Proteomic Response of Deinococcus radiodurans to Short-Term Real Microgravity during Parabolic Flight Reveals Altered Abundance of Proteins Involved in Stress Response and Cell Envelope Functions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Growth
2.2. Parabolic Flight
2.3. Protein Extraction, Purification, and Digestion
2.4. HPLC nESI MS/MS
2.5. Quality Control
2.6. Imputation and Statistical Analysis
2.7. Functional Annotation and Gene Ontology Enrichment Analysis
2.8. KEGG Pathways
3. Results
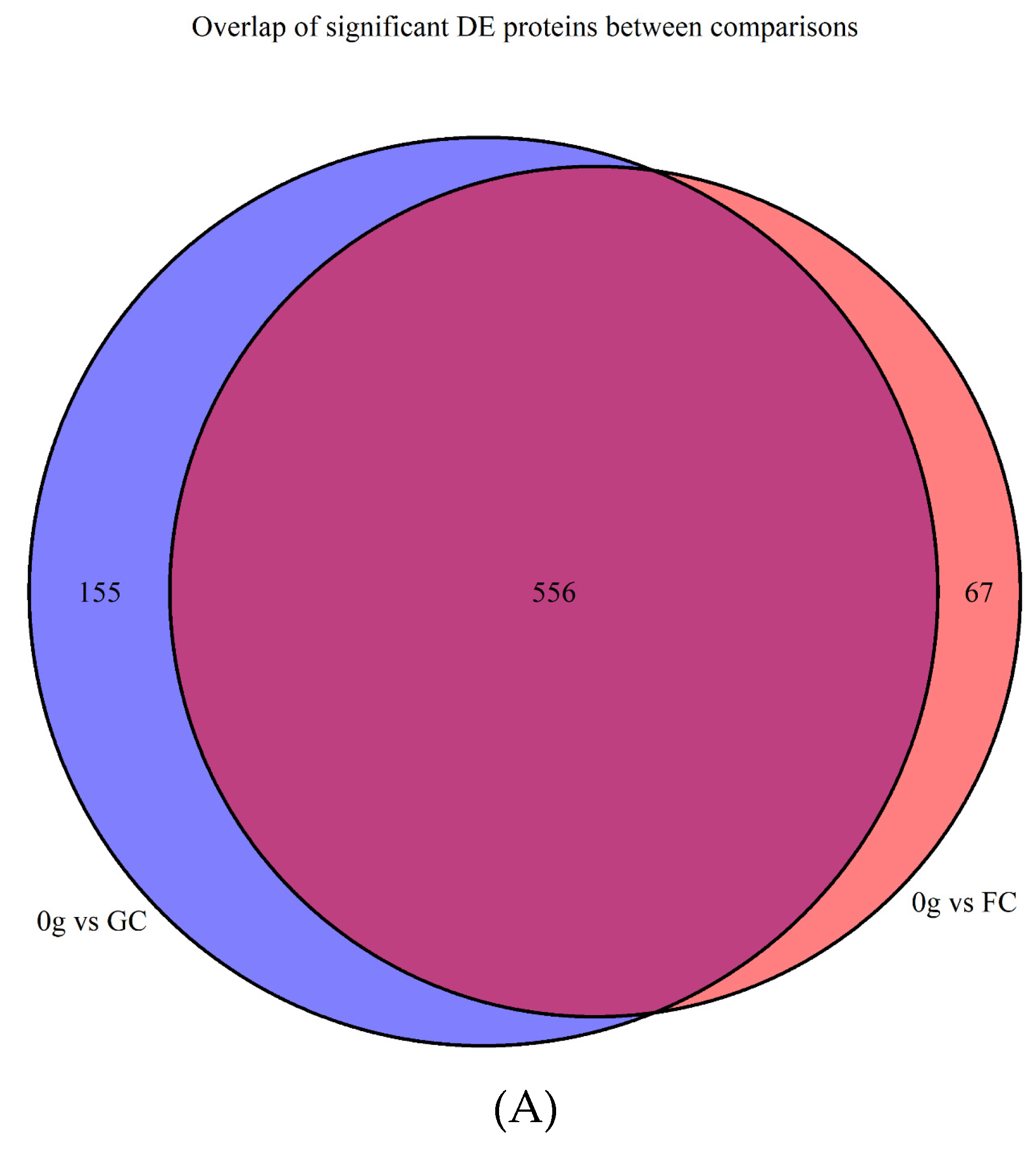
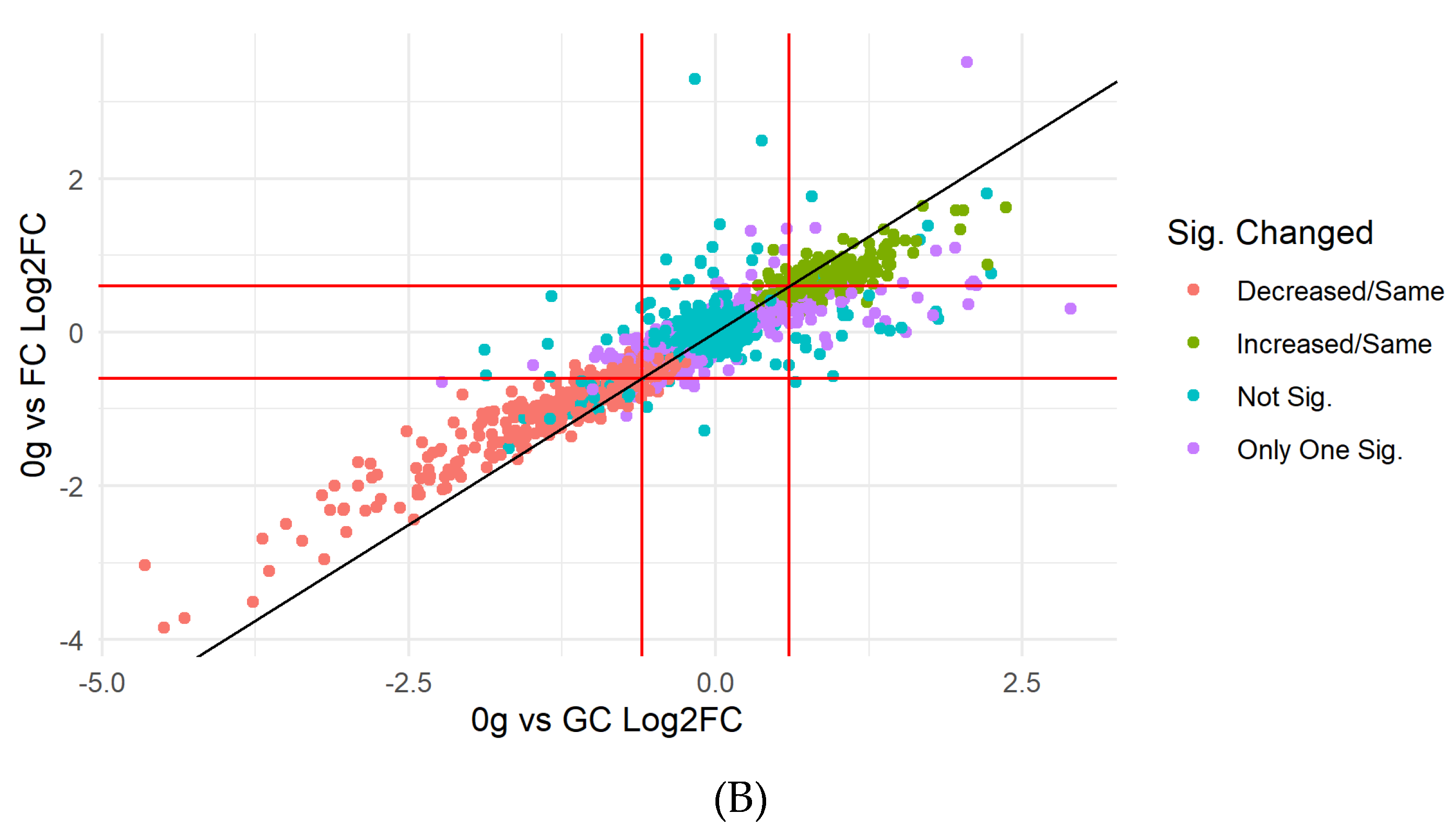
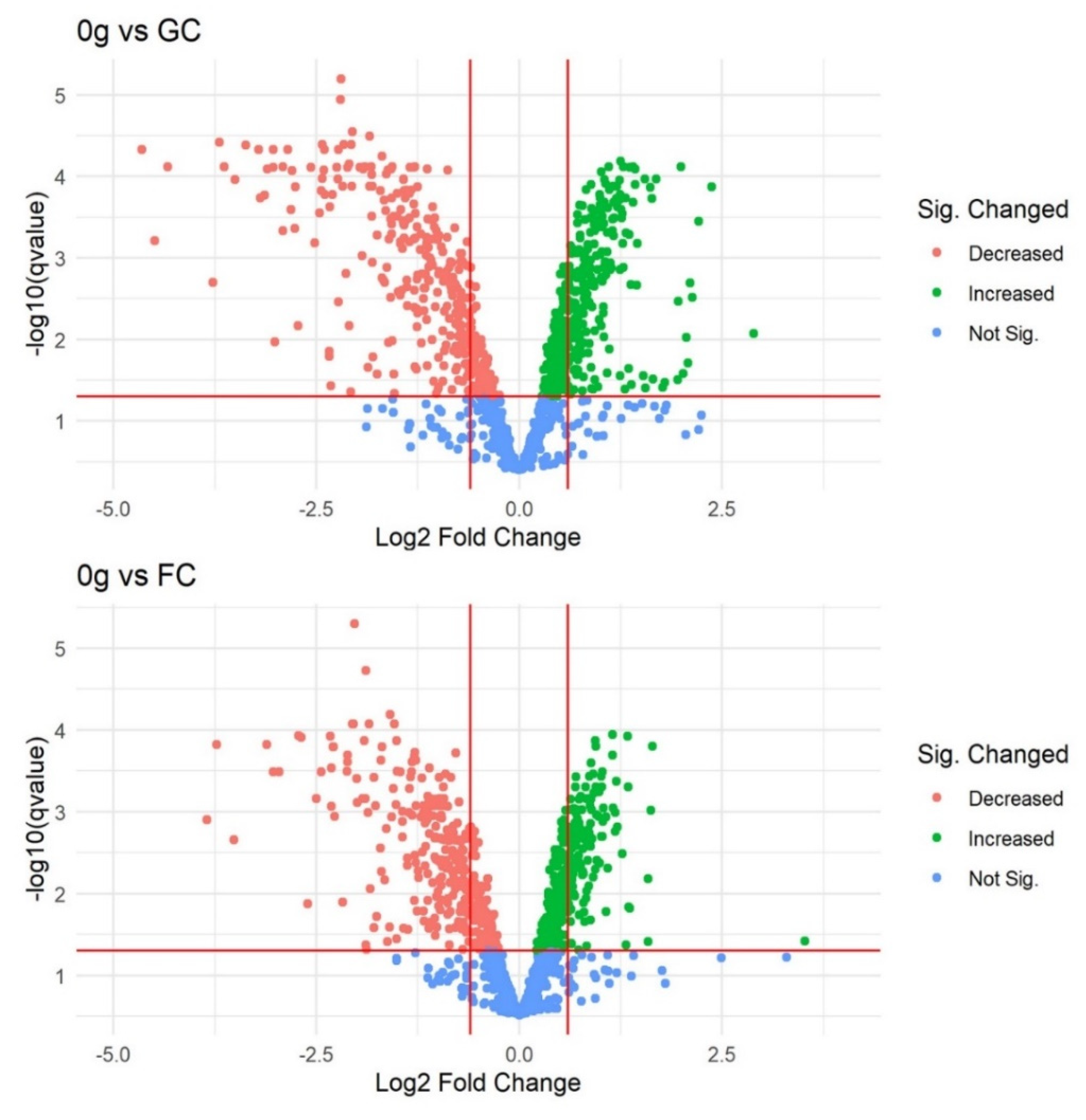
3.1. Filtering, Quality Control, and Imputation
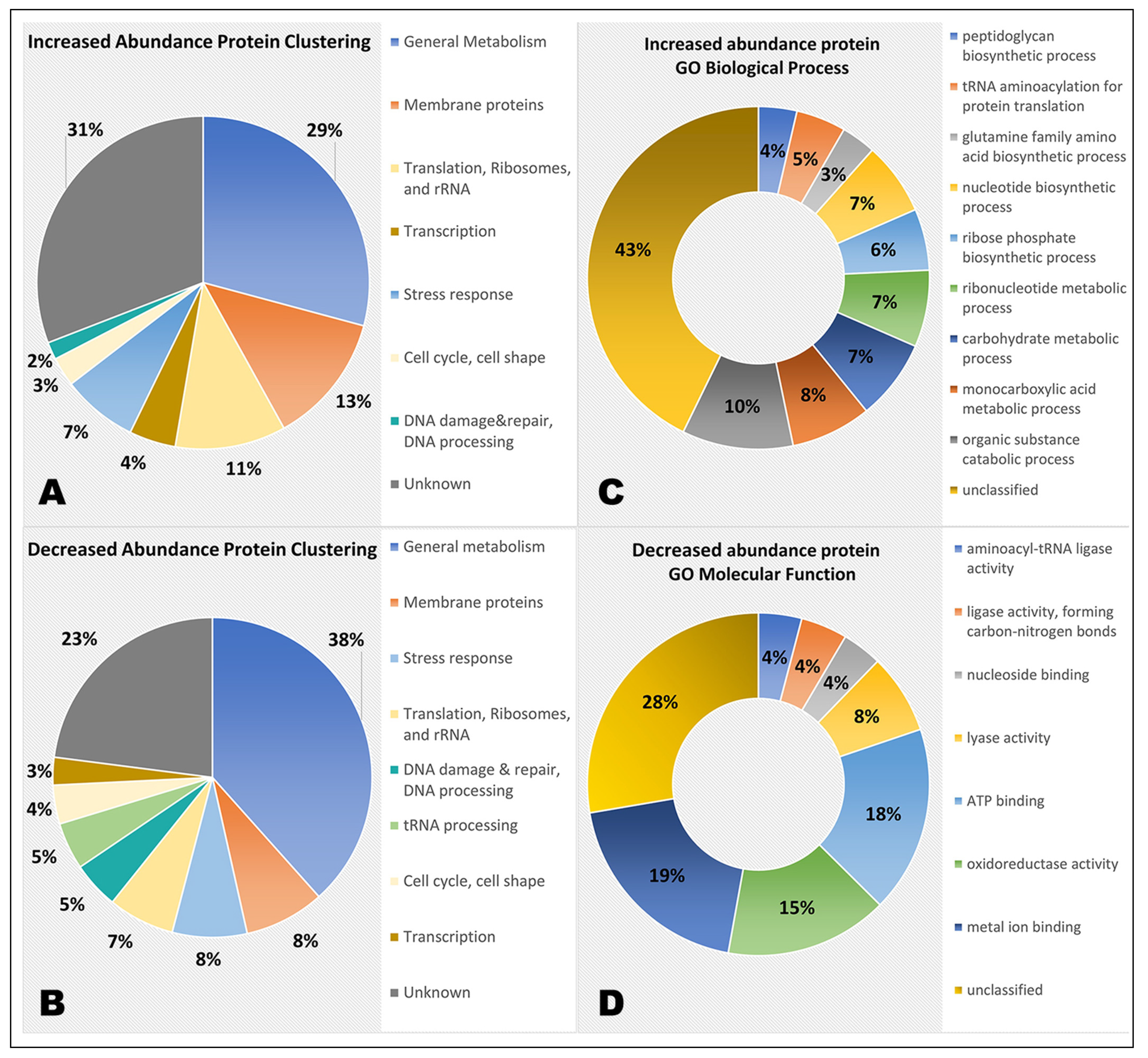
Differentially Abundant Protein Grouping and GO Enrichment
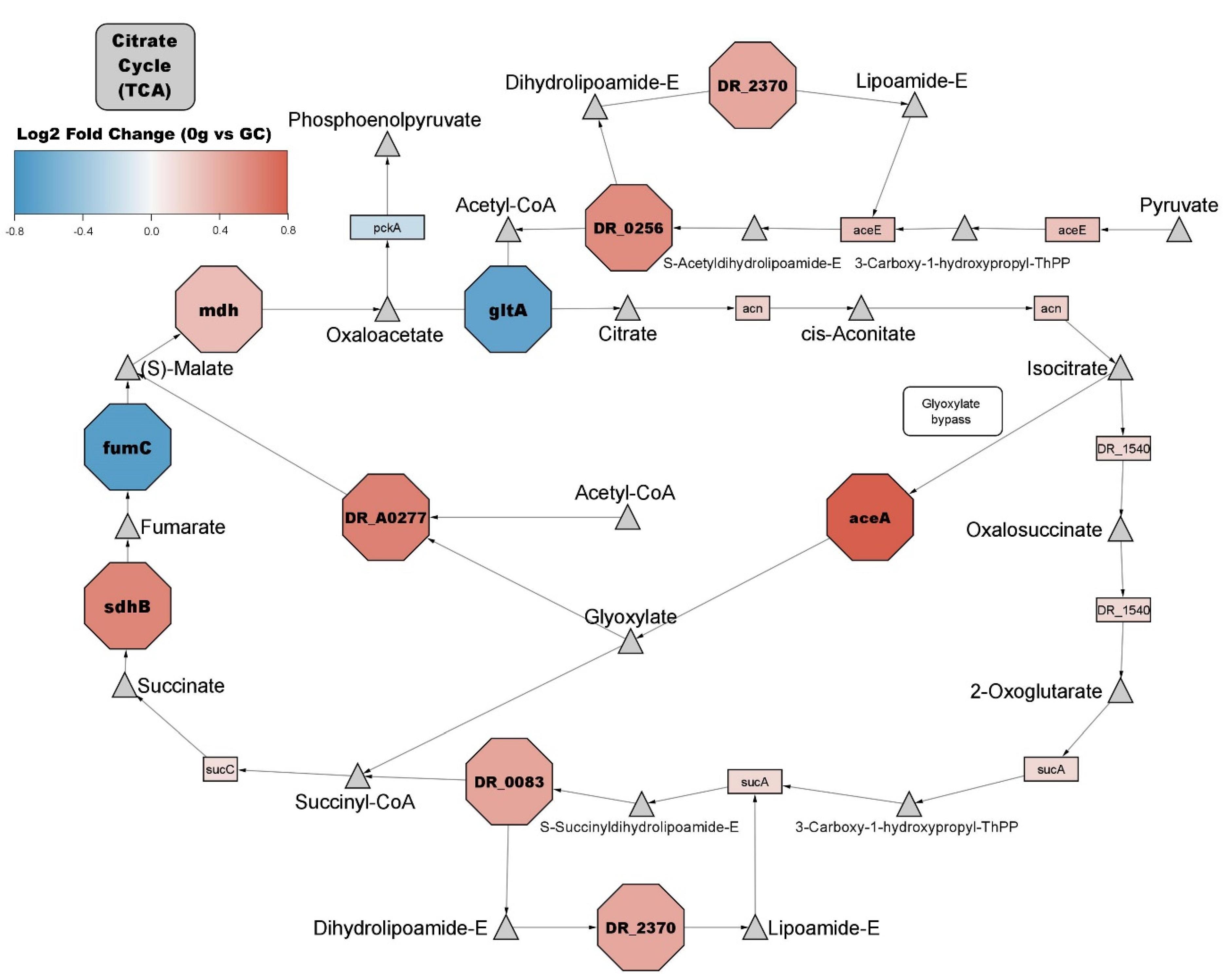
3.2. General Metabolism
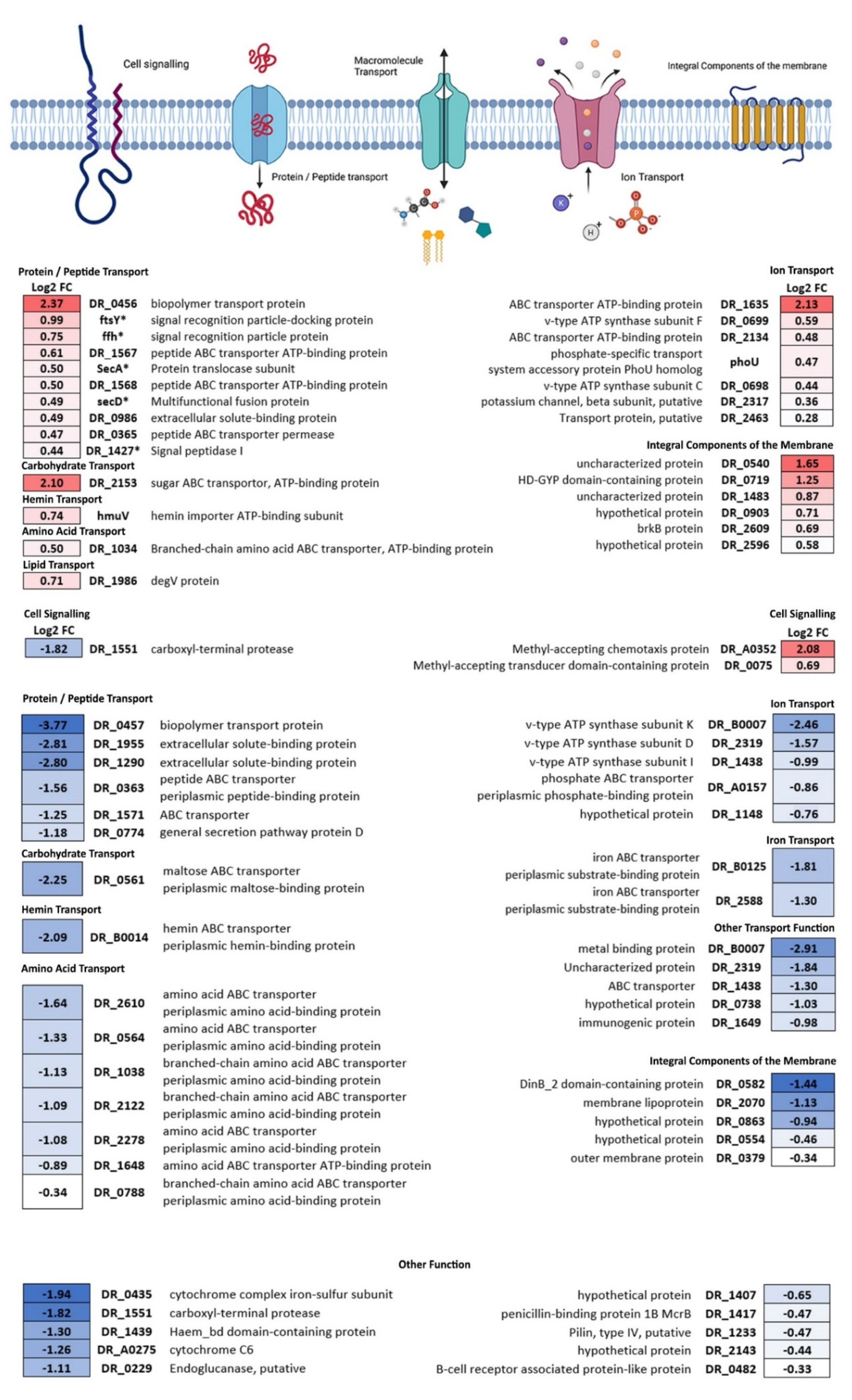
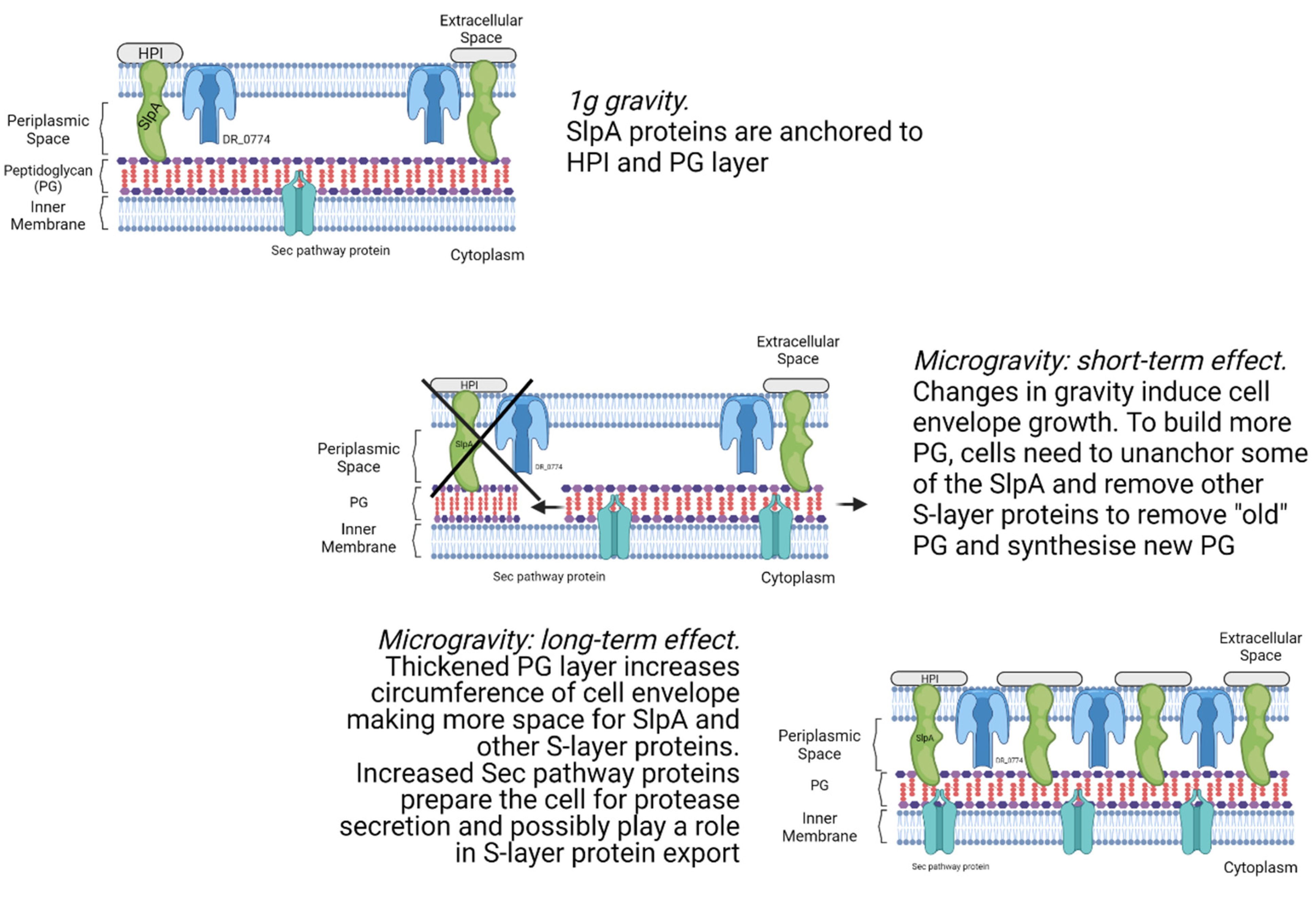
3.3. Membrane Proteins
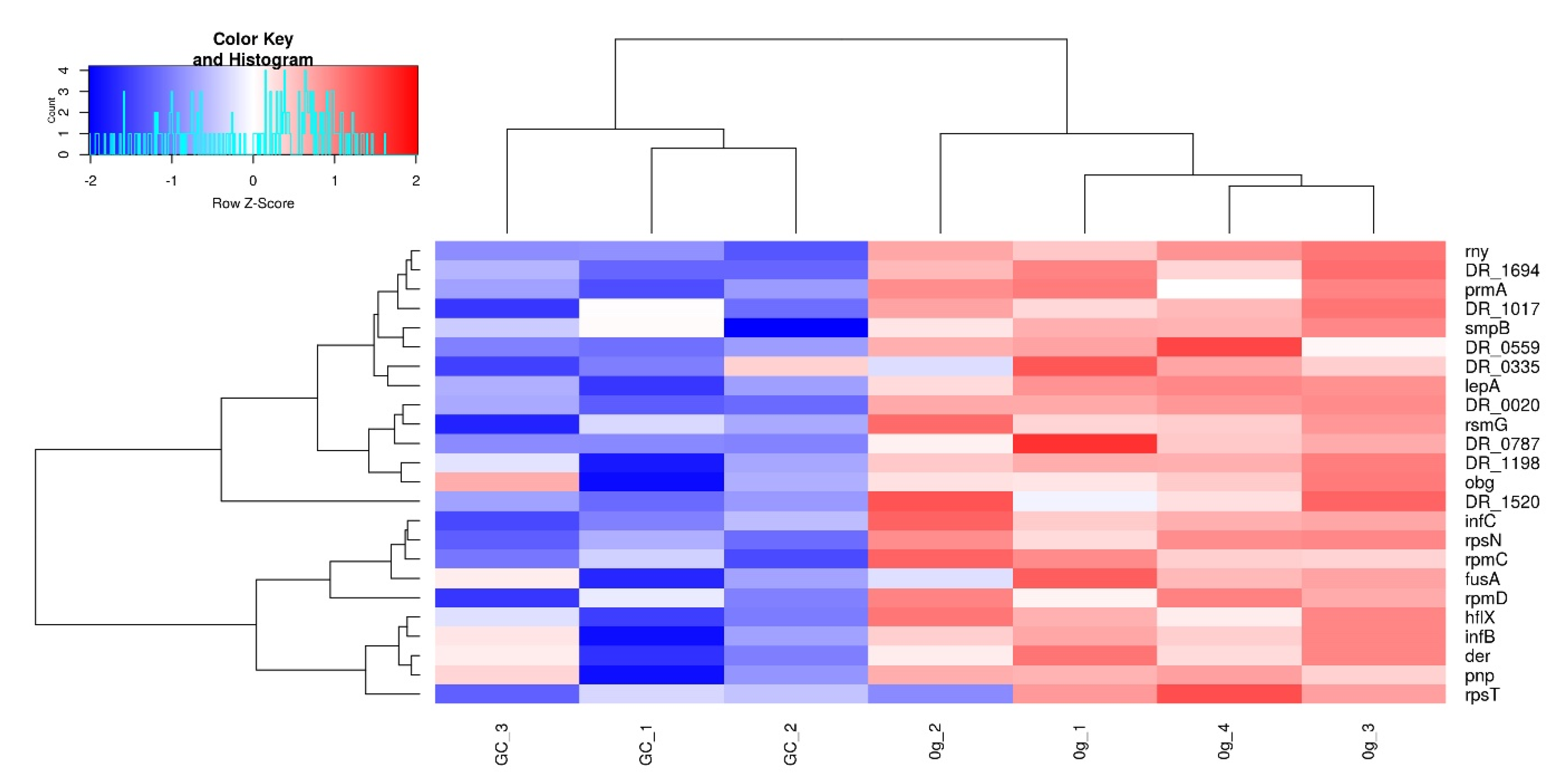
3.4. Translation, Ribosomes, and rRNA
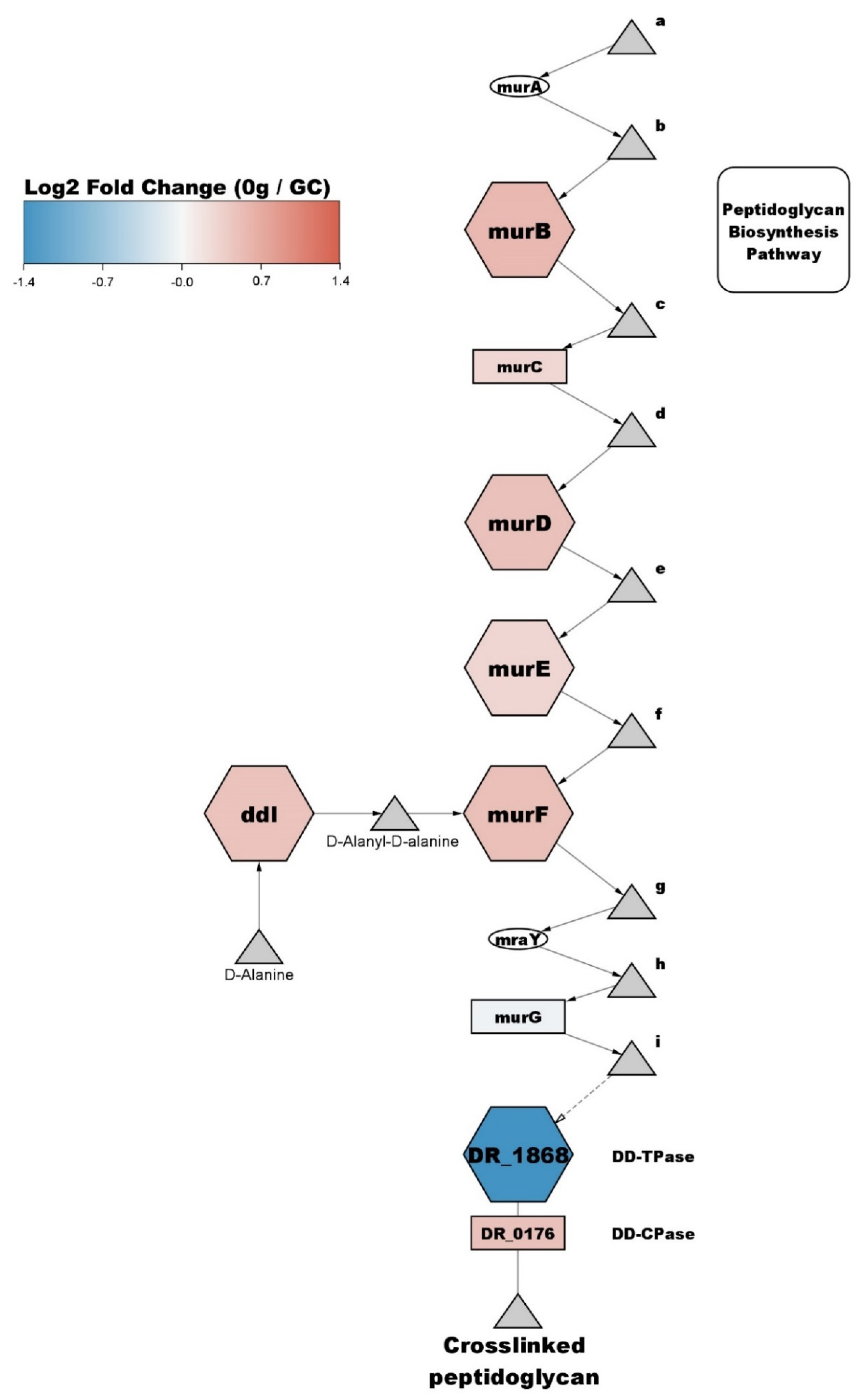
3.5. Cell Cycle and Cell Shape
3.6. tRNA-related Proteins
3.7. DNA Damage and Repair, DNA Processing
3.8. Transcription
3.9. Stress Response
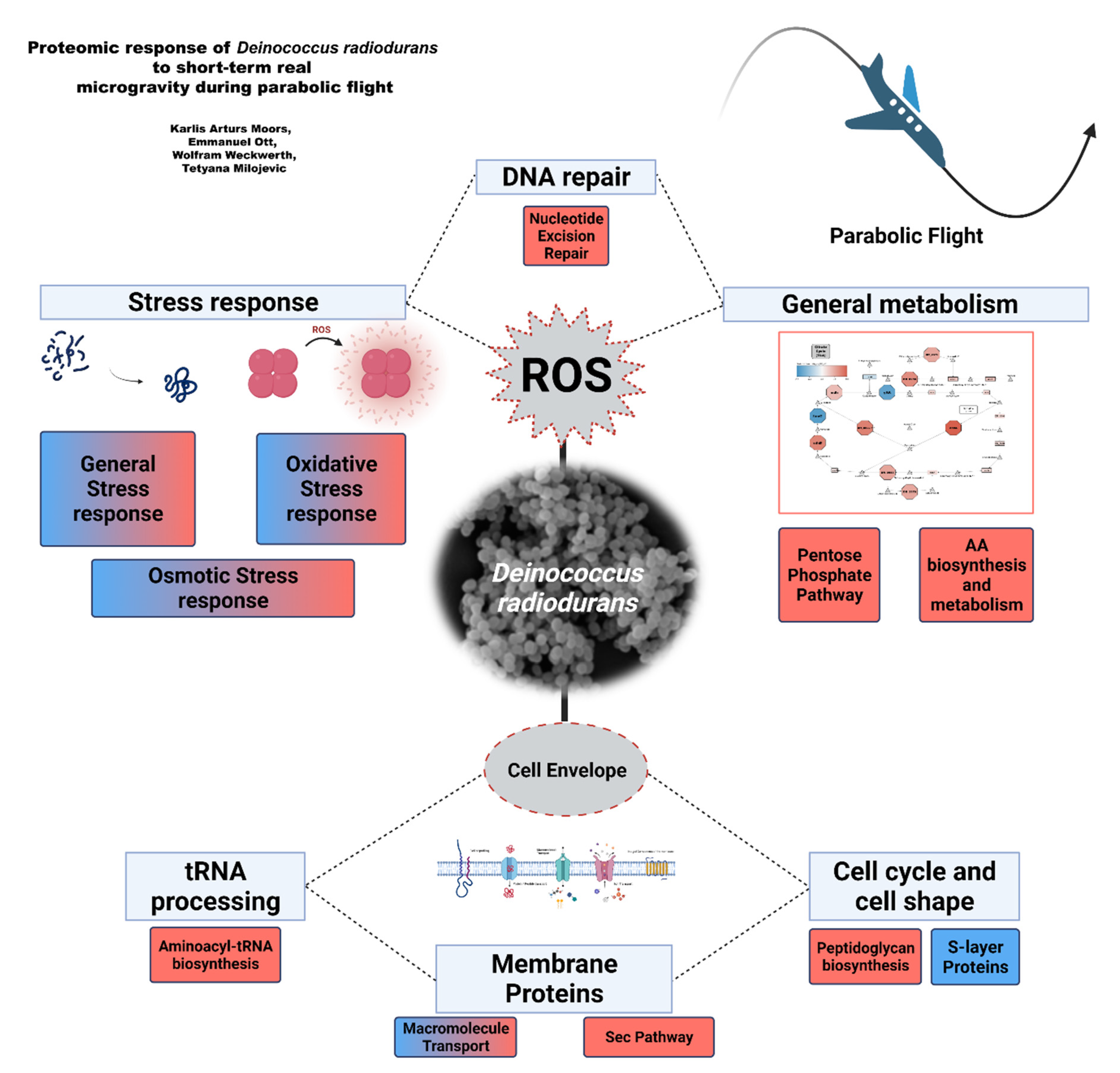
4. Discussion
4.1. Changes in General Metabolism Reflect Previously Reported Stress Response
4.2. Cell Envelope Processes Are Affected by Microgravity
4.3. Does Microgravity Induce DNA Repair?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sancho, L.G.; De La Torre, R.; Horneck, G.; Ascaso, C.; de los Rios, A.; Pintado, A.; Wierzchos, J.; Schuster, M. Lichens Survive in Space: Results from the 2005 LICHENS Experiment. Astrobiology 2007, 7, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Ott, C.M.; Zu