Role of a Dual Glucose-Dependent Insulinotropic Peptide (GIP)/Glucagon-like Peptide-1 Receptor Agonist (Twincretin) in Glycemic Control: From Pathophysiology to Treatment
Abstract
:1. Introduction
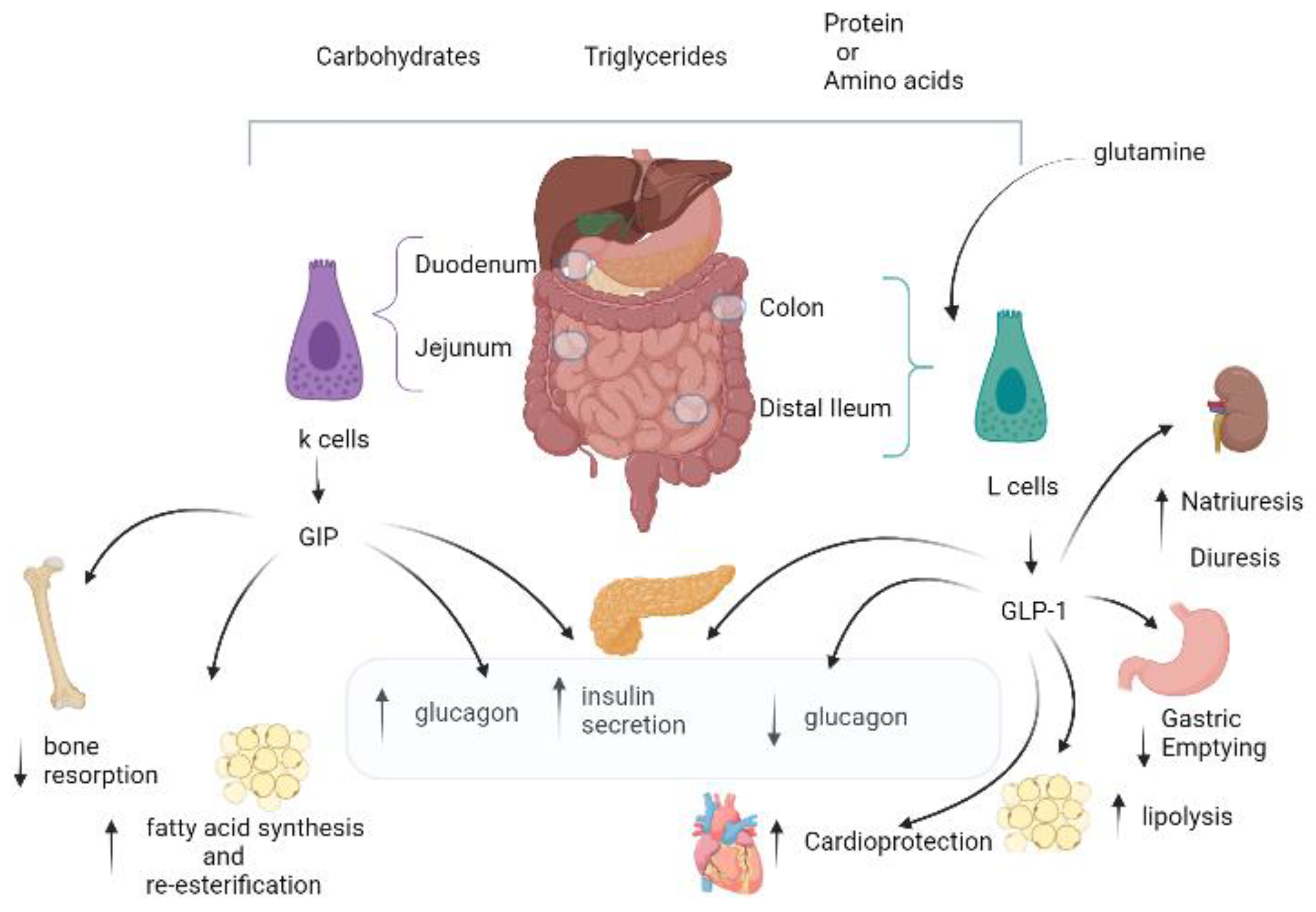
2. GIP Physiology: Similarities and Differences versus GLP-1
The Dual GIP/GLP-1 Mechanisms
3. In Vitro Studies
4. Animal Studies
5. In Human Studies
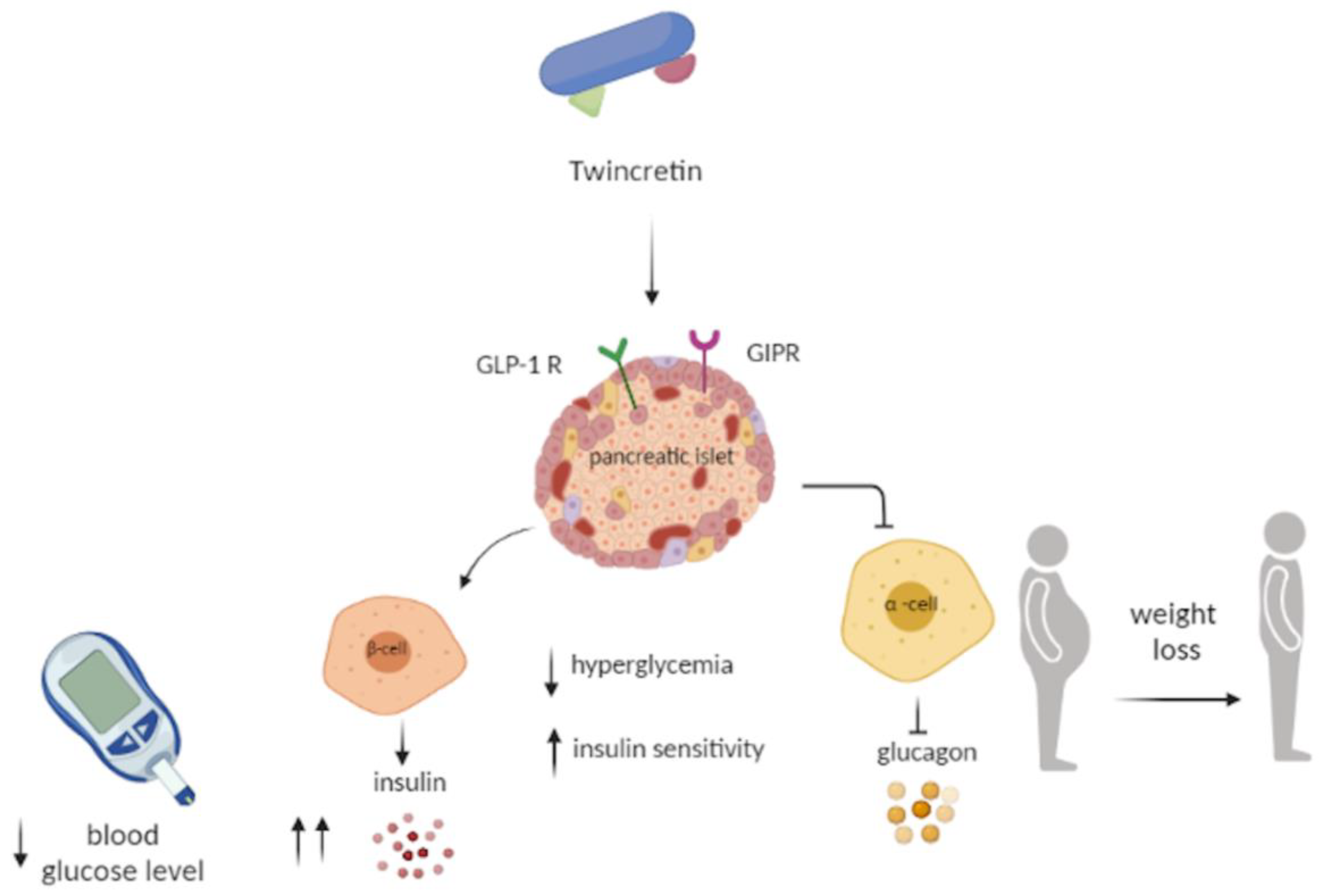
6. Twincretin
7. Tirzepatide, a Dual GIP/GLP-1 Receptor Agonist: Efficacy in Phase 3 Clinical Trials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CV | Cardiovascular |
| cAMP | Cyclic-adenosine-monophosphate |
| CVOT | Cardiovascular outcome trial |
| DPP-4 | Dipeptidyl peptidase 4 |
| FDA | United States Food and Drug Administration |
| FFA | Free fatty acids |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GIPRs | GIP receptors |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1 R | Glucagon-like peptide-1 receptor |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonists |
| GR | Glucagon receptor |
| HbA1c | Glycated hemoglobin A1c |
| i.v. | Intravenous |
| MACE | Major cardiovascular events |
| MI | Myocardial infarction |
| OGTT | Oral glucose tolerance test |
| PEG | Polyethylene glycol |
| SGLT | Sodium-glucose co-transporter type |
| SD | Standard deviation |
| T2DM | Type 2 diabetes mellitus |
References
- Zunz, E.; La Barre, J. Contributions A L’Étude des Variations Physiologiques De La Sécrétion Interne Du Pancréas. Arch. Int. Phys. 1929, 31, 162–179. (In French) [Google Scholar] [CrossRef]
- Bayliss, W.M.; Starling, E.H. The mechanism of pancreatic secretion. J. Physiol. 1902, 28, 325–353. [Google Scholar] [CrossRef]
- Moore, B. On the treatment of Diabetus mellitus by acid extract of Duodenal Mucous Membrane. Biochem. J. 1906, 1, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcintyre, N.; Holdsworth, C.D.; Turner, D.S. New interpretation of oral glucose tolerance. Lancet 1964, 2, 20–21. [Google Scholar] [CrossRef]
- Unger, R.H.; Eisentraut, A.M. Entero-insularaxis. Arch. Intern. Med. 1969, 123, 261–266. [Google Scholar] [CrossRef]
- Eissele, R.; Göke, R.; Willemer, S.; Harthus, H.P.; Vermeer, H.; Arnold, R.; Göke, B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Investig. 1992, 22, 283–291. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Gribble, F.M.; Reimann, F. Nutrient detection by incretin hormone secreting cells. Physiol. Behav. 2012, 106, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Takeda, J.; Seino, Y.; Tanaka, K.; Fukumoto, H.; Kayano, T.; Takahashi, H.; Mitani, T.; Kurono, M.; Suzuki, T.; Tobe, T. Sequence of an intestinal cDNA encoding human gastric inhibitory polypeptide precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 7005–7008. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Mutt, V.; Pederson, R.A. Further purification of a polypeptide demonstrating enterogastrone activity. J. Physiol. 1970, 209, 57–64. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet. Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Caruso, I.; Cignarelli, A.; Giorgino, F. Heterogeneity and Similarities in GLP-1 Receptor Agonist Cardiovascular Outcomes Trials. Trends Endocrinol. Metab. TEM 2019, 30, 578–589. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Carlson, O.D.; Kim, W.; Shin, Y.K.; Charles, C.P.; Kim, H.S.; Melvin, D.L.; Egan, J.M. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 2009, 58, 1342–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Højberg, P.V.; Vilsbøll, T.; Rabøl, R.; Knop, F.K.; Bache, M.; Krarup, T.; Holst, J.J.; Madsbad, S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009, 52, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Bokvist, K.; Brown, R.; Coskun, T.; Cox, A.; Cummins, R.; Farb, T.; Ficorilli, J.; Lewis, A.P.; Marcelo, M.; O’Farrell, L.; et al. LY3298176, a novel long-actingGIP/GLP-1 coagonist, shows enhanced activity on weight loss and energy utilization whilst maintaining its efficacy for glycaemic control. Diabetologia 2017, 60 (Suppl. 1), S399. [Google Scholar]
