Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Color Fundus Photography (CFP) Findings
3. Optical Coherence Tomography (OCT) Findings
3.1. Drusen Volume
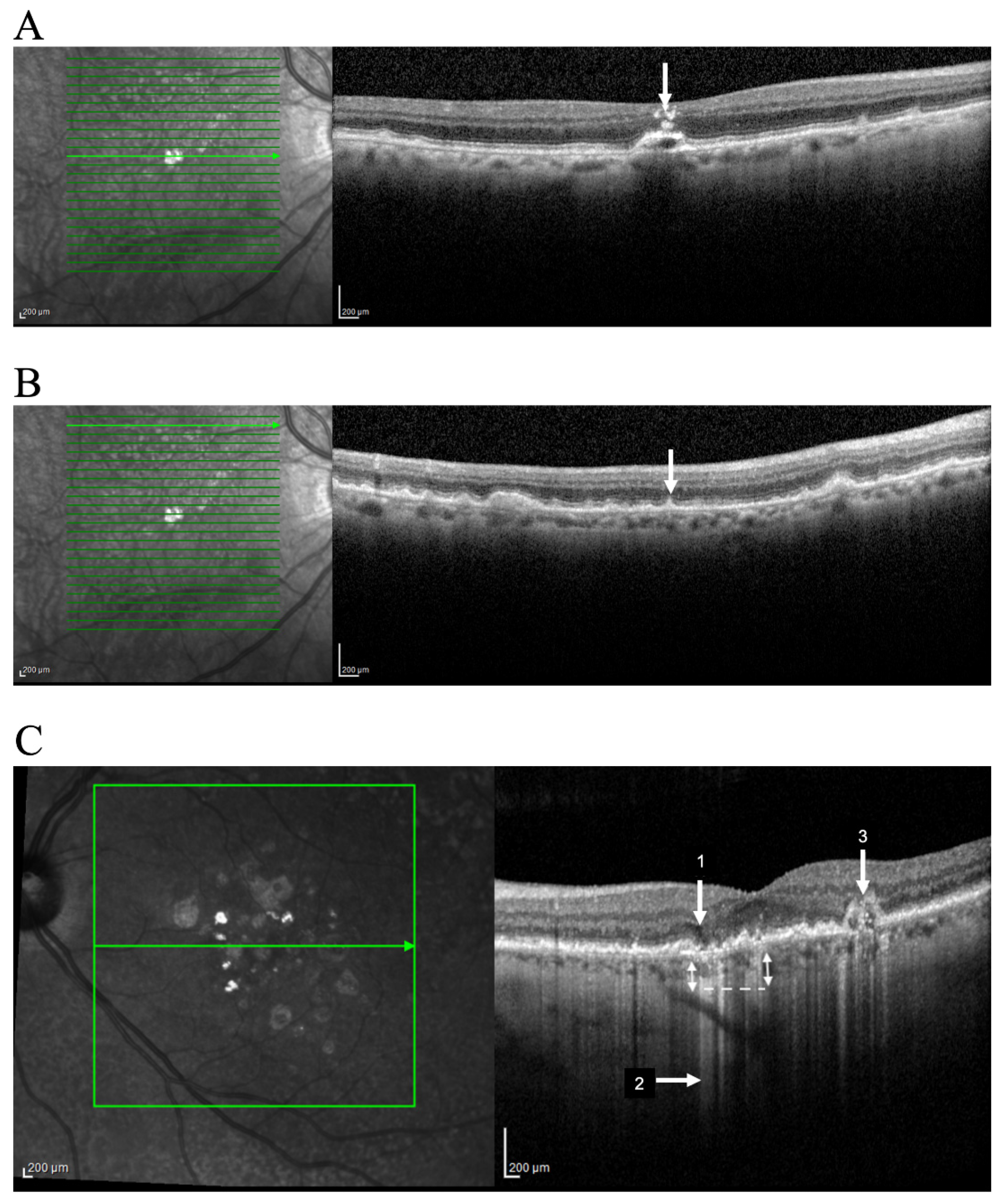
3.2. Hyper-Reflective Foci
3.3. Reticular Pseudodrusen
3.4. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy
3.5. Hyper-Transmission Defects
3.6. OCT-Reflective Drusen Substructures
3.7. Other OCT Morphologic Findings
4. Optical Coherence Tomography-Angiography Findings
5. Fundus Autofluorescence (FAF) Findings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferris, F.L., III; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Comitee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R.; Age-related eye disease study research group. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol 2005, 123, 1570–1574. [Google Scholar] [CrossRef]
- De Oliveira Dias, J.R.; Zhang, Q.; Garcia, J.M.B.; Zheng, F.; Motulsky, E.H.; Roisman, L.; Miller, A.; Chen, C.L.; Kubach, S.; de Sisternes, L.; et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology 2018, 125, 255–266. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Motulsky, E.H.; Thulliez, M.; Shi, Y.; Lyu, C.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Wang, R.K.; et al. Two-year risk of exudation in eyes with nonexudative age-related macular degeneration and subclinical neovascularization detected with swept source optical coherence tomography angiography. Am. J. Ophthalmol. 2019, 208, 1–11. [Google Scholar] [CrossRef]
- Chen, L.; Messinger, J.D.; Sloan, K.R.; Swain, T.A.; Sugiura, Y.; Yannuzzi, L.A.; Curcio, C.A.; Freund, K.B. Nonexudative macular neovascularization supporting outer retina in age-related macular degeneration: A clinicopathologic correlation. Ophthalmology 2020, 127, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Drusen characterization with multimodal imaging. Retina 2010, 30, 1441–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.; Cougnard-Gregoire, A.; Delyfer, M.N.; Combillet, F.; Rougier, M.B.; Schweitzer, C.; Dartigues, J.F.; Korobelnik, J.F.; Delcourt, C. Multimodal imaging of reticular pseudodrusen in a population-based setting: The alienor study. Invest. Ophthalmol. Vis. Sci. 2016, 57, 3058–3065. [Google Scholar] [CrossRef] [Green Version]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: Recommendations from classification of atrophy consensus meetings. Ophthalmology 2017, 124, 464–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of atrophy report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Guymer, R.H.; Rosenfeld, P.J.; Curcio, C.A.; Holz, F.G.; Staurenghi, G.; Freund, K.B.; Schmitz-Valckenberg, S.; Sparrow, J.; Spaide, R.F.; Tufail, A.; et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: Classification of atrophy meeting report 4. Ophthalmology 2020, 127, 394–409. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Chakravarthy, U.; Freund, K.B.; Guymer, R.H.; Holz, F.G.; Liakopoulos, S.; Mones, J.M.; Rosenfeld, P.J.; Sadda, S.R.; Sarraf, D.; et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration: Classification of atrophy meeting report 5. Ophthalmol. Retina 2020, 5, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pfau, M.; Blodi, B.A.; Holz, F.G.; Jaffe, G.J.; Liakopoulos, S.; Sadda, S.R.; Staurenghi, G.; Bjelopera, E.; Brown, T.; et al. OCT Signs of early atrophy in age-related macular degeneration: Interreader agreement: Classification of atrophy meetings report 6. Ophthalmol. Retina 2021. [Google Scholar] [CrossRef] [PubMed]
