.; Gardner, K.H.; Walls, D.J.; Keiper-Hrynko, N.M.; Ganzke, T.S.; Hallahan, D.L. Characterization of the structure and composition of gecko adhesive setae. J. R. Soc. Interface 2006, 3, 441–451. [Google Scholar] [CrossRef]
- Puthoff, J.B.; Prowse, M.S.; Wilkinson, M.; Autumn, K. Changes in materials properties explain the effects of humidity on gecko adhesion. J. Exp. Biol. 2010, 213, 3699–3704. [Google Scholar] [CrossRef]
- Russell, A.P.; Higham, T.E. A new angle on clinging in geckos: Incline, not substrate, triggers the deployment of the adhesive system. Proc. Biol. Sci. 2009, 276, 3705–3709. [Google Scholar] [CrossRef]
- Sun, W.; Neuzil, P.; Kustandi, T.S.; Oh, S.; Samper, V.D. The nature of the gecko lizard adhesive force. Biophys. J. 2005, 89, L14–L17. [Google Scholar] [CrossRef]
- Niewiarowski, P.H.; Lopez, S.; Ge, L.; Hagan, E.; Dhinojwala, A. Sticky gecko feet: The role of temperature and humidity. PLoS ONE 2008, 3, e2192. [Google Scholar] [CrossRef] [PubMed]
- Siminoff, R.; Kruger, L. Properties of reptilian cutaneous mechanoreceptors. Exp. Neurol. 1968, 20, 403–414. [Google Scholar] [CrossRef]
- Kenton, B.; Kruger, L.; Woo, M. Two classes of slowly adapting mechanoreceptor fibres in reptile cutaneous nerve. J. Physiol. 1971, 212, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Von Düring, M. The ultrastructure of lamellated mechanoreceptors in the skin of reptiles. Z. Anat. Entwicklungsgesch. 1973, 143, 81–94. [Google Scholar] [CrossRef]
- Faraci, F.M.; Shirer, H.W.; Orr, J.A.; Trank, J.W. Circulatory mechanoreceptors in the pond turtle Pseudemys scripta. Am. J. Physiol. 1982, 242, R216–R219. [Google Scholar] [CrossRef]
- Schroeder, D.M. The marginal nuclei in the spinal cord of reptiles: Intraspinal mechanoreceptors. Ohio J. Sci. 1986, 86, 69–72. [Google Scholar]
- Ananjeva, N.B.; Dilmuchamedov, M.E.; Matveyeva, T.N. The skin sense organs of some iguanian lizards. J. Herpetol. 1991, 25, 186–199. [Google Scholar] [CrossRef]
- Matveeva, T.N.; Ananjeva, N.B. The distribution and number of the skin sense organs of agamid, iguanid and gekkonid lizards. J. Zool. 1995, 235, 253–268. [Google Scholar] [CrossRef]
- García-Cobos, D.; Vásquez-Restrepo, J.D. Notes on the distribution, morphological variation, and mechanoreceptors of Tretanorhinus nigroluteus (Serpentes: Dipsadidae) in Colombia. Biota Colomb. 2022, 23, e943. [Google Scholar] [CrossRef]
- Hiller, U. Structure and position of receptors within scales bordering the toes of gekkonids. Cell Tissue Res. 1977, 177, 325–330. [Google Scholar] [CrossRef]
- Lauff, R.F.; Russell, A.P.; Bauer, A.M. Topography of the digital cutaneous sensilla of the tokay gecko, Gekko gecko (Reptilia, Gekkonidae), and their potential role in locomotion. Can. J. Zool. 1993, 71, 2462–2472. [Google Scholar] [CrossRef]
- Röll, B. Epidermal fine structure of the toe tips of Sphaerodactylus cinereus (Reptilia, Gekkonidae). J. Zool. 1995, 235, 289–300. [Google Scholar] [CrossRef]
- Guo, C.; Cai, L.; Xie, H.; Wang, Z.Y.; Dai, Z.D.; Sun, J.R. Divisional and hierarchical innervations of G. gecko’s toes to motion and reception. Chin. Sci. Bull. 2009, 54, 2880–2887. [Google Scholar] [CrossRef]