Bentrup, K.H.; Ramamurthy, R.; Quick, L.; Porwollik, S.; Cheng, P.; McClelland, M.; Tsaprailis, G.; Radabaugh, T.; et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc. Natl. Acad. Sci. USA 2007, 104, 16299–16304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastroleo, F.; Van Houdt, R.; Leroy, B.; Benotmane, R.; Janssen, A.; Mergeay, M.; Vanhavere, F.; Hendrickx, L.; Wattiez, R.; Leys, N. Experimental design and environmental parameters affect Rhodospirillum rubrum S1H response to space flight. ISME J. 2009, 3, 1402–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaishampayan, P.A.; Rabbow, E.; Horneck, G.; Venkateswaran, K.J. Survival ofBacillus pumilusSpores for a Prolonged Period of Time in Real Space Conditions. Astrobiology 2012, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, M.; Moeller, R.; Rabbow, E.; Panitz, C.; Horneck, G.; Reitz, G.; Douki, T.; Cadet, J.; Stan-Lotter, H.; Cockell, C.S.; et al. Survival of Spores of the UV-ResistantBacillus subtilisStrain MW01 After Exposure to Low-Earth Orbit and Simulated Martian Conditions: Data from the Space Experiment ADAPT on EXPOSE-E. Astrobiology 2012, 12, 498–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jules, K.; McPherson, K.; Hrovat, K.; Kelly, E.; Reckart, T. A status report on the characterization of the microgravity environment of the International Space Station. Acta Astronaut. 2004, 55, 335–364. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-Based Facilities for Simulation of Microgravity: Organism-Specific Recommendations for Their Use, and Recommended Terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Beysens, D.A.; van Loon, J.J. Generation and Applications of Extra-Terrestrial Environments on Earth; River Publishers: Gistrup, Nordjylland, Denmark, 2015. [Google Scholar] [CrossRef] [Green Version]
