Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation
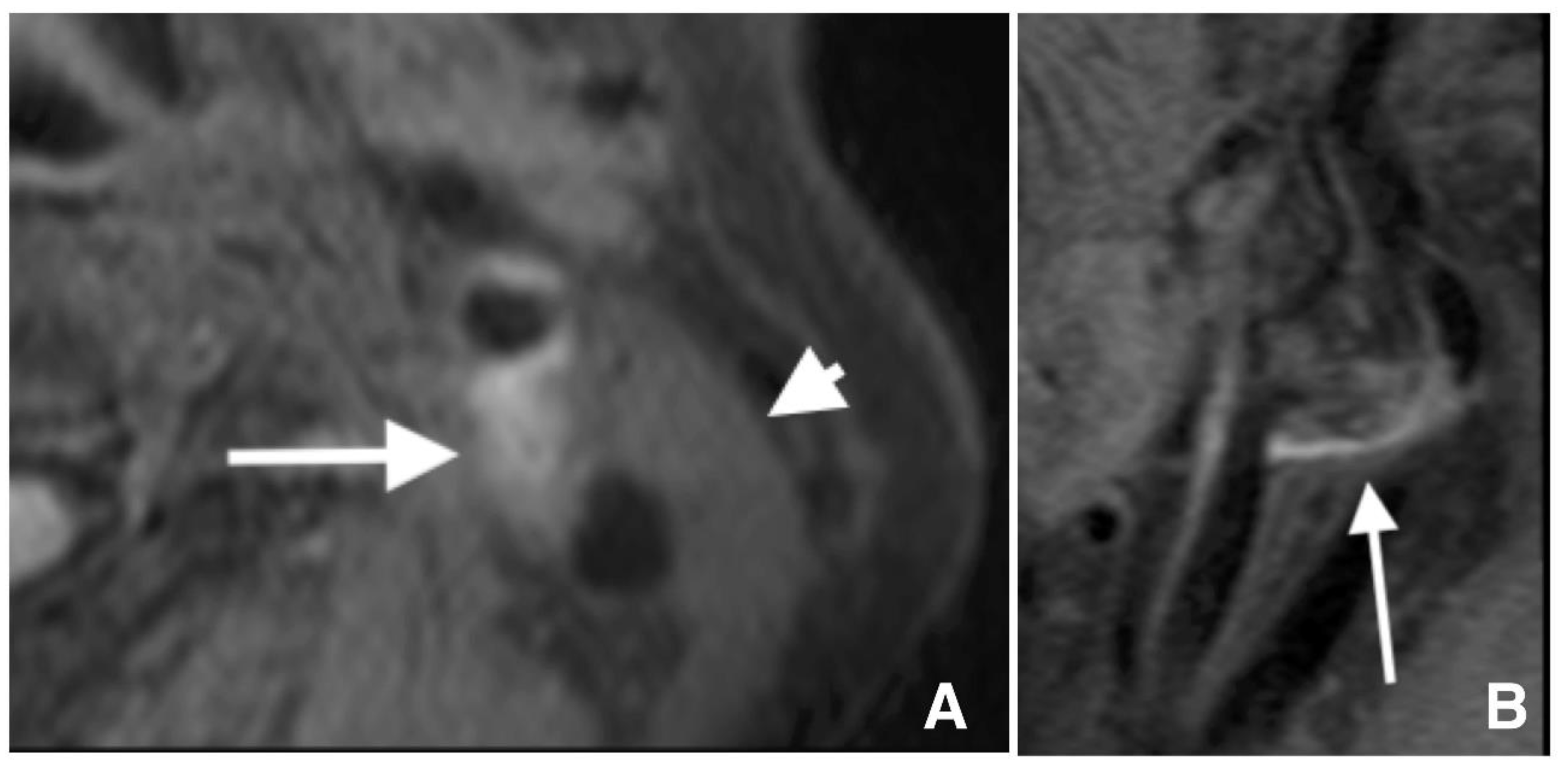
2.2. Carotid Artery Plaque Imaging
2.3. Definition of Lesions with IPH
2.4. MRA and DWI
2.5. Extremely Severe Carotid Stenosis with Probable High Oxygen Extraction Fraction
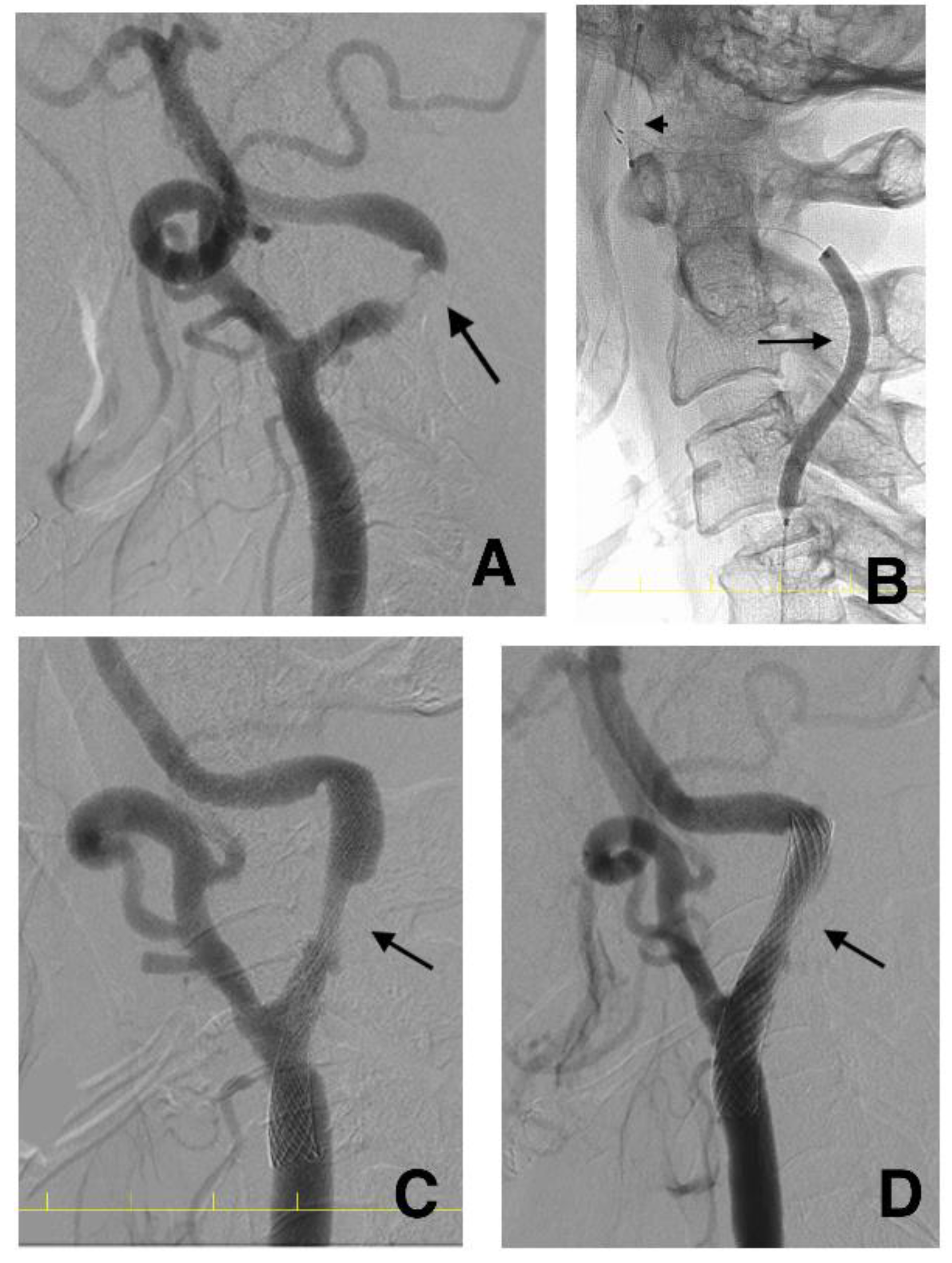
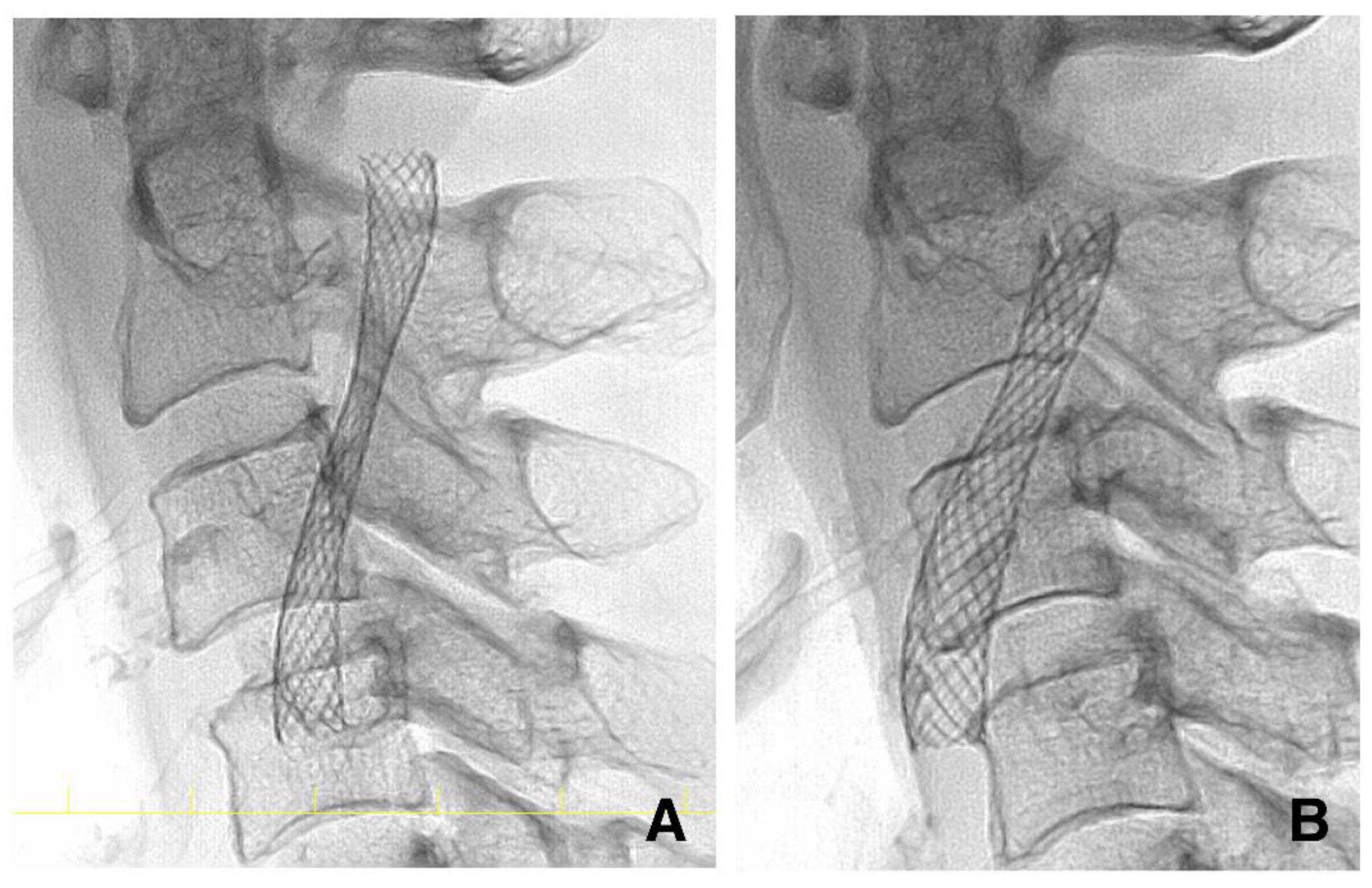
2.6. CAS Procedure
2.7. DSA Investigation after CAS
2.8. Management before and after CAS
2.9. Statistical Analysis
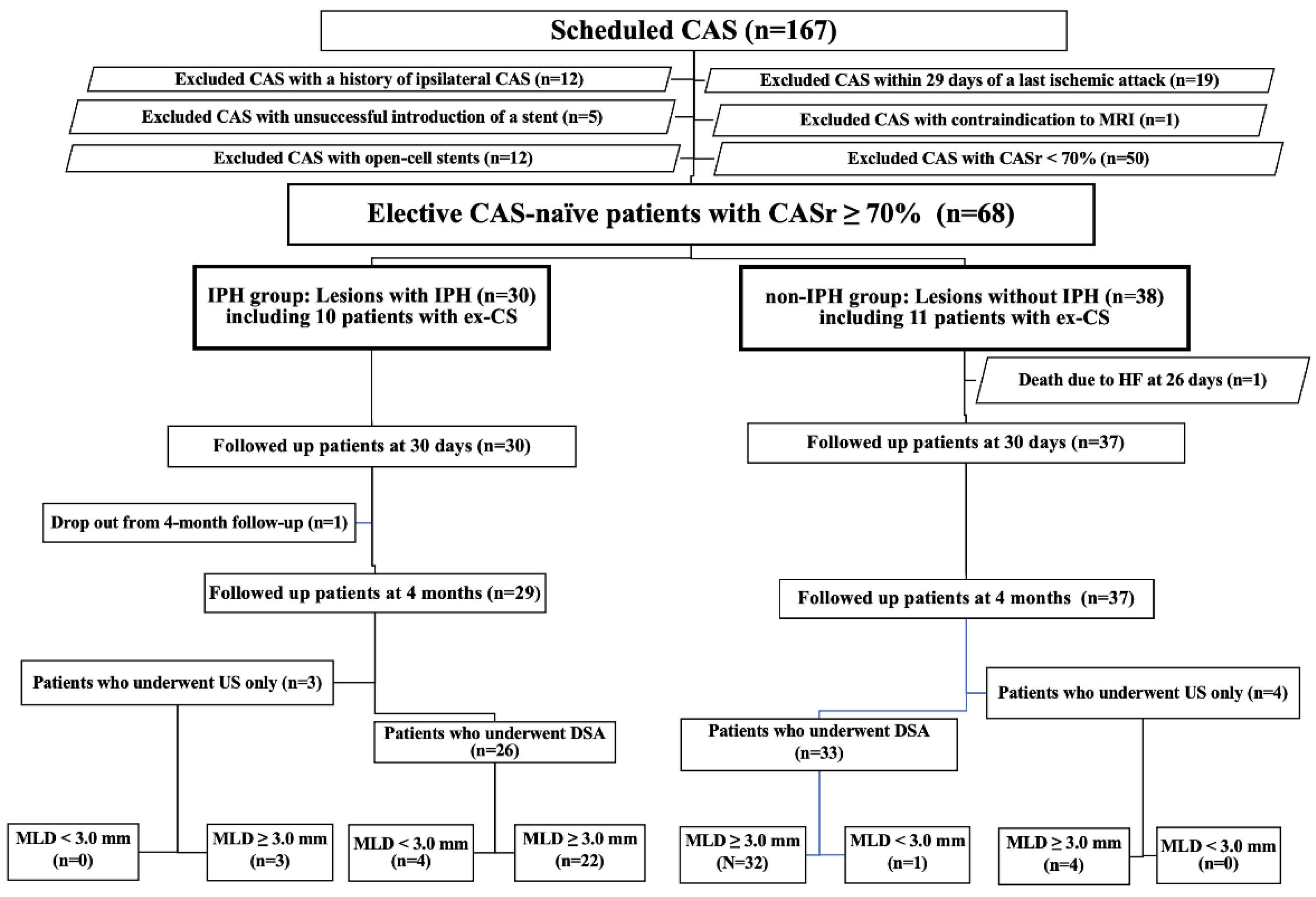
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altaf, N.; Daniels, L.; Morgan, P.S.; Auer, D.; MacSweeney, S.T.; Moody, A.R.; Gladman, J.R. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J. Vasc. Surg. 2008, 47, 337–342. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Yoshida, K.; Endo, H.; Chin, M.; Yamagata, S. Association between carotid atherosclerosis plaque with high signal intensity on T1-weighted imaging and subsequent ipsilateral ischemic events. Neurosurgery 2011, 68, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Yuan, C.; Hatsukami, T.S.; Balu, N.; Qiao, Y.; DeMarco, J.K.; Saam, T.; Moody, A.R.; Li, D.; Matouk, C.C.; et al. Carotid artery wall imaging: Perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am. J. Neuroradiol. 2018, 39, E9–E31. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tsuruta, W.; Nakai, Y.; Takigawa, T.; Marushima, A.; Masumoto, T.; Matsumaru, Y.; Ishikawa, E.; Matsumura, A. Treatment strategy based on plaque vulnerability and the treatment risk evaluation for internal carotid artery stenosis. Neurol. Med. Chir. 2018, 58, 191–198. [Google Scholar] [CrossRef]