- Frias, J.P.; Bastyr, E.J., III; Vignati, L.; Tschöp, M.H.; Schmitt, C.; Owen, K.; Christensen, R.H.; DiMarchi, R.D. The Sustained Effects of a Dual GIP/GLP-1 Receptor Agonist, NNC0090-2746, in Patients with Type 2 Diabetes. Cell Metab. 2017, 26, 343–352.e2. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Boer, G.A.; Holst, J.J. Incretin Hormones and Type 2 Diabetes-Mechanistic Insights and Therapeutic Approaches. Biology 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. J. Vasc. Interv. Radiol. JVIR 2018, 29, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elrick, H.; Stimmler, L.; Hlad, C.J.; Arai, Y., Jr. Plasma insulin response to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef]
- McIntyre, N.; Holdsworth, C.D.; Turner, D.S. Intestinal factors in the control of insulin secretion. J. Clin. Endocrinol. Metab. 1965, 25, 1317–1324. [Google Scholar] [CrossRef]
- Fujita, Y.; Wideman, R.D.; Asadi, A.; Yang, G.K.; Baker, R.; Webber, T.; Zhang, T.; Wang, R.; Ao, Z.; Warnock, G.L.; et al. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet alpha-cells and promotes insulin secretion. Gastroenterology 2010, 138, 1966–1975. [Google Scholar] [CrossRef]
- Brown, J.C. Gastric inhibitory polypeptide. In Monographs on Endocrinology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1982; Volume 24, 88p. [Google Scholar]
- Gupta, K.; Raja, A. Physiology, Gastric Inhibitory Peptide; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fehmann, H.C.; Göke, R.; Göke, B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr. Rev. 1995, 16, 390–410. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Hartmann, B.; Deacon, C.F.; Holst, J.J. Measurement of the incretin hormones: Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J. Diabetes Complicat. 2015, 29, 445–450. [Google Scholar] [CrossRef]
- Mayo, K.E.; Miller, L.J.; Bataille, D.; Dalle, S.; Göke, B.; Thorens, B.; Drucker, D.J. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 2003, 55, 167–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornberry, N.A.; Gallwitz, B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract. Research. Clin. Endocrinol. Metab. 2009, 23, 479–486. [Google Scholar] [CrossRef]
- Usdin, T.B.; Mezey, E.; Button, D.C.; Brownstein, M.J.; Bonner, T.I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993, 133, 2861–2870. [Google Scholar] [CrossRef]
- Ding, W.G.; Gromada, J. Protein kinase A-dependent stimulation of exocytosis in mouse pancreatic beta-cells by glucose-dependent insulinotropic polypeptide. Diabetes 1997, 46, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Gromada, J.; Bokvist, K.; Ding, W.G.; Holst, J.J.; Nielsen, J.H.; Rorsman, P. Glucagon-like peptide 1 (7-36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes 1998, 47, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Montrose-Rafizadeh, C.; Adams, L.; Raygada, M.; Nadiv, O.; Egan, J.M. GIP regulates glucose transporters, hexokinases, and glucose-induced insulin secretion in RIN 1046-38 cells. Mol. Cell. Endocrinol. 1996, 116, 81–87. [Google Scholar] [CrossRef]
- Nyberg, J.; Anderson, M.F.; Meister, B.; Alborn, A.M.; Ström, A.K.; Brederlau, A.; Illerskog, A.C.; Nilsson, O.; Kieffer, T.J.; Hietala, M.A.; et al. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1816–1825. [Google Scholar] [CrossRef] [Green Version]
- Irwin, N.; Gault, V.; Flatt, P.R. Therapeutic potential of the original incretin hormone glucose-dependent insulinotropic polypeptide: Diabetes, obesity, osteoporosis and Alzheimer’s disease? Expert Opin. Investig. Drugs 2010, 19, 1039–1048. [Google Scholar] [CrossRef]
- Villar, H.V.; Fender, H.R.; Rayford, P.L.; Bloom, S.R.; Ramus, N.I.; Thompson, J.C. Suppression of gastrin release and gastric secretion by gastric inhibitory polypeptide (GIP) and vasoactive intestinal polypeptide (VIP). Ann. Surg. 1976, 184, 97–102. [Google Scholar] [CrossRef]
- Nauck, M.A.; Bartels, E.; Orskov, C.; Ebert, R.; Creutzfeldt, W. Lack of effect of synthetic human gastric inhibitory polypeptide and glucagon-like peptide 1 [7-36 amide] infused at near-physiological concentrations on pentagastrin-stimulated gastric acid secretion in normal human subjects. Digestion 1992, 52, 214–221. [Google Scholar] [CrossRef]
- Besterman, H.S.; Cook, G.C.; Sarson, D.L.; Christofides, N.D.; Bryant, M.G.; Gregor, M.; Bloom, S.R. Gut hormones in tropical malabsorption. Br. Med. J. 1979, 2, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Besterman, H.S.; Bloom, S.R.; Sarson, D.L.; Blackburn, A.M.; Johnston, D.I.; Patel, H.R.; Stewart, J.S.; Modigliani, R.; Guerin, S.; Mallinson, C.N. Gut-hormone profile in coeliac disease. Lancet 1978, 1, 785–788. [Google Scholar] [CrossRef]