- Hemmersbach, R.; Strauch, S.; Seibt, D.; Schuber, M. Comparative studies on gravisensitive protists on ground (2D and 3D clinostats) and in microgravity. Microgravity-Sci. Technol. 2006, 18, 257–259. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated Microgravity: Critical Review on the Use of Random Positioning Machines for Mammalian Cell Culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [Green Version]
- Valles, J., Jr.; Maris, H.; Seidel, G.; Tang, J.; Yao, W. Magnetic levitation-based Martian and Lunar gravity simulator. Adv. Space Res. 2005, 36, 114–118. [Google Scholar] [CrossRef]
- Grimm, D.; Pietsch, J.; Wehland, M.; Richter, P.; Strauch, S.; Lebert, M.; Magnusson, N.E.; Wise, P.; Bauer, J. The impact of microgravity-based proteomics research. Expert Rev. Proteom. 2014, 11, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, N.; Fengler, S.; Hennig, A.; Franz-Wachtel, M.; Hampp, R.; Neef, M. Cytosolic calcium, hydrogen peroxide and related gene expression and protein modulation in A rabidopsis thaliana cell cultures respond immediately to altered gravitation: Parabolic flight data. Plant Biol. 2013, 16, 120–128. [Google Scholar] [CrossRef]
- Barjaktarović, Ž.; Nordheim, A.; Lamkemeyer, T.; Fladerer, C.; Madlung, J.; Hampp, R. Time-course of changes in amounts of specific proteins upon exposure to hyper-g, 2-D clinorotation, and 3-D random positioning of Arabidopsis cell cultures. J. Exp. Bot. 2007, 58, 4357–4363. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zheng, H.Q.; Sha, W.; Zeng, R.; Xia, Q.C. A proteomic approach to analysing responses of Arabidopsis thaliana callus cells to clinostat rotation. J. Exp. Bot. 2006, 57, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, L.; Xie, J.; Zheng, H. Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft. Planta 2014, 241, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Richter, P.; Stoltze, J.; Strauch, S.M.; Krüger, M.; Daiker, V.; Prasad, B.; Sonnewald, S.; Reid, S.; Lebert, M. Changes of Gene Expression in Euglena gracilis Obtained During the 29th DLR Parabolic Flight Campaign. Sci. Rep. 2019, 9, 14260. [Google Scholar] [CrossRef] [Green Version]