- Yadav, J.S.; Wholey, M.H.; Kuntz, R.E.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Whitlow, P.; Strickman, N.E.; Jaff, M.R.; et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2004, 351, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Yamada, K.; Kawasaki, M.; Asano, T.; Kanematsu, M.; Takamatsu, M.; Hara, A.; Iwama, T. High-intensity signal on time-of-flight magnetic resonance angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke 2011, 42, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Lehman, V.T.; Huston, J., 3rd; Murad, M.H.; Lanzino, G.; Cloft, H.J.; Kallmes, D.F. The association between carotid intraplaque hemorrhage and outcomes of carotid stenting: A systematic review and meta-analysis. J. Neurointerv. Surg. 2017, 9, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.J.; Taylor, D.W.; Eliasziw, M.; Fox, A.J.; Ferguson, G.G.; Haynes, R.B.; Rankin, R.N.; Clagett, G.P.; Hachinski, V.C.; Sackett, D.L. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 1998, 339, 1415–1425. [Google Scholar] [CrossRef]
- Brott, T.G.; Hobson, R.W., 2nd; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Yoshida, K.; Fukumitsu, R.; Sadamasa, N.; Handa, A.; Chin, M.; Yamagata, S. Carotid artery plaque assessment using quantitative expansive remodeling evaluation and MRI plaque signal intensity. J. Neurosurg. 2016, 124, 736–742. [Google Scholar] [CrossRef]
- Mori, T.; Yoshioka, K.; Tanno, Y.; Kasakura, S. Intentional stent-stenosis to prevent hyperperfusion syndrome after carotid artery stenting for extremely high-grade stenosis. AJNR Am. J. Neuroradiol. 2021, 42, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Ogasawara, K.; Hirooka, R.; Kobayashi, M.; Fujiwara, S.; Chida, K.; Ishigaki, D.; Otawara, Y.; Ogawa, A. Prediction of cerebral hyperperfusion after carotid endarterectomy using middle cerebral artery signal