- Inagaki, N.; Seino, Y.; Takeda, J.; Yano, H.; Yamada, Y.; Bell, G.I.; Eddy, R.L.; Fukushima, Y.; Byers, M.G.; Shows, T.B. Gastric inhibitory polypeptide: Structure and chromosomal localization of the human gene. Mol. Endocrinol. 1989, 3, 1014–1021. [Google Scholar] [CrossRef]
- Vollmer, K.; Holst, J.J.; Baller, B.; Ellrichmann, M.; Nauck, M.A.; Schmidt, W.E.; Meier, J.J. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008, 57, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24. [Google Scholar] [CrossRef]
- Nauck, M.A.; Siemsglüss, J.; Orskov, C.; Holst, J.J. Release of glucagon-like peptide 1 (GLP-1 [7-36 amide]), gastric inhibitory polypeptide (GIP) and insulin in response to oral glucose after upper and lower intestinal resections. Z. Fur Gastroenterol. 1996, 34, 159–166. [Google Scholar]
- Hansen, L.; Deacon, C.F.; Orskov, C.; Holst, J.J. Glucagon-like peptide-1-(7-36) amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999, 140, 5356–5363. [Google Scholar] [CrossRef]
- Thorens, B.; Porret, A.; Bühler, L.; Deng, S.P.; Morel, P.; Widmann, C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes 1993, 42, 1678–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorens, B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. USA 1992, 89, 8641–8645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moens, K.; Heimberg, H.; Flamez, D.; Huypens, P.; Quartier, E.; Ling, Z.; Pipeleers, D.; Gremlich, S.; Thorens, B.; Schuit, F. Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes 1996, 45, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Amiranoff, B.; Couvineau, A.; Vauclin-Jacques, N.; Laburthe, M. Gastric inhibitory polypeptide receptor in hamster pancreatic beta cells. Direct cross-linking, solubilization and characterization as a glycoprotein. Eur. J. Biochem. 1986, 159, 353–358. [Google Scholar] [CrossRef]
- Gremlich, S.; Porret, A.; Hani, E.H.; Cherif, D.; Vionnet, N.; Froguel, P.; Thorens, B. Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes 1995, 44, 1202–1208. [Google Scholar] [CrossRef]
- Volz, A.; Göke, R.; Lankat-Buttgereit, B.; Fehmann, H.C.; Bode, H.P.; Göke, B. Molecular cloning, functional expression, and signal transduction of the GIP-receptor cloned from a human insulinoma. FEBS Lett. 1995, 373, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.B.; Gelling, R.W.; McIntosh, C.H.; Georgiou, J.; Brown, J.C.; Pederson, R.A. Functional expression of the rat pancreatic islet glucose-dependent insulinotropic polypeptide receptor: Ligand binding and intracellular signaling properties. Endocrinology 1995, 136, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hayami, T.; Nakamura, K.; Kaisaki, P.J.; Someya, Y.; Wang, C.Z.; Seino, S.; Seino, Y. Human gastric inhibitory polypeptide receptor: Cloning of the gene (GIPR) and cDNA. Genomics 1995, 29, 773–776. [Google Scholar] [CrossRef]
- Yasuda, K.; Inagaki, N.; Yamada, Y.; Kubota, A.; Seino, S.; Seino, Y. Hamster gastric inhibitory polypeptide receptor expressed in pancreatic islets and clonal insulin-secreting cells: Its structure and functional properties. Biochem. Biophys. Res. Commun. 1994, 205, 1556–1562. [Google Scholar] [CrossRef] [Green Version]
- Dillon, J.S.; Tanizawa, Y.; Wheeler, M.B.; Leng, X.H.; Ligon, B.B.; Rabin, D.U.; Yoo-Warren, H.; Permutt, M.A.; Boyd, A.E., III. Cloning and functional expression of the human glucagon-like peptide-1 (GLP-1) receptor. Endocrinology 1993, 133, 1907–1910. [Google Scholar] [CrossRef]
- Stoffel, M.; Espinosa, R.; Le Beau, M.M., III; Bell, G.I. Human glucagon-like peptide-1 receptor gene. Localization to chromosome band 6p21 by fluorescence in situ hybridization and linkage of a highly polymorphic simple tandem repeat DNA polymorphism to other markers on chromosome 6. Diabetes 1993, 42, 1215–1218. [Google Scholar] [CrossRef]