- Chopra, V.; Fadl, A.A.; Sha, J.; Chopra, S.; Galindo, C.L.; Chopra, A.K. Alterations in the Virulence Potential of Enteric Pathogens and Bacterial–Host Cell Interactions Under Simulated Microgravity Conditions. J. Toxicol. Environ. Health Part A 2006, 69, 1345–1370. [Google Scholar] [CrossRef]
- Lawal, A.; Jejelowo, O.A.; Rosenzweig, J.A. The Effects of Low-Shear Mechanical Stress on Yersinia pestis Virulence. Astrobiology 2010, 10, 881–888. [Google Scholar] [CrossRef]
- Su, L.; Zhou, L.; Liu, J.; Cen, Z.; Wu, C.; Wang, T.; Zhou, T.; Chang, D.; Guo, Y.; Fang, X.; et al. Phenotypic, genomic, transcriptomic and proteomic changes in Bacillus cereus after a short-term space flight. Adv. Space Res. 2014, 53, 18–29. [Google Scholar] [CrossRef]
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—the consummate survivor. Nat. Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative Stress Resistance inDeinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishino, Y.; Narumi, I. DNA repair in hyperthermophilic and hyperradioresistant microorganisms. Curr. Opin. Microbiol. 2015, 25, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Shibuya, M.; Kinoshita, I.; Yatabe, J.; Narumi, I.; Shibata, H.; Hayashi, R.; Fujiwara, D.; Murano, Y.; Hashimoto, H.; et al. DNA Damage and Survival Time Course of Deinococcal Cell Pellets During 3 Years of Exposure to Outer Space. Front. Microbiol. 2020, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Ott, E.; Kawaguchi, Y.; Kölbl, D.; Rabbow, E.; Rettberg, P.; Mora, M.; Moissl-Eichinger, C.; Weckwerth, W.; Yamagishi, A.; Milojevic, T. Molecular repertoire of Deinococcus radiodurans after 1 year of exposure outside the International Space Station within the Tanpopo mission. Microbiome 2020, 8, 150. [Google Scholar] [CrossRef]