intensity in preoperative single-slab 3-dimensional time-of-flight magnetic resonance angiography. Neurosurgery 2009, 64, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Yoshioka, K.; Tanno, Y.; Kasakura, S.; Miyazaki, Y. Reduced magnetic resonance angiography signal intensity in the middle cerebral artery ipsilateral to severe carotid stenosis may be a practical index of high oxygen extraction fraction. Eur. Radiol. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Mori, T.; Miyazaki, Y.; Nakazaki, M.; Takahashi, Y.; Mizokami, K. Initial experience of a novel sheath guide for transbrachial carotid artery stenting: Technical note. J. Neurointerv. Surg. 2013, 5, i77–i80. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Sonobe, M.; Kato, N.; Yamazaki, T.; Kasuya, H.; Ikeda, G.; Miki, S.; Matsushima, T. Carotid artery stenting without post-stenting balloon dilatation. J. Neurointerv. Surg. 2014, 6, 517–520. [Google Scholar] [CrossRef]
- Tanno, Y.; Mori, T.; Iwata, T.; Aoyagi, Y.; Kasakura, S.; Yoshioka, K. Spontaneous dilatation of carotid artery stents three months after the procedure, without the need for post-CAS balloon dilatation. No Shinkei Geka 2015, 43, 1019–1025. [Google Scholar]
- Martin, J.B.; Pache, J.C.; Treggiari-Venzi, M.; Murphy, K.J.; Gailloud, P.; Puget, E.; Pizzolato, G.; Sugiu, K.; Guimaraens, L.; Théron, J.; et al. Role of the distal balloon protection technique in the prevention of cerebral embolic events during carotid stent placement. Stroke 2001, 32, 479–484. [Google Scholar] [CrossRef]
