Recurrent Implantation Failure—Is It the Egg or the Chicken?
Abstract
:1. Introduction
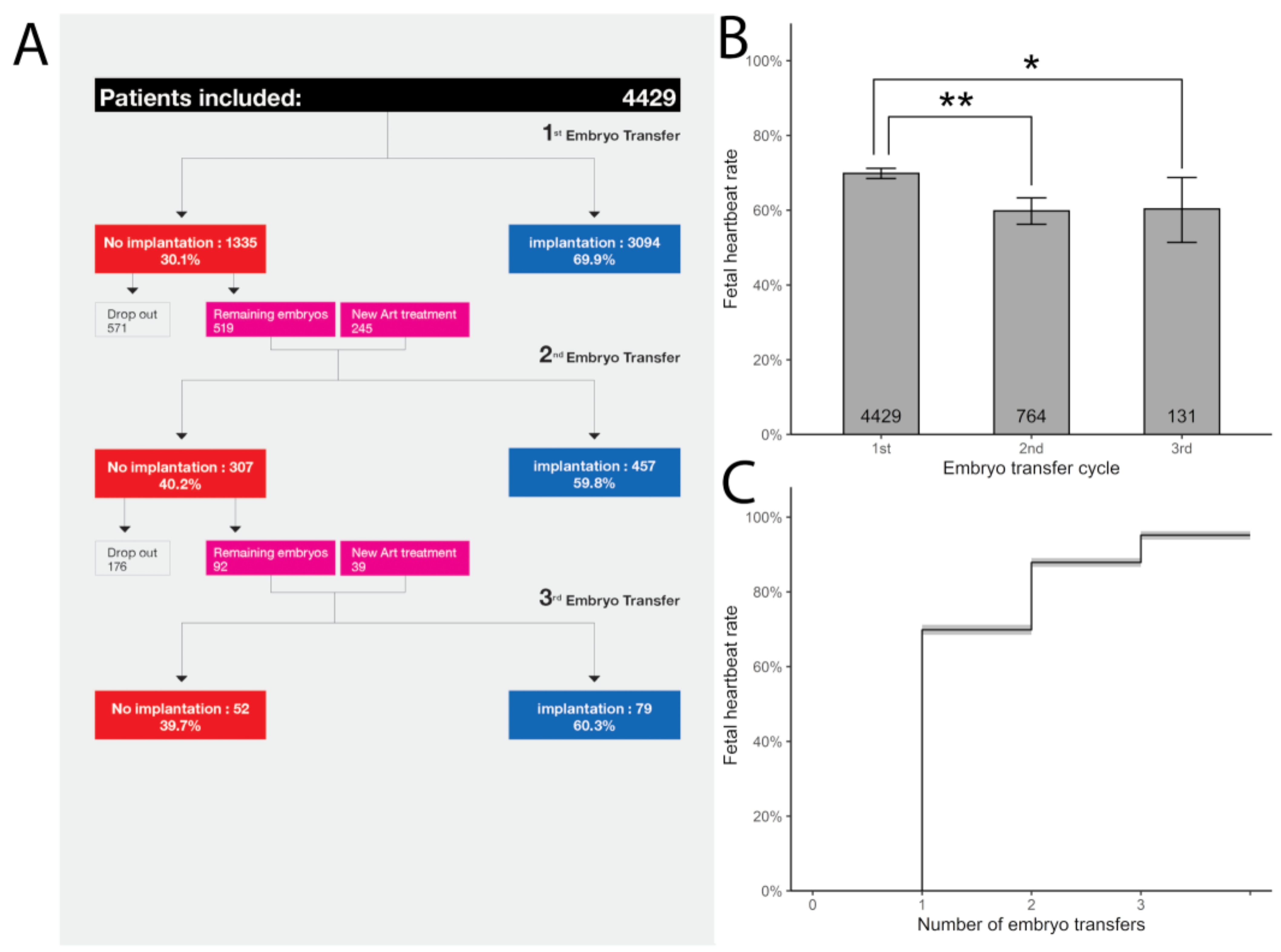
2. RIF: Is it the Egg or the Chicken?
3. RIF and the Endometrium
4. RIF and the Embryo
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirtea, P.; Scott, R.T., Jr.; de Ziegler, D.; Ayoubi, J.M. Recurrent implantation failure: How common is it? Curr. Opin. Obstet. Gynecol. 2021, 33, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Polanski, L.T.; Baumgarten, M.N.; Quenby, S.; Brosens, J.; Campbell, B.K.; Raine-Fenning, N.J. What exactly do we mean by ‘recurrent implantation failure’? A systematic review and opinion. Reprod. Biomed. Online 2014, 28, 409–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.B.; Bouaziz, J.; Bar On, A.; Orvieto, R. Fertility success rates in patients with secondary infertility and symptomatic cesarean scar niche undergoing hysteroscopic niche resection. Gynecol. Endocrinol. 2020, 36, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Bosdou, J.K.; Venetis, C.A.; Tarlatzis, B.C.; Grimbizis, G.F.; Kolibianakis, E.M. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: A meta-analysis. Hum. Reprod. 2019, 34, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, K.; Fauser, B.; Devroey, P.; Griesinger, G. The role of the endometrium and embryo in human implantation. Hum. Reprod. Updat. 2007, 13, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Pertile, M.; Norris, H.; Hale, L.; Baker, H. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum. Reprod. 1999, 14, 2097–2101. [Google Scholar] [CrossRef] [Green Version]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef]
- Harper, J.; Coonen, E.; De Rycke, M.; Fiorentino, F.; Geraedts, J.; Goossens, V.; Harton, G.; Moutou, C.; Budak, T.P.; Renwick, P.; et al. What next for preimplantation genetic screening (PGS)? A position statement from the ESHRE PGD Consortium steering committee. Hum. Reprod. 2010, 25, 821–823. [Google Scholar] [CrossRef] [Green Version]
- Munné, S.; Kaplan, B.; Frattarelli, J.L.; Child, T.; Nakhuda, G.; Shamma, F.N.; Silverberg, K.; Kalista, T.; Handyside, A.H.; Katz-Jaffe, M.; et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: A multicenter randomized clinical trial. Fertil. Steril. 2019, 112, 1071–1079.e7. [Google Scholar] [CrossRef]
- Forman, E.J.; Hong, K.H.; Ferry, K.M.; Tao, X.; Taylor, D.; Levy, B.; Treff, N.R.; Scott, R.T., Jr. In vitro fertilization with single euploid blastocyst transfer: A randomized controlled trial. Fertil. Steril. 2013, 100, 100–107.e1. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liu, J.; Collins, G.S.; Salem, S.A.; Liu, X.; Lyle, S.S.; Peck, A.C.; Sills, E.S.; Salem, R.D. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: Results from a randomized pilot study. Mol. Cytogenet. 2012, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.T., Jr.; Upham, K.M.; Forman, E.J.; Hong, K.H.; Scott, K.L.; Taylor, D.; Tao, X.; Treff, N.R. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: A randomized controlled trial. Fertil. Steril. 2013, 100, 697–703. [Google Scholar] [CrossRef]