- Ott, E.; Fuchs, F.M.; Moeller, R.; Hemmersbach, R.; Kawaguchi, Y.; Yamagishi, A.; Weckwerth, W.; Milojevic, T. Molecular response of Deinococcus radiodurans to simulated microgravity explored by proteometabolomic approach. Sci. Rep. 2019, 9, 18462. [Google Scholar] [CrossRef]
- Crabbé, A.; Schurr, M.J.; Monsieurs, P.; Morici, L.; Schurr, J.; Wilson, J.W.; Ott, C.M.; Tsaprailis, G.; Pierson, D.L.; Stefanyshyn-Piper, H.; et al. Transcriptional and Proteomic Responses of Pseudomonas aeruginosa PAO1 to Spaceflight Conditions Involve Hfq Regulation and Reveal a Role for Oxygen. Appl. Environ. Microbiol. 2011, 77, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.J.; Losic, M. Temperature Lapse Rates in Glacierized Basins. In Encyclopedia of Snow, Ice and Glaciers; Springer: Dordrecht, The Netherlands, 2011; pp. 1145–1150. [Google Scholar]
- Ott, E.; Kawaguchi, Y.; Özgen, N.; Yamagishi, A.; Rabbow, E.; Rettberg, P.; Weckwerth, W.; Milojevic, T. Proteomic and Metabolomic Profiling of Deinococcus radiodurans Recovering After Exposure to Simulated Low Earth Orbit Vacuum Conditions. Front. Microbiol. 2019, 10, 909. [Google Scholar] [CrossRef] [Green Version]
- Ott, E.; Kawaguchi, Y.; Kölbl, D.; Chaturvedi, P.; Nakagawa, K.; Yamagishi, A.; Weckwerth, W.; Milojevic, T. Proteometabolomic response of Deinococcus radiodurans exposed to UVC and vacuum conditions: Initial studies prior to the Tanpopo space mission. PLoS ONE 2017, 12, e0189381. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio Inc.(2015). Acknowledgements This work was supported by Fundação para a Ciência e Tecnologia (FCT), Azores 2020. Available online: https://www.rstudio.com (accessed on 19 December 2021).