- Bussière, M.; Pelz, D.M.; Kalapos, P.; Lee, D.; Gulka, I.; Leung, A.; Lownie, S.P. Results using a self-expanding stent alone in the treatment of severe symptomatic carotid bifurcation stenosis. J. Neurosurg. 2008, 109, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Zander, T.; Rabellino, M.; González, G.; Maynar, M. Carotid artery stenting without angioplasty and cerebral protection: A single-center experience with up to 7 years’ follow-up. AJNR Am. J. Neuroradiol. 2011, 32, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Voûte, M.T.; Hendriks, J.M.; van Laanen, J.H.H.; Pattynama, P.M.; Muhs, B.E.; Poldermans, D.; Verhagen, H.J. Radial force measurements in carotid stents: Influence of stent design and length of the lesion. J. Vasc. Interv. Radiol. 2011, 22, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yokoi, H.; Nakagawa, Y.; Tamura, T.; Kaburagi, S.; Sawada, Y.; Sato, Y.; Yokoi, H.; Hamasaki, N.; Nosaka, H. Three-year follow-up after implantation of metallic coronary-artery stents. N. Engl. J. Med. 1996, 334, 561–566. [Google Scholar] [CrossRef]
- Mori, T.; Kazita, K.; Chokyu, K.; Mima, T.; Mori, K. Short-term arteriographic and clinical outcome after cerebral angioplasty and stenting for intracranial vertebrobasilar and carotid atherosclerotic occlusive disease. AJNR Am. J. Neuroradiol. 2000, 21, 249–254. [Google Scholar] [PubMed]
- Douglas, J.S., Jr.; Holmes, D.R., Jr.; Kereiakes, D.J.; Grines, C.L.; Block, E.; Ghazzal, Z.M.; Morris, D.C.; Liberman, H.; Parker, K.; Jurkovitz, C.; et al. Coronary stent restenosis in patients treated with cilostazol. Circulation 2005, 112, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Matsumaru, Y.; Hayakawa, M.; Nemoto, S.; Matsumura, A. Cilostazol reduces restenosis after carotid artery stenting. J. Vasc. Surg. 2010, 51, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, M.; Mori, T.; Iwata, T.; Miyazaki, Y.; Takahashi, Y. Retrospective analysis of the effectiveness of Yokukansan (Japanese Herbal Medicine, TJ-54) in the treatment of delirium following acute stroke. No Shinkei Geka 2013, 41, 765–771. [Google Scholar]