- Perley, M.J.; Kipnis, D.M. Plasma insulin responses to oral and intravenous glucose: Studies in normal and diabetic sujbjects. J. Clin. Investig. 1967, 46, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Gabe, M.; Sparre-Ulrich, A.H.; Pedersen, M.F.; Gasbjerg, L.S.; Inoue, A.; Bräuner-Osborne, H.; Hartmann, B.; Rosenkilde, M.M. Human GIP(3-30)NH2 inhibits G protein-dependent as well as G protein-independent signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. Biochem. Pharmacol. 2018, 150, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Patel, R.T.; Bruno, J.; Panhwar, M.S.; Wen, J.; McGraw, T.E. A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Mol. Cell. Biol. 2014, 34, 3618–3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maida, A.; Hansotia, T.; Longuet, C.; Seino, Y.; Drucker, D.J. Differential importance of glucose-dependent insulinotropic polypeptide vs. glucagon-like peptide 1 receptor signaling for beta cell survival in mice. Gastroenterology 2009, 137, 2146–2157. [Google Scholar] [CrossRef]
- Orskov, C.; Holst, J.J.; Nielsen, O.V. Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 1988, 123, 2009–2013. [Google Scholar] [CrossRef]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Kleine, N.; Orskov, C.; Holst, J.J.; Willms, B.; Creutzfeldt, W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993, 36, 741–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Heimesaat, M.M.; Behle, K.; Holst, J.J.; Nauck, M.S.; Ritzel, R.; Hüfner, M.; Schmiegel, W.H. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J. Clin. Endocrinol. Metab. 2002, 87, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Pederson, R.A.; Brown, J.C. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology 1978, 103, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.B.; Calanna, S.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: Blood glucose stabilizing effects in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E418–E426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahrén, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am. J. Physiology. Endocrinol. Metab. 2008, 295, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Rasmussen, O.; Lousen, T.; Holst, J.J.; Fenselau, S.; Schrezenmeir, J.; Hermansen, K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Creutzfeldt, W.; Ebert, R.; Willms, B.; Frerichs, H.; Brown, J.C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: Increased response to stimulation and defective feedback control of serum levels. Diabetologia 1978, 14, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salera, M.; Giacomoni, P.; Pironi, L.; Cornia, G.; Capelli, M.; Marini, A.; Benfenati, F.; Miglioli, M.; Barbara, L. Gastric inhibitory polypeptide release after oral glucose: Relationship to glucose intolerance, diabetes mellitus, and obesity. J. Clin. Endocrinol. Metab. 1982, 55, 329–336. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Pacini, G.; De Michieli, F.; Cassader, M. Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: Dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am. J. Clin. Nutr. 2009, 89, 558–567. [Google Scholar] [CrossRef]
- Asmar, M.; Simonsen, L.; Madsbad, S.; Stallknecht, B.; Holst, J.J.; Bülow, J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes 2010, 59, 2160–2163. [Google Scholar] [CrossRef] [Green Version]
- Ebert, R.; Nauck, M.; Creutzfeldt, W. Effect of exogenous or endogenous gastric inhibitory polypeptide (GIP) on plasma triglyceride responses in rats. Horm. Metab. Res. 1991, 23, 517–521. [Google Scholar] [CrossRef]
- Wasada, T.; McCorkle, K.; Harris, V.; Kawai, K.; Howard, B.; Unger, R.H. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J. Clin. Investig. 1981, 68, 1106–1107. [Google Scholar] [CrossRef]
- Beck, B.; Max, J.P. Gastric inhibitory polypeptide enhancement of the insulin effect on fatty acid incorporation into adipose tissue in the rat. Regul. Pept. 1983, 7, 3–8. [Google Scholar] [CrossRef]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression Within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [Green Version]
- Heimbürger, S.M.; Bergmann, N.C.; Augustin, R.; Gasbjerg, L.S.; Christensen, M.B.; Knop, F.K. Glucose-dependent insulinotropic polypeptide (GIP) and cardiovascular disease. Peptides 2020, 125, 170174. [Google Scholar] [CrossRef]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef] [Green Version]
- Paratore, S.; Ciotti, M.T.; Basille, M.; Vaudry, D.; Gentile, A.; Parenti, R.; Calissano, P.; Cavallaro, S. Gastric inhibitory polypeptide and its receptor are expressed in the central nervous system and support neuronal survival. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 210–222. [Google Scholar] [CrossRef]