- Friberg, T.R.; Bilonick, R.A.; Brennen, P.M. Analysis of the relationship between drusen size and drusen area in eyes with age-related macular degeneration. Ophthalmic. Surg. Lasers Imaging 2011, 42, 369–375. [Google Scholar] [CrossRef]
- De Sisternes, L.; Simon, N.; Tibshirani, R.; Leng, T.; Rubin, D.L. Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7093–7103. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.L.; Francis, P.J.; Ferris, F.L., 3rd; Hamon, S.C.; Clemons, T.E. Risk assessment model for development of advanced age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 1543–1550. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunovic, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2017, 31, 26–44. [Google Scholar] [CrossRef]
- Nathoo, N.A.; Or, C.; Young, M.; Chui, L.; Fallah, N.; Kirker, A.W.; Albiani, D.A.; Merkur, A.B.; Forooghian, F. Optical coherence tomography-based measurement of drusen load predicts development of advanced age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 757–761 e751. [Google Scholar] [CrossRef]
- Schuman, S.G.; Koreishi, A.F.; Farsiu, S.; Jung, S.H.; Izatt, J.A.; Toth, C.A. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology 2009, 116, 488–496.e482. [Google Scholar] [CrossRef] [Green Version]
- Schlanitz, F.; Baumann, B.; Sacu, S.; Baumann, L.; Pircher, M.; Hitzenberger, C.K.; Schmidt-Erfurth, U.M. Impact of drusen and drusenoid retinal pigment epithelium elevation size and structure on the integrity of the retinal pigment epithelium layer. Br. J. Ophthalmol. 2019, 103, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Friberg, T.R.; Bilonick, R.A.; Brennen, P. Is drusen area really so important? An assessment of risk of conversion to neovascular AMD based on computerized measurements of drusen. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1742–1751. [Google Scholar] [CrossRef]
- Yehoshua, Z.; Gregori, G.; Sadda, S.R.; Penha, F.M.; Goldhardt, R.; Nittala, M.G.; Konduru, R.K.; Feuer, W.J.; Gupta, P.; Li, Y.; et al. Comparison of drusen area detected by spectral domain optical coherence tomography and color fundus imaging. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2429–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, B.; Ribeiro, R.; Heussen, F.M.; Maia, M.; Sadda, S. Drusen measurements comparison by fundus photograph manual delineation versus optical coherence tomography retinal pigment epithelial segmentation automated analysis. Retina 2014, 34, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Curcio, C.A.; Mullins, R.F.; Spaide, R.F. REFRACTILE DRUSEN: Clinical Imaging and Candidate Histology. Retina 2015, 35, 859–865. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Yannuzzi, L.A.; Curcio, C.A.; Morgan, W.H.; Querques, G.; Capuano, V.; Souied, E.; Jung, J.; Freund, K.B. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Nassisi, M.; Lei, J.; Abdelfattah, N.S.; Karamat, A.; Balasubramanian, S.; Fan, W.; Uji, A.; Marion, K.M.; Baker, K.; Huang, X.; et al. OCT Risk factors for development of late age-related macular degeneration in the fellow eyes of patients enrolled in the Harbor study. Ophthalmology 2019, 126, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Christenbury, J.G.; Folgar, F.A.; O’Connell, R.V.; Chiu, S.J.; Farsiu, S.; Toth, C.A.; Age-related eye disease study 2 ancillary spectral domain optical coherence tomography study group. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 2013, 120, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Sadda, S.R.; Abdelfattah, N.S.; Lei, J.; Shi, Y.; Marion, K.M.; Morgenthien, E.; Gune, S.; Balasubramanian, S. Spectral-domain OCT analysis of risk factors for macular atrophy development in the Harbor study for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 1360–1370. [Google Scholar] [CrossRef]