- Rubio, C.; Bellver, J.; Rodrigo, L.; Castillón, G.; Guillén, A.; Vidal, C.; Giles, J.; Ferrando, M.; Cabanillas, S.; Remohí, J.; et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: A randomized, controlled study. Fertil. Steril. 2017, 107, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
- Practice Committee of the American Society for Reproductive Medicine. Guidance on the limits to the number of embryos to transfer: A committee opinion. Fertil. Steril. 2017, 107, 901–903. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, B.S.; Daneshmand, S.T.; Desai, J.; Garner, F.C.; Aguirre, M.; Hudson, C. The risk of embryo-endometrium asynchrony increases with maternal age after ovarian stimulation and IVF. Reprod. Biomed. Online 2016, 33, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Chen, J.J.; Nabu, S.; Yeung, Q.S.Y.; Li, Y.; Tan, J.H.; Suksalak, W.; Chanchamroen, S.; Quangkananurug, W.; Wong, P.S.; et al. The pregnancy outcome of mosaic embryo transfer: A prospective multicenter study and meta-analysis. Genes 2020, 11, 973. [Google Scholar] [CrossRef]
- Simon, A.; Laufer, N. Assessment and treatment of repeated implantation failure (RIF). J. Assist. Reprod. Genet. 2012, 29, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Mahdian, S.; Pirjani, R.; Favaedi, R.; Movahedi, M.; Moini, A.; Shahhoseini, M. Platelet-activating factor and antiphospholipid antibodies in recurrent implantation failure. J. Reprod. Immunol. 2021, 143, 103251. [Google Scholar] [CrossRef]
- Sauer, R.; Roussev, R.; Jeyendran, R.S.; Coulam, C.B. Prevalence of antiphospholipid antibodies among women experiencing unexplained infertility and recurrent implantation failure. Fertil. Steril. 2010, 93, 2441–2443. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Hornstein, M.D.; Davis, O.K.; Massey, J.B.; Paulson, R.J.; Collins, J.A. Antiphospholipid antibodies and in vitro fertilization success: A meta-analysis. Fertil. Steril. 2020, 73, 330–333. [Google Scholar] [CrossRef]
- Arachchillage, D.R.; Mackie, I.J.; Efthymiou, M.; Isenberg, D.A.; Machin, S.J.; Cohen, H. Interactions between rivaroxaban and antiphospholipid antibodies in thrombotic antiphospholipid syndrome. J. Thromb. Haemost. 2015, 13, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Macklon, N.S.; Brosens, J. The human endometrium as a sensor of embryo quality. Biol. Reprod. 2014, 91, 98. [Google Scholar] [CrossRef]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 202–223. [Google Scholar] [CrossRef]
- Rock, J.; Bartlett, M.K. Biopsy studies of human endometrium: Criteria of dating and information about amenorrhea, menorrhagia, and time of ovulation. J. Am. Med. Assoc. 1937, 108, 2022–2028. [Google Scholar] [CrossRef]
- Noyes, R.; Hertig, A.; Rock, J. Reprint of: Dating the endometrial biopsy. Fertil. Steril. 2019, 112, e93–e115. [Google Scholar] [CrossRef] [Green Version]