- Zhang, X.; Smits, A.H.; van Tilburg, G.B.; Ovaa, H.; Huber, W.; Vermeulen, M. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 2018, 13, 530. [Google Scholar] [CrossRef]
- Välikangas, T.; Suomi, T.; Elo, L.L. A systematic evaluation of normalization methods in quantitative label-free proteomics. Briefings Bioinform. 2016, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.I.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Schwämmle, V.; León, I.R.; Jensen, O.N. Assessment and Improvement of Statistical Tools for Comparative Proteomics Analysis of Sparse Data Sets with Few Experimental Replicates. J. Proteome Res. 2013, 12, 3874–3883. [Google Scholar] [CrossRef] [PubMed]
- Dowell, J.A.; Wright, L.J.; Armstrong, E.A.; Denu, J.M. Benchmarking Quantitative Performance in Label-Free Proteomics. ACS Omega 2021, 6, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44. [Google Scholar]
- UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [CrossRef]
- Gene Ontology Consortium. The Gene Ontology Resource. Available online: http://geneontology.org/ (accessed on 19 December 2021).
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M. STRING 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009, 37, D412–D416. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Genomes, K.E.o.G.a. KEGG PATHWAY: Dra02024. Available online: https://www.kegg.jp/entry/dra02024 (accessed on 27 May 2021).
- Milojevic, T.; Weckwerth, W. Molecular Mechanisms of Microbial Survivability in Outer Space: A Systems Biology Approach. Front. Microbiol. 2020, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- He, Y. High cell density production of Deinococcus radiodurans under optimized conditions. J. Ind. Microbiol. Biotechnol. 2009, 36, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Albrecht-Buehler, G. Possible mechanisms of indirect gravity sensing by cells. ASGSB Bull. 1991, 4, 25–34. [Google Scholar]
- Klaus, D.M.; Benoit, M.R.; Nelson, E.S.; Hammond, T.G. Extracellular mass transport considerations for space flight research concerning suspended and adherent in vitro cell cultures. J. Gravit. Physiol. J. Int. Soc. Gravit. Physiol. 2004, 11, 17–27. [Google Scholar]
- Zea, L.; Prasad, N.; Levy, S.; Stodieck, L.; Jones, A.; Shrestha, S.; Klaus, D. A Molecular Genetic Basis Explaining Altered Bacterial Behavior in Space. PLoS ONE 2016, 11, e0164359. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Omelchenko, M.V.; Beliaev, A.S.; Venkateswaran, A.; Stair, J.; Wu, L.; Thompson, D.K.; Xu, D.; Rogozin, I.; et al. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc. Natl. Acad. Sci. USA 2003, 100, 4191–4196. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, D.; Omelchenko, M.; Gaidamakova, E.; Matrosova, V.; Vasilenko, A.; Venkateswaran, A.; Zhai, M.; Kostandarithes, H.; Brim, H.; Makarova, K. How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol. Rev. 2005, 29, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Frees, D.; Gerth, U.; Ingmer, H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int. J. Med Microbiol. 2014, 304, 142–149. [Google Scholar] [CrossRef]
- Lazzaroni, J.C.; Germon, P.; Ray, M.-C.; Vianney, A. The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiol. Lett. 1999, 177, 191–197. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Wong, T.-Y.; Chen, L.-Y.; Lin, C.-S.; Liu, J.-K. Induction of a Futile Embden-Meyerhof-Parnas Pathway in Deinococcus radiodurans by Mn: Possible Role of the Pentose Phosphate Pathway in Cell Survival. Appl. Environ. Microbiol. 2000, 66, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Liu, J.-K.; Wong, T.-Y. The DNA excision repair system of the highly radioresistant bacterium Deinococcus radiodurans is facilitated by the pentose phosphate pathway. Mol. Microbiol. 2003, 48, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Maloy, S.R.; Bohlander, M.; Nunn, W.D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J. Bacteriol. 1980, 143, 720–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, M.; Fink, G.R. The glyoxylate cycle is required for fungal virulence. Nature 2001, 412, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Jung, J.; Jang, I.-A.; Madsen, E.L.; Park, W. Role of Glyoxylate Shunt in Oxidative Stress Response. J. Biol. Chem. 2016, 291, 11928–11938. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Thornley, M.J.; Horne, R.W.; Glauert, A.M. The fine structure of micrococcus radiodurans. Arch. Microbiol. 1965, 51, 267–289. [Google Scholar] [CrossRef]
- Thompson, B.G.; Murray, R.G.E. Isolation and characterization of the plasma membrane and the outer membrane of Deinococcus radiodurans strain Sark. Can. J. Microbiol. 1981, 27, 729–734. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the Extremely Radiation-Resistant Bacterium Deinococcus radiodurans Viewed from the Perspective of Comparative Genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79. [Google Scholar] [CrossRef] [Green Version]
- Pavkov-Keller, T.; Howorka, S.; Keller, W. The structure of bacterial S-layer proteins. Prog. Mol. Biol. Transl. Sci. 2011, 103, 73–130. [Google Scholar]
- Rothfuss, H.; Lara, J.C.; Schmid, A.K.; Lidstrom, M.E. Involvement of the S-layer proteins Hpi and SlpA in the maintenance of cell envelope integrity in Deinococcus radiodurans R1. Microbiology 2006, 152, 2779–2787. [Google Scholar] [CrossRef] [Green Version]
- Misra, C.S.; Basu, B.; Apte, S.K. Surface (S)-layer proteins of Deinococcus radiodurans and their utility as vehicles for surface localization of functional proteins. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 3181–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farci, D.; Bowler, M.W.; Kirkpatrick, J.; McSweeney, S.; Tramontano, E.; Piano, D. New features of the cell wall of the radio-resistant bacterium Deinococcus radiodurans. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Farci, D.; Slavov, C.; Tramontano, E.; Piano, D. The S-layer Protein DR_2577 Binds Deinoxanthin and under Desiccation Conditions Protects against UV-Radiation in Deinococcus radiodurans. Front. Microbiol. 2016, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Farci, D.; Aksoyoglu, M.A.; Farci, S.F.; Bafna, J.A.; Bodrenko, I.; Ceccarelli, M.; Kirkpatrick, J.; Winterhalter, M.; Kereïche, S.; Piano, D. Structural insights into the main S-layer unit of Deinococcus radiodurans reveal a massive protein complex with porin-like features. J. Biol. Chem. 2020, 295, 4224–4236. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.Y.; Cho, C.S.; Kim, S.S.; Sung, H.C.; Yu, Y.G.; Cho, Y. Structure and mechanism of glutamate racemase from Aquifex pyrophilus. Nat. Struct. Biol. 1999, 6, 422–426. [Google Scholar] [CrossRef]
- Tsirigotaki, A.; De Geyter, J.; Šoštaric, N.; Economou, A.; Karamanou, S. Protein export through the bacterial Sec pathway. Nat. Rev. Microbiol. 2017, 15, 21–36. [Google Scholar] [CrossRef]
- Dramsi, S.; Magnet, S.; Davison, S.; Arthur, M. Covalent attachment of proteins to peptidoglycan. FEMS Microbiol. Rev. 2008, 32, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Chen, S.; Dare, K.; Gilreath, M.; Praetorius-Ibba, M.; Raina, M.; Reynolds, N.M.; Rogers, T.; Roy, H.; Yadavalli, S.S.; et al. tRNAs: Cellular barcodes for amino acids. FEBS Lett. 2010, 584, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Mogk, A.; Schmidt, R.; Bukau, B. The N-end rule pathway for regulated proteolysis: Prokaryotic and eukaryotic strategies. Trends Cell Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef]
- Raina, M.; Ibba, M. tRNAs as regulators of biological processes. Front. Genet. 2014, 5, 171. [Google Scholar] [CrossRef] [Green Version]