- Dieaconescu, D.A.; Dieaconescu, I.M.; Williams, M.A.; Hogg, R.E.; Chakravarthy, U. Drusen height and width are highly predictive markers for progression to neovascular AMD. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2910. [Google Scholar]
- Yehoshua, Z.; Wang, F.; Rosenfeld, P.J.; Penha, F.M.; Feuer, W.J.; Gregori, G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology 2011, 118, 2434–2441. [Google Scholar] [CrossRef] [Green Version]
- Corvi, F.; Srinivas, S.; Nittala, M.G.; Corradetti, G.; Velaga, S.B.; Stambolian, D.; Haines, J.; Pericak-Vance, M.A.; Sadda, S.R. Reproducibility of qualitative assessment of drusen volume in eyes with age related macular degeneration. Eye 2021, 35, 2594–2600. [Google Scholar] [CrossRef]
- Schlanitz, F.G.; Baumann, B.; Kundi, M.; Sacu, S.; Baratsits, M.; Scheschy, U.; Shahlaee, A.; Mittermuller, T.J.; Montuoro, A.; Roberts, P.; et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br. J. Ophthalmol. 2017, 101, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.; Rodger, D.C.; Chavali, V.R.; MacKay, T.; Lee, S.Y.; Stambolian, D.; Sadda, S.V. Drusen and RPE atrophy automated quantification by optical coherence tomography in an elderly population. Eye 2015, 29, 272–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Filho, C.A.; Yehoshua, Z.; Gregori, G.; Nunes, R.P.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Feuer, W.; Rosenfeld, P.J. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic. Surg. Lasers Imaging Retina 2014, 45, 18–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavali, V.R.; Diniz, B.; Huang, J.; Ying, G.S.; Sadda, S.R.; Stambolian, D. Association of OCT derived drusen measurements with AMD associated-genotypic SNPs in Amish population. J. Clin. Med. 2015, 4, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Folgar, F.A.; Yuan, E.L.; Sevilla, M.B.; Chiu, S.J.; Farsiu, S.; Chew, E.Y.; Toth, C.A. Age related eye disease study 2 ancillary spectral-domain optical coherence tomography study, G. drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology 2016, 123, 39–50.e31. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Zhang, H.; Boyer, D.S.; Rosenfeld, P.J.; Feuer, W.J.; Gregori, G.; Sadda, S.R. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1839–1846. [Google Scholar] [CrossRef]
- Beck, M.; Joshi, D.S.; Berger, L.; Klose, G.; De Zanet, S.; Mosinska, A.; Apostolopoulos, S.; Ebneter, A.; Zinkernagel, M.S.; Wolf, S.; et al. Comparison of drusen volume assessed by two different OCT devices. J. Clin. Med. 2020, 9, 2657. [Google Scholar] [CrossRef]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar] [CrossRef]
- Coscas, G.; De Benedetto, U.; Coscas, F.; Li Calzi, C.I.; Vismara, S.; Roudot-Thoraval, F.; Bandello, F.; Souied, E. Hyperreflective dots: A new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica 2013, 229, 32–37. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Wang, X.; Clark, M.E.; McGwin, G., Jr.; Owsley, C.; Curcio, C.A.; Zhang, Y. Retinal pigment epithelium degeneration associated with subretinal drusenoid deposits in age-related macular degeneration. Am. J. Ophthalmol. 2017, 175, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, S.A.; Imamura, Y.; Spaide, T.C.; Fujiwara, T.; Spaide, R.F. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010, 117, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Yapp, M.; Nivison-Smith, L.; Assaad, N.; Hennessy, M.; Kalloniatis, M. Developing prognostic biomarkers in intermediate age-related macular degeneration: Their clinical use in predicting progression. Clin. Exp. Optom. 2018, 101, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mones, J.; Biarnes, M.; Trindade, F. Hyporeflective wedge-shaped band in geographic atrophy secondary to age-related macular degeneration: An underreported finding. Ophthalmology 2012, 119, 1412–1419. [Google Scholar] [CrossRef]