- Wettergren, A.; Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig. Dis. Sci. 1993, 38, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Orskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 1997, 273, E981–E988. [Google Scholar] [CrossRef]
- Willms, B.; Werner, J.; Holst, J.J.; Orskov, C.; Creutzfeldt, W.; Nauck, M.A. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: Effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J. Clin. Endocrinol. Metab. 1996, 81, 327–332. [Google Scholar] [CrossRef]
- Meier, J.J.; Goetze, O.; Anstipp, J.; Hagemann, D.; Holst, J.J.; Schmidt, W.E.; Gallwitz, B.; Nauck, M.A. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E621–E625. [Google Scholar] [CrossRef]
- Holst, J.J.; Windeløv, J.A.; Boer, G.A.; Pedersen, J.; Svendsen, B.; Christensen, M.; Torekov, S.; Asmar, M.; Hartmann, B.; Nissen, A. Searching for the physiological role of glucose-dependent insulinotropic polypeptide. J. Diabetes Investig. 2016, 7, 8–12. [Google Scholar] [CrossRef]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Inendocrinol. Metab. TEM 2020, 31, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahi, D.; McAloon-Dyke, M.; Fukagawa, N.K.; Meneilly, G.S.; Sclater, A.L.; Minaker, K.L.; Habener, J.F.; Andersen, D.K. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul. Pept. 1994, 51, 63–74. [Google Scholar] [CrossRef]
- Finan, B.; Ma, T.; Ottaway, N.; Müller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013, 5, 209ra151. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Bartels, E.; Orskov, C.; Ebert, R.; Creutzfeldt, W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 1993, 76, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Skow, M.A.; Bergmann, N.C.; Knop, F.K. Diabetes and obesity treatment based on dual incretin receptor activation: ‘twincretins’. Diabetes Obes. Metab. 2016, 18, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B.; Witt, M.; Fölsch, U.R.; Creutzfeldt, W.; Schmidt, W.E. Binding specificity and signal transduction of receptors for glucagon-like peptide-1(7-36)amide and gastric inhibitory polypeptide on RINm5F insulinoma cells. J. Mol. Endocrinol. 1993, 10, 259–268. [Google Scholar] [CrossRef]
- Delmeire, D.; Flamez, D.; Moens, K.; Hinke, S.A.; Van Schravendijk, C.; Pipeleers, D.; Schuit, F. Prior in vitro exposure to GLP-1 with or without GIP can influence the subsequent beta cell responsiveness. Biochem. Pharmacol. 2004, 68, 33–39. [Google Scholar] [CrossRef]
- Lupi, R.; Del Guerra, S.; D’Aleo, V.; Boggi, U.; Filipponi, F.; Marchetti, P. The direct effects of GLP-1 and GIP, alone or in combination, on human pancreatic islets. Regul. Pept. 2010, 165, 129–132. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. GPCR monomers and oligomers: It takes all kinds. Trends Neurosci. 2008, 31, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikumar, K.G.; Wootten, D.; Pinon, D.I.; Koole, C.; Ball, A.M.; Furness, S.G.; Graham, B.; Dong, M.; Christopoulos, A.; Miller, L.J.; et al. Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signaling but does not affect small molecule allostery. Proc. Natl. Acad. Sci. USA 2012, 109, 18607–18612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, G.M.; Lynn, F.C.; McIntosh, C.H.; Accili, E.A. Regulation of GIP and GLP1 receptor cell surface expression by N-glycosylation and receptor heteromerization. PLoS ONE 2012, 7, e32675. [Google Scholar] [CrossRef] [PubMed]
- Schelshorn, D.; Joly, F.; Mutel, S.; Hampe, C.; Breton, B.; Mutel, V.; Lütjens, R. Lateral allosterism in the glucagon receptor family: Glucagon-like peptide 1 induces G-protein-coupled receptor heteromer formation. Mol. Pharmacol. 2012, 81, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Roed, S.N.; Nøhr, A.C.; Wismann, P.; Iversen, H.; Bräuner-Osborne, H.; Knudsen, S.M.; Waldhoer, M. Functional consequences of glucagon-like peptide-1 receptor cross-talk and trafficking. J. Biol. Chem. 2015, 290, 1233–1243. [Google Scholar] [CrossRef] [Green Version]
- Gault, V.A.; Kerr, B.D.; Harriott, P.; Flatt, P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type2 diabetes and obesity. Clin. Sci. 2011, 121, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Finan, B.; Müller, T.D.; Clemmensen, C.; Perez-Tilve, D.; DiMarchi, R.D.; Tschöp, M.H. Reappraisal of GIP Pharmacology for Metabolic Diseases. Trends Mol. Med. 2016, 22, 359–376. [Google Scholar] [CrossRef]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef]