- Dare, K.; Ibba, M. Roles of tRNA in cell wall biosynthesis. Wiley Interdiscip. Rev. RNA 2012, 3, 247–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, J.; Ibba, M. Direction of aminoacylated transfer RNAs into antibiotic synthesis and peptidoglycan-mediated antibiotic resistance. FEBS Lett. 2013, 587, 2895–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrophanov, A.Y.; Groisman, E.A. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008, 22, 2601–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefers, M.M.; Liao, T.L.; Boisvert, N.M.; Roux, D.; Yoder-Himes, D.; Priebe, G. An Oxygen-Sensing Two-Component System in the Burkholderia cepacia Complex Regulates Biofilm, Intracellular Invasion, and Pathogenicity. PLoS Pathog. 2017, 13, e1006116. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, A.I.M.S.; Roujeinikova, A. Methyl-accepting chemotaxis proteins: A core sensing element in prokaryotes and archaea. Cell. Mol. Life Sci. 2017, 74, 3293–3303. [Google Scholar] [CrossRef]
- Hickman, J.W.; Tifrea, D.F.; Harwood, C.S. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. USA 2005, 102, 14422–14427. [Google Scholar] [CrossRef] [Green Version]
- Black, W.P.; Yang, Z. Myxococcus xanthus Chemotaxis Homologs DifD and DifG Negatively Regulate Fibril Polysaccharide Production. J. Bacteriol. 2004, 186, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Harkey, C.W.; Everiss, K.D.; Peterson, K.M. The Vibrio cholerae toxin-coregulated-pilus gene tcpI encodes a homolog of methyl-accepting chemotaxis proteins. Infect. Immun. 1994, 62, 2669–2678. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yan, Y.; Rong, D.; Wang, J.; Wang, H.; Liu, Z.; Wang, J.; Yang, R.; Han, Y. Increased biofilm formation ability in Klebsiella pneumoniae after short-term exposure to a simulated microgravity environment. Microbiologyopen 2016, 5, 793–801. [Google Scholar] [CrossRef]
- Searles, S.C.; Woolley, C.M.; Petersen, R.A.; Hyman, L.E.; Nielsen-Preiss, S.M. Modeled microgravity increases filamentation, biofilm formation, phenotypic switching, and antimicrobial resistance in Candida albicans. Astrobiology 2011, 11, 825–836. [Google Scholar] [CrossRef]
- Kim, W.; Tengra, F.K.; Young, Z.; Shong, J.; Marchand, N.; Chan, H.K.; Pangule, R.C.; Parra, M.; Dordick, J.S.; Plawsky, J.L.; et al. Spaceflight Promotes Biofilm Formation by Pseudomonas aeruginosa. PLoS ONE 2013, 8, e62437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.K.; Rao, T.S. The first recorded incidence of Deinococcus radiodurans R1 biofilm formation and its implications in heavy metals bioremediation. bioRxiv 2017, 234781. [Google Scholar] [CrossRef] [Green Version]
- Typas, A.; Banzhaf, M.; Gross, C.A.; Vollmer, W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Genet. 2011, 10, 123–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.E.; Kukielczak, B.M.; Chignell, C.F.; Sik, B.H.; Hu, D.-N.; Principato, M.A. Simulated microgravity induced damage in human retinal pigment epithelial cells. Mol. Vis. 2006, 12, 633–638. [Google Scholar]
- Rai, B.; Kaur, J.; Catalina, M.; Anand, S.; Jacobs, R.; Teughels, W. Effect of Simulated Microgravity on Salivary and Serum Oxidants, Antioxidants, and Periodontal Status. J. Periodontol. 2011, 82, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Kang, T.-H. DNA oxidation and excision repair pathways. Int. J. Mol. Sci. 2019, 20, 6092. [Google Scholar] [CrossRef] [Green Version]
- Zahradka, K.; Slade, D.; Bailone, A.; Sommer, S.; Averbeck, D.; Petranovic, M.; Lindner, A.; Radman, M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 2006, 443, 569–573. [Google Scholar] [CrossRef]
- Li, M.; Sun, H.; Feng, Q.; Lu, H.; Zhao, Y.; Zhang, H.; Xu, X.; Jiao, J.; Wang, L.; Hua, Y. Extracellular dGMP Enhances Deinococcus radiodurans Tolerance to Oxidative Stress. PLoS ONE 2013, 8, e54420. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Kiang, J.G.; Fukumoto, R.; Lee, D.-Y.; Wehr, N.B.; Viteri, G.A.; Berlett, B.S.; Levine, R.L. Small-Molecule Antioxidant Proteome-Shields in Deinococcus radiodurans. PLoS ONE 2010, 5, e12570. [Google Scholar] [CrossRef]
- Hanawalt, P.C. Controlling the efficiency of excision repair. Mutat. Res. 2001, 485, 3–13. [Google Scholar] [CrossRef]
- Pani, B.; Nudler, E. Mechanistic insights into transcription coupled DNA repair. DNA Repair 2017, 56, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Gupta, R.; Sen, R. Rho-dependent transcription termination in bacteria recycles RNA polymerases stalled at DNA lesions. Nat. Commun. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Epshtein, V.; Kamarthapu, V.; McGary, K.; Svetlov, V.; Ueberheide, B.; Proshkin, S.; Mironov, A.; Nudler, E. UvrD facilitates DNA repair by pulling RNA polymerase backwards. Nature 2014, 505, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Anaganti, N.; Padwal, M.K.; Dani, P.; Basu, B. Pleiotropic effects of a cold shock protein homolog PprM on the proteome of Deinococcus radiodurans. Biochim. Biophys. Acta-Proteins Proteom. 2018, 1867, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, L.; Nodwell, J.R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, R.R.; Fernandez, R.; Paz, L.I.; Singh, C.R.; Rosato, A.E. TCA Cycle-Mediated Generation of ROS Is a Key Mediator for HeR-MRSA Survival under β-Lactam Antibiotic Exposure. PLoS ONE 2014, 9, e99605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Carotti, D.; Desiato, G.; Miele, A.; Bellelli, A. Thioredoxin Reductase and its Inhibitors. Curr. Protein Pept. Sci. 2014, 15, 621–646. [Google Scholar] [CrossRef] [Green Version]
- Hofer, A.; Crona, M.; Logan, D.T.; Sjöberg, B.-M. DNA building blocks: Keeping control of manufacture. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, C.; Vahlensieck, C.; Bradley, T.; Tauber, S.; Lehmann, M.; Ullrich, O. Metabolic Dynamics in Short- and Long-Term Microgravity in Human Primary Macrophages. Int. J. Mol. Sci. 2021, 22, 6752. [Google Scholar] [CrossRef]
- Schnabl, H.; Hunte, C.; Schulz, M.; Wolf, D.; Ghiena-Rhalenbeck, C.; Bramer, M.; Graab, M.; Janßen, M.; Kalweit, H. Microgravity affects the ubiquitin pools and actin isoforms of mesophyll protoplasts. In Life Sciences Experiments Performed on Sounding Rockets (1985–1994); Texus 11–32, Maser 3–6 Maxus 1; ESA Publications Division: Noordwijk, The Netherlands, 1997; p. 105. [Google Scholar]
- Pajares, M.; Jiménez-Moreno, N.; Dias, I.H.; Debelec, B.; Vucetic, M.; Fladmark, K.E.; Basaga, H.; Ribaric, S.; Milisav, I.; Cuadrado, A. Redox control of protein degradation. Redox Biol. 2015, 6, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Adrian, A.; Schoppmann, K.; Sromicki, J.; Brungs, S.; Von der Wiesche, M.; Hock, B.; Kolanus, W.; Hemmersbach, R.; Ullrich, O. The oxidative burst reaction in mammalian cells depends on gravity. Cell Commun. Signal. 2013, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, X.; Sanon, S.; Zambrano, K.; Asquel, S.; Bassantes, M.; Morales, J.E.; Otáñez, G.; Pomaquero, C.; Villarroel, S.; Zurita, A.; et al. Key points for the development of antioxidant cocktails to prevent cellular stress and damage caused by reactive oxygen species (ROS) during manned space missions. Npj Microgravity 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2018, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
| Protein Name | Gene Name | Log2 Fold Change | Protein Name | Gene Name | Log2 Fold Change |
|---|---|---|---|---|---|
| General Metabolism | General Metabolism | ||||
| Glutamate 5-kinase | proB | 3.52 | Cytochrome-related protein | DR_0429 | −0.54 |
| Alanine dehydrogenase | DR_1895 | 0.64 | 3-isopropylmalate dehydratase small subunit 2 | leuD2 | −0.48 |
| Aspartokinase | DR_1365 | 0.56 | Purine-nucleoside phosphorylase | DR_2166 | −0.47 |
| Glycogen synthase | glgA | 0.46 | UDP-N-acetyl-D-mannosaminuronic acid transferase, putative | DR_1645 | −0.43 |
| Acetyl-CoA acetyltransferase | DR_A0053 | 0.44 | Lipopolysaccharide biosynthesis protein, putative | DR_A0043 | −0.40 |
| Citrate lyase subunit beta-like protein | DR_1240 | 0.38 | dTDP-glucose 4,6-dehydratase | DR_A0041 | −0.38 |
| 3-oxoacyl-[acyl-carrier-protein] synthase 2 | DR_1941 | 0.35 | Succinate--CoA ligase [ADP-forming] subunit alpha | sucD | −0.34 |
| Histidinol dehydrogenase | hisD | 0.31 | |||