- Bassil, R.; Casper, R.; Samara, N.; Hsieh, T.-B.; Barzilay, E.; Orvieto, R.; Haas, J. Does the endometrial receptivity array really provide personalized embryo transfer? J. Assist. Reprod. Genet. 2018, 35, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Alonso, M.; Blesa, D.; Gimeno, P.D.; Gómez, E.; Fernández-Sánchez, M.; Carranza, F.; Carrera, J.; Vilella, F.; Pellicer, A.; Simón, C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 2013, 100, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Koizumi, M.; Doshida, M.; Toya, M.; Sagara, E.; Oka, N.; Nakajo, Y.; Aono, N.; Igarashi, H.; Kyono, K. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: A retrospective, two-centers study. Reprod. Med. Biol. 2017, 16, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Simón, C.; Gómez, C.; Cabanillas, S.; Vladimirov, I.; Castillón, G.; Giles, J.; Boynukalin, K.; Findikli, N.; Bahçeci, M.; Ortega, I.; et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod. Biomed. Online 2020, 41, 402–415. [Google Scholar] [CrossRef]
- Neves, A.R.; Devesa, M.; Martínez, F.; Garcia-Martinez, S.; Rodriguez, I.; Polyzos, N.P.; Coroleu, B. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? J. Assist. Reprod. Genet. 2019, 36, 1901–1908. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.W.; Lessey, B.A. KRAS Activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef]
- Almquist, L.D.; Likes, C.E.; Stone, B.; Brown, K.R.; Savaris, R.; Forstein, D.A.; Miller, P.B.; Lessey, B.A. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: A cohort study. Fertil. Steril. 2017, 108, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Likes, C.E.; Cooper, L.J.; Efird, J.; Forstein, D.A.; Miller, P.B.; Savaris, R.; Lessey, B.A. Medical or surgical treatment before embryo transfer improves outcomes in women with abnormal endometrial BCL6 expression. J. Assist. Reprod. Genet. 2019, 36, 483–490. [Google Scholar] [CrossRef]
- Lédée, N.; Petitbarat, M.; Prat-Ellenberg, L.; Dray, G.; Cassuto, G.N.; Chevrier, L.; Kazhalawi, A.; Vezmar, K.; Chaouat, G. Endometrial immune profiling: A method to design personalized care in assisted reproductive medicine. Front. Immunol. 2020, 11, 1032. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Kushnir, V.A.; Solouki, S.; Sarig-Meth, T.; Vega, M.G.; Albertini, D.F.; Darmon, S.K.; Deligdisch, L.; Barad, D.H.; Gleicher, N. Systemic inflammation and autoimmunity in women with chronic endometritis. Am. J. Reprod. Immunol. 2016, 75, 672–677. [Google Scholar] [CrossRef]
- Bouet, P.E.; El Hachem, H.; Monceau, E.; Gariépy, G.; Kadoch, I.J.; Sylvestre, C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: Prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil. Steril. 2016, 105, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.Y.; Jayaprakasan, K.; Tan, A.; Thornton, J.G.; Coomarasamy, A.; Raine-Fenning, N.J. Reproductive outcomes in women with congenital uterine anomalies: A systematic review. Ultrasound Obstet. Gynecol. 2011, 38, 371–382. [Google Scholar] [CrossRef]
- Simon, C. Introduction: Do microbes in the female reproductive function matter? Fertil. Steril. 2018, 110, 325–326. [Google Scholar] [CrossRef] [Green Version]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rouholamin, S.; Rezaeinejad, M.; Maroufizadeh, S.; Sepidarkish, M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 137, 103078. [Google Scholar] [CrossRef]