- Wu, Z.; Luu, C.D.; Ayton, L.N.; Goh, J.K.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology 2014, 121, 2415–2422. [Google Scholar] [CrossRef]
- Thiele, S.; Pfau, M.; Larsen, P.P.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Multimodal imaging patterns for development of central atrophy secondary to age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD1–AMD11. [Google Scholar] [CrossRef]
- Ooto, S.; Vongkulsiri, S.; Sato, T.; Suzuki, M.; Curcio, C.A.; Spaide, R.F. Outer retinal corrugations in age-related macular degeneration. JAMA Ophthalmol. 2014, 132, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Padnick-Silver, L.; Weinberg, A.B.; Lafranco, F.P.; Macsai, M.S. Pilot study for the detection of early exudative age-related macular degeneration with optical coherence tomography. Retina 2012, 32, 1045–1056. [Google Scholar] [CrossRef]
- Veerappan, M.; El-Hage-Sleiman, A.M.; Tai, V.; Chiu, S.J.; Winter, K.P.; Stinnett, S.S.; Hwang, T.S.; Hubbard, G.B., III; Michelson, M.; Gunther, R.; et al. Optical coherence tomography reflective drusen substructures predict progression to geographic atrophy in age-related macular degeneration. Ophthalmology 2016, 123, 2554–2570. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.S.; Pilgrim, M.G.; Fearn, S.; Bertazzo, S.; Tsolaki, E.; Morrell, A.P.; Li, M.; Messinger, J.D.; Dolz-Marco, R.; Lei, J.; et al. Calcified nodules in retinal drusen are associated with disease progression in age-related macular degeneration. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, D.; Silver, R.E.; Louzada, R.N.; Novais, E.A.; Collins, G.K.; Seddon, J.M. Optical coherence tomography features preceding the onset of advanced age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3519–3529. [Google Scholar] [CrossRef]
- Querques, G.; Srour, M.; Massamba, N.; Georges, A.; Ben Moussa, N.; Rafaeli, O.; Souied, E.H. Functional characterization and multimodal imaging of treatment-naive "quiescent" choroidal neovascularization. Investig. Ophthalmol Vis. Sci 2013, 54, 6886–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roisman, L.; Zhang, Q.; Wang, R.K.; Gregori, G.; Zhang, A.; Chen, C.L.; Durbin, M.K.; An, L.; Stetson, P.F.; Robbins, G.; et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology 2016, 123, 1309–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laiginhas, R.; Yang, J.; Rosenfeld, P.J.; Falcao, M. Nonexudative macular neovascularization—A systematic review of prevalence, natural history, and recent insights from OCT angiography. Ophthalmol. Retina 2020, 4, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ooto, S.; Curcio, C.A. Subretinal drusenoid deposits AKA pseudodrusen. Surv. Ophthalmol. 2018, 63, 782–815. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Messinger, J.D.; Sloan, K.R.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Histologic and optical coherence tomographic correlates in drusenoid pigment epithelium detachment in age-related macular degeneration. Ophthalmology 2017, 124, 644–656. [Google Scholar] [CrossRef]
- Ouyang, Y.; Heussen, F.M.; Hariri, A.; Keane, P.A.; Sadda, S.R. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology 2013, 120, 2656–2665. [Google Scholar] [CrossRef] [Green Version]