- Joshi, K.C.; Beer-Furlan, A.; Crowley, R.W.; Chen, M.; Munich, S.A. Transradial approach for neuro-interventions: A systematic review of the literature. J. Neurointerv. Surg. 2020, 12, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Gensicke, H.; van der Worp, H.B.; Nederkoorn, P.J.; Macdonald, S.; Gaines, P.A.; van der Lugt, A.; Mali, W.P.; Lyrer, P.A.; Peters, N.; Featherstone, R.L.; et al. Ischemic brain lesions after carotid artery stenting increase future cerebrovascular risk. J. Am. Coll. Cardiol. 2015, 65, 521–529. [Google Scholar] [CrossRef]
- Eckstein, H.H.; Ringleb, P.; Dörfler, A.; Klemm, K.; Müller, B.T.; Zegelman, M.; Bardenheuer, H.; Hacke, W.; Bruckner, T.; Sandmann, W.; et al. The Carotid Surgery for Ischemic Stroke trial: A prospective observational study on carotid endarterectomy in the early period after ischemic stroke. J. Vasc. Surg. 2002, 36, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kurata, A.; Iwamoto, K.; Nakahara, K.; Niki, J.; Miyazaki, T.; Yamada, M.; Oka, H.; Fujii, K.; Kan, S. Carotid artery stenting alone or without post-stenting angioplasty: Sequential change of luminal diameter following carotid artery stenting. J. Neuroendovasc. Ther. 2010, 4, 16–20. [Google Scholar] [CrossRef][Green Version]
- Nakazaki, M.; Nonaka, T.; Takahashi, A.; Yonemasu, Y.; Nomura, T.; Onda, T.; Honda, O.; Hashimoto, Y.; Ohnishi, H.; Sasaki, M.; et al. Double balloon protection during carotid artery stenting for vulnerable carotid stenosis reduces the incidence of new brain lesions. Acta Neurochir. 2016, 158, 1377–1386. [Google Scholar] [CrossRef]