- Russell, A.P.; McGregor, L.D.; Bauer, A.M. Morphology and distribution of cutaneous sensory organs on the digits of Anolis carolinensis and A. sagrei (Squamata: Dactyloidae) in relation to the adhesive toepads and their deployment. Russ. J. Herpetol. 2021, 28, 249–266. [Google Scholar] [CrossRef]
- Porseva, V.V.; Shilkin, V.V.; Strelkov, A.A.; Krasnov, I.B.; Masliukov, P.M. Izmeneniia motoneironov spinnogo mozga u myshei posle kosmicheskogo poleta (Changes of spinal motor neurons in mice after a space flight). Morfologiia 2016, 150, 50–54. [Google Scholar]
- Tyapkina, O.V.; Rezvyakov, P.N.; Nurullin, L.F.; Petrov, K.A.; Nikolskiy, E.E.; Islamov, R.R. Immunogistokhimicheskoe issledovanie reakcii motoneironov poiasnichnogo otdela spinnogo mozga myshei nakhodivshikhsia v 30-sutochnom polete na biosputnike BION-M1, na nedel’nuju readeptaciju k usloviiam zemnoi gravitacii (Immunohistochemical research of reaction of motoneurons of lumbar spinal cord of the mice that were in 30-day flight on the BION-M1 biosatellite on a week readaptation to conditions of Earth gravitation). Genes Cells 2016, 11, 80–83. [Google Scholar]
- Lõrincz, D.; Kálmán, M. Distribution of GFAP in squamata: Extended immunonegative areas, astrocytes, high diversity, and their bearing on evolution. Front. Neuroanat. 2020, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Povysheva, T.V.; Nigmetzyanova, M.V.; Tyapkina, O.V.; Islamov, R.R.; Nikolskiy, E.E.; Chelyshev, Y.A. Astrocity i mikrogliya spinnogo mozga myshi v usloviyakh opornoy razgruzki zadnikh konechnostey. Dokl. Akad. Nauk 2014, 56, 114–116. [Google Scholar]
- Andreev-Andrievskiy, A.; Popova, A.; Boyle, R.; Alberts, J.; Shenkman, B.; Vinogradova, O.; Dolgov, O.; Anokhin, K.; Tsvirkun, D.; Soldatov, P.; et al. Mice in Bion-M 1 space mission: Training and selection. PLoS ONE 2014, 9, e104830. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Podlubnaya, Z.A.; Vikhlyantsev, I.M.; Litvinova, K.S.; Udaltsov, S.N.; Nemirovskaya, T.L.; Lemesheva, Y.S.; Mukhina, A.M.; Kozlovskaya, I.B. Contractile characteristics and sarcomeric cytoskeletal proteins of human soleus fibers in muscle unloading: Role of mechanical stimulation from the support surface. Biophysics 2004, 49, 807–815. [Google Scholar]
- Mirzoev, T.M.; Shenkman, B.S.; Ushakov, I.B.; Ogneva, I.V. Desmin and α-actinin-2 content in rat soleus muscle in the dynamics of gravitational unloading and subsequent reloading. Dokl. Biochem. Biophys. 2012, 444, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, K.S.; Vikhlyantsev, I.M.; Kozlovskaya, I.B.; Podlubnaya, Z.A.; Shenkman, B.S. Effects of artificial support stimulation on fiber and molecular characteristics of soleus muscle in men exposed to 7-day dry immersion. J. Gravit. Physiol. 2004, 11, P131–P132. [Google Scholar] [PubMed]
- Ogneva, I.V.; Ponomareva, E.V.; Altaeva, E.G.; Fokina, N.M.; Kurushin, V.A.; Kozlovskaya, I.B.; Shenkman, B.S. Mechanical properties of human soleus fibers after 7-day “dry” immersion. Effects of plantar support stimulation and high frequency electrical stimulation. Acta Astronaut. 2011, 68, 1478–1485. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Kozlovskaya, I.B. Cellular Responses of Human Postural Muscle to Dry Immersion. Front. Physiol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Layne, C.S.; Mulavara, A.P.; Pruett, C.J.; McDonald, P.V.; Kozlovskaya, I.B.; Bloomberg, J.J. The use of in-flight foot pressure as a countermeasure to neuromuscular degradation. Acta Astronaut. 1998, 42, 231–246. [Google Scholar] [CrossRef]