| Membrane Proteins | Membrane Proteins | ||||
| Uncharacterized protein | DR_0458 | 0.44 | Sodium extrusion protein NatA | DR_0927 | −0.44 |
| DNA damage and repair, DNA processing | DNA damage and repair, DNA processing | ||||
| Replicative DNA helicase | DR_0549 | 0.56 | Probable chromosome 1-partitioning protein ParB | parB1 | −0.32 |
| DNA gyrase subunit A | gyrA | 0.54 | |||
| DNA topoisomerase 1 | topA | 0.41 | |||
| Endonuclease MutS2 | mutS2 | 0.36 | |||
| Transcription | Transcription | ||||
| Transcriptional regulator MraZ | mraZ | 0.34 | Probable transcriptional regulatory protein DR_2548 | DR_2548 | −1.09 |
| DNA-binding response regulator | DR_0432 | −0.55 | |||
| Transcription termination/antitermination protein NusG | nusG | −0.40 | |||
| Cell cycle and cell shape | Cell cycle and cell shape | ||||
| UDP-N-acetylmuramate--L-alanine ligase | murC | 0.75 | |||
| Stress Response | Stress Response | ||||
| Thiol:disulfide interchange protein | DR_0189 | 0.46 | 60 kDa chaperonin | groL | −0.65 |
| Leucyl aminopeptidase, putative | DR_0717 | 0.38 | Phosphinothricin acetyltransferase | DR_1182 | −0.55 |
| Carboxyl-terminal protease, putative | DR_1491 | 0.33 | Chaperone protein DnaK | dnaK | −0.50 |
| N-acetyltransferase domain-containing protein | DR_0653 | −0.48 | |||
| Lon protease | DR_0349 | −0.44 | |||
| Putative phosphoenolpyruvate synthase regulatory protein | DR_1728 | −0.35 | |||
| tRNA processing | tRNA processing | ||||
| Methylenetetrahydrofolate--tRNA-(uracil-5-)-methyltransferase TrmFO | trmFO | −0.71 | |||
| Translation, Ribosomes and rRNA | Translation, Ribosomes and rRNA | ||||
| Glutamyl-tRNA(Gln) amidotransferase subunit A | gatA | −0.52 | |||
| 30S ribosomal protein S2 | rpsB | −0.49 | |||
| Elongation factor Ts | tsf | −0.42 | |||
| Ribosome-recycling factor | frr | −0.40 | |||
| Unknown | Unknown | ||||
| KAP NTPase domain-containing protein | DR_C0009 | 1.36 | Uncharacterized protein | DR_2563 | −0.85 |
| Uncharacterized protein | DR_1256 | 1.35 | Uncharacterized protein | DR_1252 | −0.73 |
| Uncharacterized protein | DR_1331 | 1.32 | Uncharacterized protein | DR_0389 | −0.67 |
| Metallophos domain-containing protein | DR_1119 | 1.07 | Heat shock protein, HSP20 family | DR_1114 | −0.66 |
| Uncharacterized protein | DR_0360 | 0.91 | Uncharacterized protein | DR_0994 | −0.46 |
| Ferripyochelin-binding protein | DR_2089 | 0.73 | Glyoxalase-like_dom domain-containing protein | DR_2014 | −0.44 |
| Uncharacterized protein | DR_1018 | 0.63 | Lipopolysaccharide biosynthesis protein, putative | DR_0444 | -0.39 |
| GHL10 domain-containing protein | DR_A0207 | 0.53 | DUF1990 domain-containing protein | DR_A0230 | −0.38 |
| Uncharacterized protein | DR_1773 | 0.40 | zf-RING_7 domain-containing protein | DR_0291 | −0.36 |
| Uncharacterized protein | DR_A0190 | 0.37 | Site-determining protein | DR_0752 | −0.34 |
| Propionyl-CoA carboxylase, beta subunit, putative | DR_1542 | 0.36 | DUF11 domain-containing protein | DR_0685 | −0.33 |
| Uncharacterized protein | DR_0574 | 0.33 | Uncharacterized protein | DR_2057 | −0.30 |
| Uncharacterized protein | DR_A0022 | 0.32 | Chromosome partitioning ATPase, putative, ParA family | DR_A0001 | −0.27 |
| Protein Name | Gene Name | Log2 Fold Change |
|---|---|---|
| Pentose Phosphate Pathway (PPP) | ||
| phosphopentomutase | DR_2135 | 1.28 |
| glucose-6-phosphate 1-dehydrogenase | DR_1596 | 0.80 |
| carbohydrate kinase | DR_1525 | 0.61 |
| glucose-6-phosphate isomerase | pgi | 0.52 |
| 2-deoxyribose-5-phosphate aldolase | DR_1205 | 0.49 |
| DNA Damage and Repair, DNA Processing | ||
| DNA ligase | DR_2069 | 1.04 |
| DNA-directed DNA polymerase | DR_1707 | 1.01 |
| hypothetical protein | DR_0428 | 0.83 |
| exconuclease ABC subunit B | DR_2275 | 0.77 |
| Mrr restriction system protein | DR_0508 | 0.77 |
| hypothetical protein | DR_0326 | 0.68 |
| transcription-repair coupling factor | DR_1532 | 0.67 |
| ParB family chromosome partitioning protein | DR_A0002 | 0.58 |
| Beta sliding clamp | DR_0001 | 0.54 |
| exonuclease SbcC | DR_1922 | 0.54 |
| hypothetical protein | DR_2235 | 0.54 |
| Ribonucleoside-diphosphate reductase (EC 1.17.4.1) | DR_B0108 | 0.48 |
| MTS domain-containing protein | DR_0914 | 0.44 |
| Holliday junction ATP-dependent DNA helicase RuvB (EC 3.6.4.12) | RuvB | 0.39 |
| Ribonucleoside-diphosphate reductase (EC 1.17.4.1) | DR_2374 | 0.35 |
| Protein RecA (Recombinase A) | RecA | 0.31 |
| Transcription | ||
| hypothetical protein | DR_1872 | 1.96 |
| transcription termination factor Rho | rho | 1.08 |
| DNA-directed RNA polymerase subunit beta’ | DR_0911 | 1.02 |
| Lpr/AsnC family transcriptional regulator | DR_0200 | 0.93 |
| Transcriptional regulator, HTH_3 family | DR_2574 | 0.79 |
| DNA-directed RNA polymerase subunit alpha | DR_2128 | 0.73 |
| DNA-directed RNA polymerase subunit beta | rpoB | 0.53 |
| Transcription termination/antitermination protein NusA | NusA | 0.52 |
| Bifunctional protein PyrR [Includes: Pyrimidine operon regulatory protein; Uracil phosphoribosyltransferase (UPRTase)] | PyrR | 0.38 |
| magnesium protoporphyrin chelatase | DR_2594 | 0.34 |
| Stress Response | ||
| Osmotic Stress | ||
| HAMP domain-containing protein | DR_1829 | 0.92 |
| Oxidative Stress | ||
| NADH-dependent flavin oxidoreductase | DR_2190 | 1.55 |
| thioredoxin reductase | DR_1982 | 1.17 |
| thiol-specific antioxidant protein | DR_2242 | 0.85 |
| LuxA-like protein | DR_0611 | 0.79 |
| short chain dehydrogenase/reductase family oxidoreductase | DR_1938 | 0.79 |
| 3-hydroxyisobutyrate dehydrogenase | DR_0499 | 0.74 |
| Uncharacterized protein | DR_1002 | 0.58 |
| Oxidoreductase, short-chain dehydrogenase/reductase family | DR_0113 | 0.54 |
| zinc-containing alcohol dehydrogenase | DR_A0005 | 0.51 |
| Dihydrolipoyl dehydrogenase | DR_2370 | 0.43 |
| General Stress | ||
| oligoendopeptidase F | DR_2055 | 1.26 |
| prolyl endopeptidase | DR_2503 | 1.19 |
| proline iminopeptidase-like protein | DR_0654 | 1.17 |
| oligoendopeptidase | DR_1627 | 1.15 |
| hypothetical protein | DR_2363 | 1.11 |
| carboxypeptidase G2 | DR_2493 | 0.91 |
| hypothetical protein | DR_0985 | 0.87 |
| metalloprotease | DR_0617 | 0.86 |
| ATP-dependent Clp protease, ATP-binding subunit ClpB | ClpB | 0.83 |
| NAD-dependent protein deacylase | cobB | 0.79 |
| oligopeptidase A | DR_1659 | 0.70 |
| Protein GrpE (HSP-70 cofactor) | GrpE | 0.70 |
| cyclophilin-type peptidyl-prolyl cis-trans isomerase | DR_2542 | 0.67 |
| hypothetical protein | DR_1832 | 0.60 |
| Aminoglycoside N(3)-acetyltransferase | DR_2034 | 0.52 |
| ATP-dependent protease LA | Lon | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moors, K.A.; Ott, E.; Weckwerth, W.; Milojevic, T. Proteomic Response of Deinococcus radiodurans to Short-Term Real Microgravity during Parabolic Flight Reveals Altered Abundance of Proteins Involved in Stress Response and Cell Envelope Functions. Life 2022, 12, 23. https://doi.org/10.3390/life12010023
Moors KA, Ott E, Weckwerth W, Milojevic T. Proteomic Response of Deinococcus radiodurans to Short-Term Real Microgravity during Parabolic Flight Reveals Altered Abundance of Proteins Involved in Stress Response and Cell Envelope Functions. Life. 2022; 12(1):23. https://doi.org/10.3390/life12010023
Chicago/Turabian StyleMoors, Karlis Arturs, Emanuel Ott, Wolfram Weckwerth, and Tetyana Milojevic. 2022. "Proteomic Response of Deinococcus radiodurans to Short-Term Real Microgravity during Parabolic Flight Reveals Altered Abundance of Proteins Involved in Stress Response and Cell Envelope Functions" Life 12, no. 1: 23. https://doi.org/10.3390/life12010023
APA StyleMoors, K. A., Ott, E., Weckwerth, W., & Milojevic, T. (2022). Proteomic Response of Deinococcus radiodurans to Short-Term Real Microgravity during Parabolic Flight Reveals Altered Abundance of Proteins Involved in Stress Response and Cell Envelope Functions. Life, 12(1), 23. https://doi.org/10.3390/life12010023