- Liem, M.I.; Kennedy, F.; Bonati, L.H.; van der Lugt, A.; Coolen, B.F.; Nederveen, A.J.; Jager, H.R.; Brown, M.M.; Nederkoorn, P.J. Investigations of carotid stenosis to identify vulnerable atherosclerotic plaque and determine individual stroke risk. Circ. J. 2017, 81, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Sigovan, M.; Bidet, C.; Bros, S.; Boussel, L.; Mechtouff, L.; Robson, P.M.; Fayad, Z.A.; Millon, A.; Douek, P. 3D black blood MR angiography of the carotid arteries. A simple sequence for plaque hemorrhage and stenosis evaluation. Magn. Reson. Imaging 2017, 42, 95–100. [Google Scholar] [CrossRef] [PubMed]

| Groups | Overall | IPH Group | Non-IPH Group | |
|---|---|---|---|---|
| n = 68 | n = 30 | n = 38 | ||
| CAS Procedure | GE | Standard | p-Value | |
| Plaque rSI on T1WBB MRI, | 1.32 (1.11, 1.74) | 1.84 (1.6, 2.0) | 1.1 (1.0, 1.2) | <0.001 |
| Age, years | 77 (72, 81) | 78 (71, 81) | 77 (73, 80.5) | 0.92 |
| Male (sex), n (%) | 59 (86.8%) | 28 (90.3%) | 38 (77.6%) | 0.13 |
| BMI on admission, kg/m2 | 23.2 (21.0, 24.6) | 23.4 (20.7, 24.4) | 23.1 (21.0, 25.1) | 0.58 |
| TCHO on admission, mmol/L | 4.3 (3.7, 4.7) | 4.2 (3.7, 4.6) | 4.3 (3.7, 5.0) | 0.99 |
| LDL on admission, mmol/L | 2.2 (1.8, 2.5) | 2.1 (1.6, 2.6) | 2.2 (2.0, 2.4) | 0.44 |
| HDL on admission, mmol/L | 1.3 (1.1, 1.7) | 1.5 (1.2, 1.8) | 1.2 (1.1, 1.6) | 0.06 |
| TG on admission, mmol/L | 1.3 (0.9, 1.9) | 1.3 (0.8, 1.6) | 1.3 (0.9, 1.9) | 0.45 |
| Glucose on admission, mmol/L | 6.2 (5.6, 7.4) | 6.7 (5.7, 8.1) | 6.0 (5.5, 7.2) | 0.20 |
| HbA1c on admission, % | 5.9 (5.7, 6.3) | 5.8 (5.7, 6.3) | 5.9 (5.7, 6.3) | 0.52 |
| Symptomatic, n (%) | 35 (51.5%) | 19 (63.3%) | 16 (42.1%) | 0.08 |
| Statin users, n (%) | 36 (52.9%) | 17 (56.7%) | 19 (50.0%) | 0.58 |