- Ross, H.E. Haptic perception in space travel. In Human Haptic Perception: Basics and Applications; Grunwald, M., Ed.; Birkhäuser: Basel, Switzerland; Boston, MA, USA; Berlin, Germeny, 2008; pp. 273–280. [Google Scholar] [CrossRef]
- Zu Eulenburg, P.; Buchheim, J.I.; Ashton, N.J.; Vassilieva, G.; Blennow, K.; Zetterberg, H.; Choukér, A. Changes in Blood Biomarkers of Brain Injury and Degeneration Following Long-Duration Spaceflight. JAMA Neurol. 2021, 78, 1525–1527. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Crowther, R.A. Tau proteins and neurofibrillary degeneration. Brain Pathol. 1991, 1, 279–286. [Google Scholar] [CrossRef]
- Metaxas, A.; Kempf, S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016, 11, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Alonso, A.C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef]
- Chun, W.; Johnson, G.V. The role of tau phosphorylation and cleavage in neuronal cell death. Front. Biosci. 2007, 12, 733–756. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Turner, R.C.; Logsdon, A.F.; Bailes, J.E.; Huber, J.D.; Rosen, C.L. Linking traumatic brain injury to chronic traumatic encephalopathy: Identification of potential mechanisms leading to neurofibrillary tangle development. J. Neurotrauma 2014, 31, 1129–1138. [Google Scholar] [CrossRef]
- Cork, L.C.; Powers, R.E.; Selkoe, D.J.; Davies, P.; Geyer, J.J.; Price, D.L. Neurofibrillary tangles and senile plaques in aged bears. J. Neuropathol. Exp. Neurol. 1988, 47, 629–641. [Google Scholar] [CrossRef]
- Loehr, J.A.; Guilliams, M.E.; Petersen, N.; Hirsch, N.; Kawashima, S.; Ohshima, H. Physical training for long-duration spaceflight. Aerosp. Med. Hum. Perform. 2015, 86, A14–A23. [Google Scholar] [CrossRef] [PubMed]



| Antibody Specificity | Origin | Dilution | Incubation | Source |
|---|---|---|---|---|
| GFAP | Mouse | Ready to use | 60 min, RT | Thermo Fisher Scientific |
| NF 200 | Rabbit | 1:160 | 30 min, RT | Sigma |
| TUBB3 | Rabbit | 1:1000 | 60 min, RT | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proshchina, A.; Gulimova, V.; Kharlamova, A.; Krivova, Y.; Barabanov, V.; Saveliev, S. Cytoskeleton Markers in the Spinal Cord and Mechanoreceptors of Thick-Toed Geckos after Prolonged Space Flights. Life 2022, 12, 100. https://doi.org/10.3390/life12010100
Proshchina A, Gulimova V, Kharlamova A, Krivova Y, Barabanov V, Saveliev S. Cytoskeleton Markers in the Spinal Cord and Mechanoreceptors of Thick-Toed Geckos after Prolonged Space Flights. Life. 2022; 12(1):100. https://doi.org/10.3390/life12010100
Chicago/Turabian StyleProshchina, Alexandra, Victoria Gulimova, Anastasia Kharlamova, Yuliya Krivova, Valeriy Barabanov, and Sergey Saveliev. 2022. "Cytoskeleton Markers in the Spinal Cord and Mechanoreceptors of Thick-Toed Geckos after Prolonged Space Flights" Life 12, no. 1: 100. https://doi.org/10.3390/life12010100
APA StyleProshchina, A., Gulimova, V., Kharlamova, A., Krivova, Y., Barabanov, V., & Saveliev, S. (2022). Cytoskeleton Markers in the Spinal Cord and Mechanoreceptors of Thick-Toed Geckos after Prolonged Space Flights. Life, 12(1), 100. https://doi.org/10.3390/life12010100