- Nørregaard, P.K.; Deryabina, M.A.; Tofteng Shelton, P.; Fog, J.U.; Daugaard, J.R.; Eriksson, P.O.; Larsen, L.F.; Jessen, L. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes. Metab. 2018, 20, 60–68. [Google Scholar] [CrossRef]
- Pathak, N.M.; Pathak, V.; Gault, V.A.; McClean, S.; Irwin, N.; Flatt, P.R. Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential. Biochem. Pharmacol. 2018, 155, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.H.; Finan, B.; Clemmensen, C.; Gelfanov, V.; Perez-Tilve, D.; Müller, T.D.; DiMarchi, R.D. Unimolecular Polypharmacy for Treatment of Diabetes and Obesity. Cell Metab. 2016, 24, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Meessen, E.; Andersen, M.M.; Bergmann, S.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.B.; et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: A randomised, crossover study. Diabetologia 2019, 62, 665–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, N.; Hunter, K.; Frizzell, N.; Flatt, P.R. Antidiabetic effects of sub-chronic activation of the GIP receptor alone and in combination with background exendin-4 therapy in high fat fed mice. Regul. Pept. 2009, 153, 70–76. [Google Scholar] [CrossRef]
- Irwin, N.; McClean, P.L.; Cassidy, R.S.; O’harte, F.P.; Green, B.D.; Gault, V.A.; Harriott, P.; Flatt, P.R. Comparison of the anti-diabetic effects of GIP- and GLP-1-receptor activation in obese diabetic (ob/ob) mice: Studies with DPP IV resistant N-AcGIP and exendin(1-39)amide. DiabetesMetab. Res. Rev. 2007, 23, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Young, A.A.; Gedulin, B.R.; Bhavsar, S.; Bodkin, N.; Jodka, C.; Hansen, B.; Denaro, M. Glucose-lowering and insulin-sensitizing actions of exendin-4: Studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999, 48, 1026–1034. [Google Scholar] [CrossRef]
- Greig, N.H.; Holloway, H.W.; De Ore, K.A.; Jani, D.; Wang, Y.; Zhou, J.; Garant, M.J.; Egan, J.M. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 1999, 42, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Killion, E.A.; Wang, J.; Yie, J.; Shi, S.D.; Bates, D.; Min, X.; Komorowski, R.; Hager, T.; Deng, L.; Atangan, L.; et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci. Transl. Med. 2018, 10, eaat3392. [Google Scholar] [CrossRef]
- Irwin, N.; McClean, P.L.; Flatt, P.R. Comparison of the subchronic antidiabetic effects of DPP IV-resistant GIP and GLP-1 analogues in obese diabetic (ob/ob) mice. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2007, 13, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, D.S.; Bagger, J.I.; Bergmann, N.C.; Lund, A.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. The Effects of Dual GLP-1/GIP Receptor Agonism on Glucagon Secretion-A Review. Int. J. Mol. Sci. 2019, 20, 4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, A.; Vilsbøll, T.; Bagger, J.I.; Holst, J.J.; Knop, F.K. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1038–E1046. [Google Scholar] [CrossRef] [Green Version]
- Daousi, C.; Wilding, J.P.; Aditya, S.; Durham, B.H.; Cleator, J.; Pinkney, J.H.; Ranganath, L.R. Effects of peripheral administration of synthetic human glucose-dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin. Endocrinol. 2009, 71, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 2002, 45, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Mentis, N.; Vardarli, I.; Köthe, L.D.; Holst, J.J.; Deacon, C.F.; Theodorakis, M.; Meier, J.J.; Nauck, M.A. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes 2011, 60, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Krarup, T.; Saurbrey, N.; Moody, A.J.; Kühl, C.; Madsbad, S. Effect of porcine gastric inhibitory polypeptide on beta-cell function in type I and type II diabetes mellitus. Metab. Clin. Exp. 1987, 36, 677–682. [Google Scholar] [CrossRef]