| History of hypertension, n (%) | 62 (91%) | 28 (93.3%) | 35 (92.1%) | 0.84 |
| History of diabetes mellitus, n (%) | 33 (49%) | 16 (53.3%) | 17 (44.7%) | 0.48 |
| Number of hospital days, days | 4 (4, 5) | 5 (4, 5) | 4 (4, 5) | 0.23 |
| Groups | Overall | IPH Group | Non-IPH Group | |
|---|---|---|---|---|
| n = 68 | n = 30 | n = 38 | ||
| CAS Procedure | GE | Standard | p-Value | |
| MCA slow flow before CAS, n (%) | 35 (51.5%) | 15 (50.0%) | 20 (52.6%) | 0.83 |
| MCA rSI < 0.9 | 39 (56.3%) | 16 (53.3%) | 23 (60.5%) | 0.55 |
| MLD < 1.0 mm | 41 (60.3%) | 19 (63.3%) | 22 (57.9%) | 0.65 |
| CASr ≥ 80% | 39 (57.3%) | 17 (56.7%) | 22 (57.9%) | 0.92 |
| ex-CS | 21 (30.8%) | 10 (33.3%) | 11 (28.9%) | 0.70 |
| MCA rSI before CAS | 0.87 (0.74, 0.98) | 0.88 (0.74, 0.98) | 0.87 (0.74, 0.96) | 0.54 |
| MCA rSI after CAS | 1.01 (0.92, 1.06) | 1.02 (0.97, 1.06) | 0.98 (0.89, 1.07) | 0.33 |
| PSV before CAS, cm/s | 281 (210, 348) | 271 (215, 337) | 293 (178, 361) | 0.69 |
| PSV after CAS, cm/s | 83 (67.2, 113.8) | 97.6 (70.0, 147) | 80.1 (64.9, 91.2) | 0.02 |
| CASr before CAS, % | 80.9 (76.9, 86.7) | 81.2 (77.4, 88.1) | 80.7 (76.5, 86.0) | 0.68 |
| CASr after CAS, % | 29.2 (18.6, 38.8) | 37.5 (26.6, 44.6) | 26.4 (15.9, 34.2) | 0.001 |
| CASr at four months, % | 26.0 (16.5, 33.5) | 27.7 (18.5, 40.7) | 24.3 (14.2, 32.2) | 0.28 |
| Change of CASr over four months, % | −11.6 (−18.0, −8.46) | −12.6 (−20.2, −9.0) | −10.9 (−17.1, −6.3) | 0.14 |
| MLD before CAS, mm | 0.85 (0.54, 1.09) | 0.76 (0.54, 1.05) | 0.90 (0.53, 1.14) | 0.74 |
| MSD after CAS, mm | 3.42 (2.82, 3.73) | 2.97 (2.62, 3.54) | 3.58 (3.13, 3.86) | 0.003 |
| MSD at four months, mm | 3.97 (3.46, 4.47) | 3.89 (3.22, 4.75) | 4.04 (3.57, 4.33) | 0.61 |