- Pfau, M.; Moller, P.T.; Kunzel, S.H.; von der Emde, L.; Lindner, M.; Thiele, S.; Dysli, C.; Nadal, J.; Schmid, M.; Schmitz-Valckenberg, S.; et al. Type 1 choroidal neovascularization is associated with reduced localized progression of atrophy in age-related macular degeneration. Ophthalmol. Retina 2020, 4, 238–248. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Q.; Yang, J.; Zhou, H.; Chu, Z.; Zhou, X.; Feuer, W.; Jiang, X.; Shi, Y.; de Sisternes, L.; et al. Swept-source OCT angiographic characteristics of treatment-naive nonexudative macular neovascularization in AMD prior to exudation. Invest. Ophthalmol. Vis. Sci. 2021, 62, 14. [Google Scholar] [CrossRef]
- Malamos, P.; Tsolkas, G.; Kanakis, M.; Mylonas, G.; Karatzenis, D.; Oikonomopoulos, N.; Lakoumentas, J.; Georgalas, I. OCT-angiography for monitoring and managing neovascular age-related macular degeneration. Curr. Eye Res. 2017, 42, 1689–1697. [Google Scholar] [CrossRef]
- Marques, J.; Silva, R. Optical coherence tomography angiography in wet age-related macular degeneration (AMD). Eye Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Messinger, J.D.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Stages of drusen-associated atrophy in age-related macular degeneration visible via histologically validated fundus autofluorescence. Ophthalmol. Retina 2021, 5, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Bindewald-Wittich, A.; Fleckenstein, M.; Dreyhaupt, J.; Scholl, H.P.; Schmitz-Valckenberg, S.; Group, F.A.-S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Cachulo, L.; Silva, R.; Fonseca, P.; Pires, I.; Carvajal-Gonzalez, S.; Bernardes, R.; Cunha-Vaz, J.G. Early markers of choroidal neovascularization in the fellow eye of patients with unilateral exudative age-related macular degeneration. Ophthalmologica 2011, 225, 144–149. [Google Scholar] [CrossRef]
- Corradetti, G.; Corvi, F.; Nittala, M.G.; Nassisi, M.; Alagorie, A.R.; Scharf, J.; Lee, M.Y.; Sadda, S.R.; Sarraf, D. Natural history of incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration. Can. J. Ophthalmol. 2021, 56, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Freund, K.B.; Curcio, C.A. The Project MACULA Retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3253–3268. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Dolz-Marco, R.; Messinger, J.D.; Wang, L.; Feist, R.M.; Girkin, C.A.; Gattoussi, S.; Ferrara, D.; Curcio, C.A.; Freund, K.B. Clinicopathologic correlation of anti-vascular endothelial growth factor-treated type 3 neovascularization in age-related macular degeneration. Ophthalmology 2018, 125, 276–287. [Google Scholar] [CrossRef]
- Lei, J.; Balasubramanian, S.; Abdelfattah, N.S.; Nittala, M.G.; Sadda, S.R. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1551–1558. [Google Scholar] [CrossRef]
- Sleiman, K.; Veerappan, M.; Winter, K.P.; McCall, M.N.; Yiu, G.; Farsiu, S.; Chew, E.Y.; Clemons, T.; Toth, C.A. Age-related eye disease study 2 ancillary spectral domain optical coherence tomography study, G. optical coherence tomography predictors of risk for progression to non-neovascular atrophic age-related macular degeneration. Ophthalmology 2017, 124, 1764–1777. [Google Scholar] [CrossRef]