- Pirtea, P.; De Ziegler, D.; Tao, X.; Sun, L.; Zhan, Y.; Ayoubi, J.M.; Seli, E.; Franasiak, J.M.; Scott, R.T., Jr. Rate of true recurrent implantation failure is low: Results of three successive frozen euploid single embryo transfers. Fertil. Steril. 2021, 115, 45–53. [Google Scholar] [CrossRef]
- Gimeno, P.D.; Horcajadas, J.A.; Martínez-Conejero, J.A.; Esteban, F.J.; Alamá, P.; Pellicer, A.; Simón, C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011, 95, 50–60.e15. [Google Scholar] [CrossRef]
- Patel, J.A.; Patel, A.J.; Banker, J.M.; Shah, S.I.; Banker, M. Personalized embryo transfer helps in improving In vitro fertilization/ICSI outcomes in patients with recurrent implantation failure. J. Hum. Reprod. Sci. 2019, 12, 59–66. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, L.M.; Colucci, F. Uterine natural killer cells: Functional distinctions and influence on pregnancy in humans and mice. Front. Immunol. 2017, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Paria, B.C.; Song, H.; Dey, S.K. Implantation: Molecular basis of embryo-uterine dialogue. Int. J. Dev. Biol. 2001, 45, 597–605. [Google Scholar] [PubMed]
- Lessey, B.A.; Kim, J.J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil. Steril. 2017, 108, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lédée, N.; Prat-Ellenberg, L.; Chevrier, L.; Balet, R.; Simon, C.; Lenoble, C.; El Irani, E.; Bouret, D.; Cassuto, G.; Vitoux, D.; et al. Uterine immune profiling for increasing live birth rate: A one-to-one matched cohort study. J. Reprod. Immunol. 2017, 119, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Evans-Hoeker, E.; Lessey, B.A.; Jeong, J.W.; Savaris, R.F.; Palomino, W.A.; Yuan, L.; Schammel, D.P.; Young, S.L. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod. Sci. 2016, 23, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Lédée, N.; Petitbarat, M.; Chevrier, L.; Vitoux, D.; Vezmar, K.; Rahmati, M.; Dubanchet, S.; Gahéry, H.; Bensussan, A.; Chaouat, G. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef] [Green Version]
- Haouzi, D.; Dechaud, H.; Assou, S.; DE Vos, J.; Hamamah, S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod. Biomed. Online 2012, 24, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Griffin, D.K.; Fishel, S.; Gordon, T.; Yaron, Y.; Grifo, J.; Hourvitz, A.; Rechitsky, S.; Elson, J.; Blazek, J.; Fiorentino, F.; et al. Continuing to deliver: The evidence base for pre-implantation genetic screening. BMJ 2017, 356, j752. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano, P.; Busnelli, A.; Paffoni, A.; Vegetti, W.; Vercellini, P. Repeated implantation failure at the crossroad between statistics, clinics and over-diagnosis. Reprod. Biomed. Online 2018, 36, 32–38. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirtea, P.; de Ziegler, D.; Ayoubi, J.M. Recurrent Implantation Failure—Is It the Egg or the Chicken? Life 2022, 12, 39. https://doi.org/10.3390/life12010039
Pirtea P, de Ziegler D, Ayoubi JM. Recurrent Implantation Failure—Is It the Egg or the Chicken? Life. 2022; 12(1):39. https://doi.org/10.3390/life12010039
Chicago/Turabian StylePirtea, Paul, Dominique de Ziegler, and Jean Marc Ayoubi. 2022. "Recurrent Implantation Failure—Is It the Egg or the Chicken?" Life 12, no. 1: 39. https://doi.org/10.3390/life12010039