| Change in MSD over four months, mm | 0.76 (0.46, 1.02) | 0.90 (0.62, 1.08) | 0.58 (0.31, 0.94) | 0.03 |
| Groups | IPH Group | Non-IPH | |
|---|---|---|---|
| n = 30 | n = 38 | ||
| CAS Procedure | GE | Standard | p-Value |
| New ischemic lesions on DWI (binary analysis), n (%) | 18 (60.0%) | 26 (68.4%) | 0.47 |
| Number of new lesions on DWI per patient (count analysis), median (IQR) | 1 (0, 3) | 1 (0, 3) | 0.40 |
| Transient ischemic attack, n | 0 | 0 | 1 |
| Symptomatic cerebral infarction after CAS, n | 0 | 0 | 1 |
| Triad of CHS after CAS, n | 0 | 0 | 1 |
| Intracranial hemorrhage after CAS, n | 0 | 0 | 1 |
| Transient delirium, n (%) | 1 (3.3%) | 0 | 0.44 |
| Hypotension after CAS, n (%) | 0 | 2 (5.3%) | 0.50 |
| Pseudoaneurysm in the brachial artery, n (%) | 0 | 2 (5.3%) | 0.50 |
| Stroke, MI, or death within 30 days after CAS, n (%) | 0 | 1 (2.6%) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, T.; Yoshioka, K.; Tanno, Y.; Kasakura, S. Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study. Life 2022, 12, 131. https://doi.org/10.3390/life12010131
Mori T, Yoshioka K, Tanno Y, Kasakura S. Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study. Life. 2022; 12(1):131. https://doi.org/10.3390/life12010131
Chicago/Turabian StyleMori, Takahisa, Kazuhiro Yoshioka, Yuhei Tanno, and Shigen Kasakura. 2022. "Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study" Life 12, no. 1: 131. https://doi.org/10.3390/life12010131
APA StyleMori, T., Yoshioka, K., Tanno, Y., & Kasakura, S. (2022). Gradual Expansion of a Stent to Prevent Periprocedural Complications after Carotid Artery Stenting for Vulnerable Severe Stenotic Lesions with Intraplaque Hemorrhages: A Retrospective Observational Study. Life, 12(1), 131. https://doi.org/10.3390/life12010131