- Asmar, M.; Arngrim, N.; Simonsen, L.; Asmar, A.; Nordby, P.; Holst, J.J.; Bülow, J. The blunted effect of glucose-dependent insulinotropic polypeptide in subcutaneous abdominal adipose tissue in obese subjects is partly reversed by weight loss. Nutr. Diabetes 2016, 6, e208. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafré, V.; Duran, X.; Pachón, G.; Roche, K.; Garrido-Sánchez, L.; Vilarrasa, N.; Tinahones, F.J.; Vicente, V.; Pujol, J.; Vendrell, J.; et al. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2014, 99, E908–E919. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Portron, A.; Jadidi, S.; Sarkar, N.; DiMarchi, R. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes Obes. Metab. 2017, 19, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Portron, A.; Jadidi, S.; Sarkar, N.; DiMarchi, R.; Schmitt, C. Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects. Diabetes Obes. Metab. 2017, 19, 1446–1453. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 811–824. [Google Scholar] [CrossRef]
- A Study of Tirzepatide (LY3298176) Versus Placebo in Participants with Type 2 Diabetes Inadequately Controlled on Insulin Glargine With or Without Metformin. Available online: https://clinicaltrials.gov/ct2/show/NCT04039503 (accessed on 15 November 2021).
- Provenzano, M.; Pelle, M.C.; Zaffina, I.; Tassone, B.; Pujia, R.; Ricchio, M.; Serra, R.; Sciacqua, A.; Michael, A.; Andreucci, M.; et al. Sodium-Glucose Co-transporter-2 Inhibitors and Nephroprotection in DiabeticPatients: More Than a Challenge. Front. Med. 2021, 8, 654557. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Liberti, M.E.; Andreucci, M.; Conte, G.; Minutolo, R.; Provenzano, M.; De Nicola, L. SGLT2 Inhibitors: Nephroprotective Efficacy and Side Effects. Medicina 2019, 55, 268. [Google Scholar] [CrossRef] [Green Version]
- Coppolino, G.; Leporini, C.; Rivoli, L.; Ursini, F.; Di Paola, E.D.; Cernaro, V.; Arturi, F.; Bolignano, D.; Russo, E.; De Sarro, G.; et al. Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol. Res. 2018, 129, 274–294. [Google Scholar] [CrossRef] [PubMed]
- Li Vecchi, M.; Fuiano, G.; Francesco, M.; Mancuso, D.; Faga, T.; Sponton, A.; Provenzano, R.; Andreucci, M.; Tozzo, C. Prevalence and severity of anaemia in patients with type 2 diabetic nephropathy and different degrees of chronic renal insufficiency. Nephron. Clin. Pract. 2007, 105, c62-7. [Google Scholar] [CrossRef]
- Evers, A.; Haack, T.; Lorenz, M.; Bossart, M.; Elvert, R.; Henkel, B.; Stengelin, S.; Kurz, M.; Glien, M.; Dudda, A.; et al. Design of Novel Exendin-Based Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists. J. Med. Chem. 2017, 60, 4293–4303. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.J.; Konkar, A.; Hornigold, D.C.; Trevaskis, J.L.; Jackson, R.; Fritsch Fredin, M.; Jansson-Löfmark, R.; Naylor, J.; Rossi, A.; Bednarek, M.A.; et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016, 18, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Usui, R.; Yabe, D.; Seino, Y. Twincretin as a potential therapeutic for the management of type2 diabetes with obesity. J. Diabetes Investig. 2019, 10, 902–905. [Google Scholar] [CrossRef] [Green Version]

| Study | Population | Sample Size | Intervention | Primary Outcome | Results |
|---|---|---|---|---|---|
| SURPASS-1 (2021) | T2DM inadequately controlled with diet and exercise alone | 478 tirzepatide 5 mg (n = 121); tirzepatide 10 mg (n = 121); tirzepatide 15 mg (n = 121); placebo (n = 115) | Tirzepatide (5, 10, or 15 mg), or placebo | To assess efficacy, safety, and tolerability oftirzepatide versus placebo, and change from baseline in HbA1c timeframe: baseline week 40 | At 40 weeks, tirzepatide induced a dose-dependent decreasing of HbA1c (mean HbA1c decreased from baseline by 1.87% with tirzepatide 5 mg, 1.89% with tirzepatide 10 mg, and 2.07% with tirzepatide 15 mg versus +0·04% with placebo), and bodyweight loss ranging from 7.0 to 9.5 kg. |
| SURPASS-2 (2021) | Patients with T2DM treated with unchanged dose of metformin > 1500 mg/day for at least 3 months prior to screening | 1879 Patients are randomized in a 1:1:1:1 ratio, to receive tirzepatide at a dose of 5 mg, 10 mg, or 15 mg, or semaglutide at a dose of 1 mg. | Tirzepatide (5 mg, 10 mg, 15 mg) versus semaglutide once weekly as add-on therapy to metformin | To compare the effect of the tirzepatide to semaglutide onchange from baseline in HbA1c (10 mg and 15 mg) at 40 week. | Tirzepatide at all doses was noninferior and superior to semaglutide as regards t othe mean change in theHbA1c; instead reductions in body weight were greater with tirzepatide than with semaglutide (p < 0.001). |