- Bogunovic, H.; Montuoro, A.; Baratsits, M.; Karantonis, M.G.; Waldstein, S.M.; Schlanitz, F.; Schmidt-Erfurth, U. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest. Ophthalmol. Vis. Sci. 2017, 58, BIO141–BIO150. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunovic, H. Prediction of individual disease conversion in early AMD using artificial intelligence. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldstein, S.M.; Vogl, W.D.; Bogunovic, H.; Sadeghipour, A.; Riedl, S.; Schmidt-Erfurth, U. Characterization of drusen and hyperreflective foci as biomarkers for disease progression in age-related macular degeneration using artificial intelligence in optical coherence tomography. JAMA Ophthalmol. 2020, 138, 740–747. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Imaging Findings | Mechanism(s) | Prevalence in AMD% | Expected Progression (OR 1) |
|---|---|---|---|---|
| Drusen volume | Baseline drusen volume | Displacement or deterioration of photoreceptor layer | ND 2 | 1.31 risk of progression to nAMD (for each 0.1 mm3 of drusen volume increase) [36] |
| RPE-Drusen complex (DC) Advanced analysis | RAT 3 | RPE suffering and drusen regression | ND 2 | 1.32 risk of developing central GA (for each 0.001 mm3 increase in RAT volume) [36] |
| HRF | Punctate hyperreflective lesions | Anterior migration of fully pigmentated RPE cells, inflammatory or microglia cell and calcification | 50% in AMD | 5 risk of 2-year progression to GA [27] |
| SDD | Small yellow deposits: reticular, ribbon-like or interdigitated | Dysfunction of cholesterol homeostasis, retinoid processing or choroidal hypoxia [55] | 32% to 79% in AMD patients | 2.24–3.4 risk of progression to advanced disease [1,42] |
| iRORA | Subsidence of the OPL 4 and INL 5 with a hypo-reflective wedge | New onset of atrophy (nascent atrophy) | 7% in intermediate AMD [56] | 5.2 risk of progression to central GA [45] |
| Hypertransmission | Columns or strips of hyperreflectivity | Deficiencies within RPE layer | 27% in AMD patients [48] | ND |
| ODS | Internal heterogeneity | Metabolic instability | 24% in soft drusen | 5.6 risk of progression to new atrophy onset [57] |
| Non exudative Retinal neovascularization | Neovascular lesion with no fluid | Protective mechanism against ischemia | 6.25 to 27% in the fellow eye of exudative AMD [54] | 1.21 risk of progression to exudative AMD at 1 year [52] |
| Strength of Evidence | |||
|---|---|---|---|
| High | Low | ||
| Strength of Risk | High | HRF OR 1 = 4.72 to central GA, 2 years 95% CI 2: 2.43–9.80, p < 0.001 [39] SDD OR 1 = 2.64 to late AMD, 2 years 95% CI 2: 1.07–6.49, p = 0.034 [42] | OCT drusen substructures OR 1 = 5.614 for new atrophy onset, 6 months 95% CI 2: 1.277–24.673, p < 0.001 [57] iRORA + HRF OR 1 = 10 to cRORA, 2 years 95% CI: 2.36–42.20, p = 0.002 [45,65] iRORA + SDD OR 1 = 1.9 to cRORA, 2 years 95% CI: 0.60–6.2, p = 0.263 data [45,65] |
| Low | Drusen volume OR 1 1.31 to late AMD, 2 years 95% CI 2: 1.06–1.63; p = 0.013 [13] RPE Drusen Complex OR 1 = 1.32 to central GA, 2 years 95% CI 2: 1.14–1.53; p < 0.001 [27] | Hypertransmission OR 1 ND 3 [48] Ellipsoid zone disruption OR 1 ND 3 [51] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, R.; Carneiro, Â.; Tenreiro, S.; Seabra, M.C. Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration. Life 2022, 12, 36. https://doi.org/10.3390/life12010036
Flores R, Carneiro Â, Tenreiro S, Seabra MC. Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration. Life. 2022; 12(1):36. https://doi.org/10.3390/life12010036
Chicago/Turabian StyleFlores, Rita, Ângela Carneiro, Sandra Tenreiro, and Miguel C. Seabra. 2022. "Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration" Life 12, no. 1: 36. https://doi.org/10.3390/life12010036
APA StyleFlores, R., Carneiro, Â., Tenreiro, S., & Seabra, M. C. (2022). Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration. Life, 12(1), 36. https://doi.org/10.3390/life12010036