| SURPASS-3 (2021) | Subjects with T2DM inadequately controlled by metformin with or without SGLT2 inhibitors | 1947 | Tirzepatide (one weekly, 5, 10, or 15 mg) versus titrated insulin degludec add-on metformin with or without SGLT2 inhibitors | Change from baseline in HbA1c at 52 week. | People with T2DMobtained better glycemic control with tirzepatide than insulin degludec, while losing rather than gaining weight. |
| SURPASS-4 (2021) | People with T2DM with increased CV risk who are treated with metformin, or a sulfonylurea or an SGLT-2 inhibitor | 2002 | Tirzepatide (5 mg, 10 mg, and 15 mg) with titrated insulin glargine | Change from baseline in HbA1c (10 mg and 15 mg) at 52 weeks, and to assess the efficacy and safety of tirzepatide taken once a week to insulin glargine taken once daily in participants with T2DMand increased cardiovascular risk. | The highest dose of tirzepatide led to an HbA1c reduction of 2.58% and reduced body weight by −11.7 kg compared with insulin glargine at 52 weeks. |
| SURPASS 5 (2021) | Patients with T2DM inadequately controlled on insulin glargine with or without metformin | 475 | Tirzepatide versus placebo in patients with T2D Minadequately controlled on insulin glargine with or without metformin | To evaluate the safety and efficacy of tirzepatide to placebo in participants with T2DMthat are already on insulin glargine, with or without metformin, and change from baseline in HbA1c (10 mg and 15 mg) at 40 weeks | Tirzepatide was associated with greater HbA1c reductions and body weight reductions than in placebo therapy. Additionally, results indicated 97% of participants receiving tirzepatide achieved an HbA1c of less than 7% and 62% achieved an HbA1c of less than 5.7%. |
| SURPASS-6 (recruiting) | T2DM inadequately controlled on insulin glargine (U100) with or without metformin | 1182 | Tirzepatide once weekly versus insulin lispro (U100) three times daily | To compare the safety and efficacy of the tirzepatide to insulin lispro (U100) three times a day in participants with T2DM that are already on insulin glargine (U100), with or without metformin, monitoring change from baseline in HbA1c | Estimated study completion date: 18 November 2022 |
| SURPASS-CVOT (Recruiting) | Patients with T2DM and increased cardiovascular risk | 12,500 | Tirzepatide (5 mg, 10 mg, 15 mg) versus dulaglutide (1.5 mg) | Time to first occurrence of death from cardiovascular (CV) causes, myocardial infarction (MI), or stroke (MACE-3) | Estimated study completion date: 17 October 2024 |
| SURPASS AP-Combo (Active, not recruiting) | Subjects with T2DM treated with metformin with or without a sulfonylurea | 917 | Tirzepatide (5 mg, 10 mg, 15 mg) once weekly versus titrated insulin glargine add-on metformin with or without a sulfonylurea | Mean change from baseline in HbA1c (10 mg and 15 mg) | Estimated study completion date: 26 November 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelle, M.C.; Provenzano, M.; Zaffina, I.; Pujia, R.; Giofrè, F.; Lucà, S.; Andreucci, M.; Sciacqua, A.; Arturi, F. Role of a Dual Glucose-Dependent Insulinotropic Peptide (GIP)/Glucagon-like Peptide-1 Receptor Agonist (Twincretin) in Glycemic Control: From Pathophysiology to Treatment. Life 2022, 12, 29. https://doi.org/10.3390/life12010029
Pelle MC, Provenzano M, Zaffina I, Pujia R, Giofrè F, Lucà S, Andreucci M, Sciacqua A, Arturi F. Role of a Dual Glucose-Dependent Insulinotropic Peptide (GIP)/Glucagon-like Peptide-1 Receptor Agonist (Twincretin) in Glycemic Control: From Pathophysiology to Treatment. Life. 2022; 12(1):29. https://doi.org/10.3390/life12010029
Chicago/Turabian StylePelle, Maria Chiara, Michele Provenzano, Isabella Zaffina, Roberta Pujia, Federica Giofrè, Stefania Lucà, Michele Andreucci, Angela Sciacqua, and Franco Arturi. 2022. "Role of a Dual Glucose-Dependent Insulinotropic Peptide (GIP)/Glucagon-like Peptide-1 Receptor Agonist (Twincretin) in Glycemic Control: From Pathophysiology to Treatment" Life 12, no. 1: 29. https://doi.org/10.3390/life12010029
APA StylePelle, M. C., Provenzano, M., Zaffina, I., Pujia, R., Giofrè, F., Lucà, S., Andreucci, M., Sciacqua, A., & Arturi, F. (2022). Role of a Dual Glucose-Dependent Insulinotropic Peptide (GIP)/Glucagon-like Peptide-1 Receptor Agonist (Twincretin) in Glycemic Control: From Pathophysiology to Treatment. Life, 12(1), 29. https://doi.org/10.3390/life12010029








