Assessment of a Novel Fixation Method of a Frameless Intrauterine Contraceptive Device Inserted during Cesarean Delivery as a Means of Preventing Displacements and Expulsions: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Enrollment
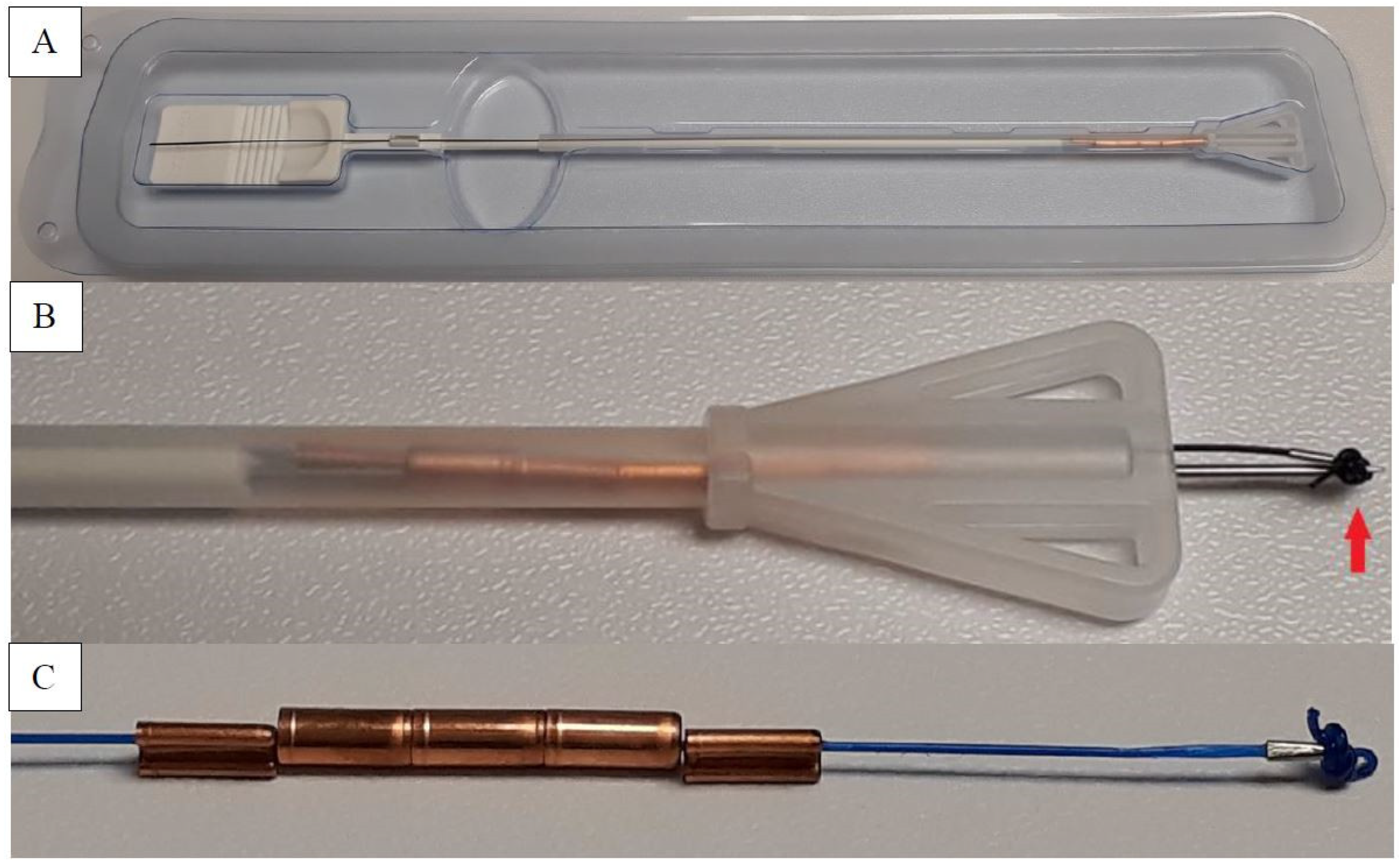
2.3. The Device
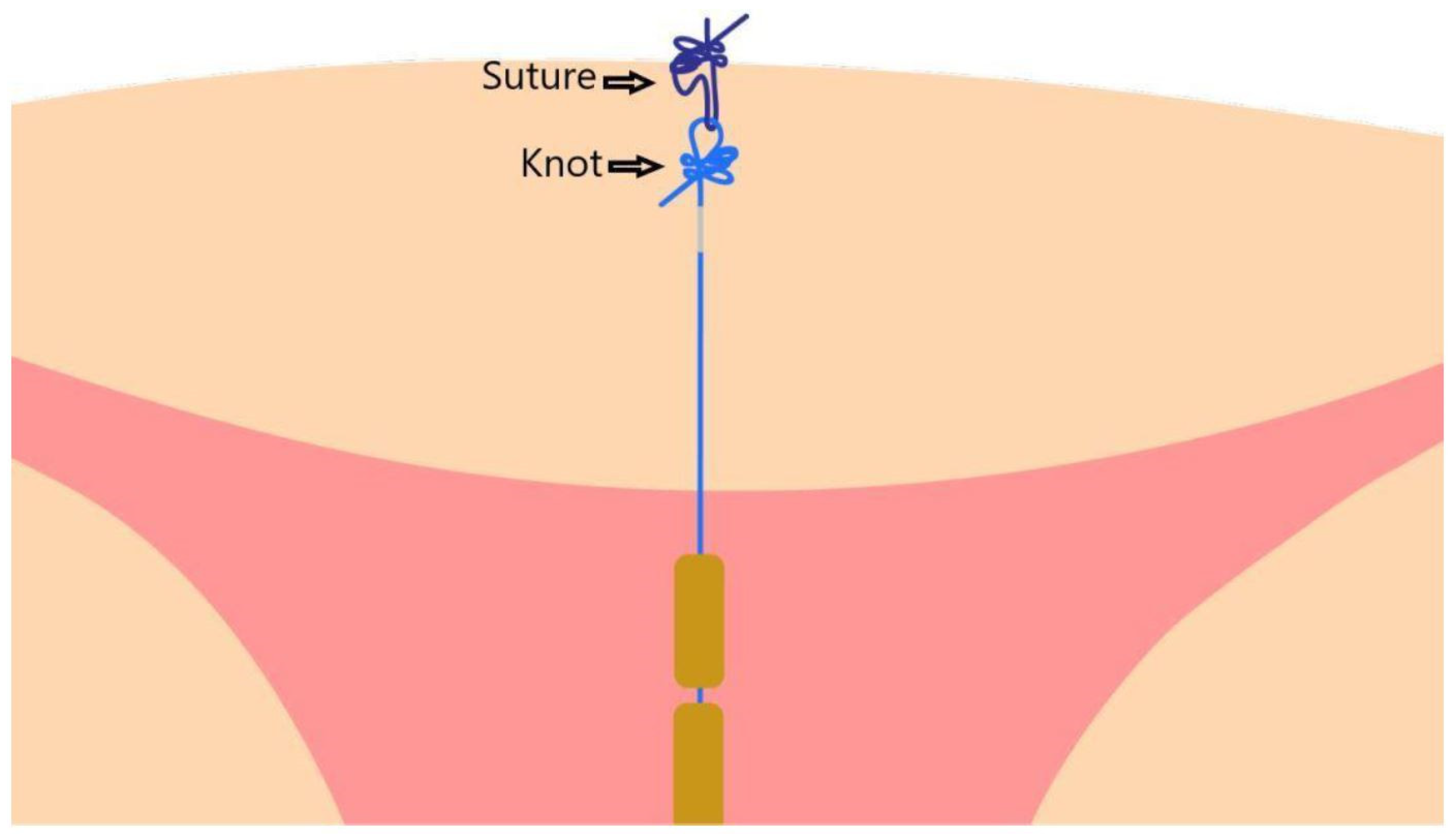
2.4. Insertion Technique
2.5. Follow-Up Examinations
2.6. Data Collection, Monitoring, and Analysis
3. Results
4. Discussion
Study Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Postpartum Family Planning: Essential for Ensuring Health of Women and Their Babies. 2018. Available online: https://www.who.int/reproductivehealth/topics/family_planning/world-contraception-day-2018/en/ (accessed on 3 January 2022).
- Bujold, E.; Gauthier, R.J. Risk of uterine rupture associated with an interdelivery interval between 18 and 24 months. Obstet. Gynecol. 2010, 115, 1003–1006. [Google Scholar] [CrossRef]
- Fotso, J.C.; Cleland, J.; Mberu, B.; Mutua, M.; Elungata, P. Birth spacing and child mortality: An analysis of prospective data from the Nairobi urban health and demographic surveillance system. J. Biosoc. Sci. 2013, 45, 779–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadi, A.F. A systematic review and meta-analysis of the effect of short birth interval on infant mortality in Ethiopia. PLoS ONE 2015, 10, e0126759. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Rosas-Bermudez, A.; Castaño, F.; Norton, M.H. Effects of birth spacing on maternal, perinatal, infant, and child health: A systematic review of causal mechanisms. Stud. Fam. Plan. 2012, 43, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Peipert, J.F.; Zhao, Q.; Buckel, C.; Madden, T.; Allsworth, J.E.; Secura, G.M. Effectiveness of long-acting reversible contraception. N. Engl. J. Med. 2012, 366, 1998–2007. [Google Scholar] [CrossRef] [Green Version]
- Trussell, J. Contraceptive failure in the United States. Contraception 2011, 83, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattinson, A.; Mansour, D. Female sterilisation: Is it what women really want? BMJ Sex. Reprod. Health 2003, 29, 136–139. Available online: https://srh.bmj.com/content/29/3/136 (accessed on 3 January 2022). [CrossRef] [Green Version]
- Jatlaoui, T.C.; Whiteman, M.K.; Jeng, G.; Tepper, N.K.; Berry-Bibee, E.; Jamieson, D.J.; Marchbanks, P.A.; Curtis, K.M. Intrauterine device expulsion after postpartum placement: A systematic review and meta-analysis. Obstet. Gynecol. 2018, 132, 895–905. [Google Scholar] [CrossRef]
- Gurney, E.P.; Sonalkar, S.; McAllister, A.; Sammel, M.D.; Schreiber, C.A. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am. J. Obstet. Gynecol. 2018, 219, 183.e1–183.e9. [Google Scholar] [CrossRef]
- Ragab, A.; Hamed, H.O.; Alsammani, M.A.; Shalaby, H.; Nabeil, H.; Barakat, R.; Fetih, A.N. Expulsion of Nova-T380, Multiload 375, and copper-T380A contraceptive devices inserted during cesarean delivery. Int. J. Gynecol. Obstet. 2015, 130, 174–178. [Google Scholar] [CrossRef]
- Lopez, L.M.; Bernholc, A.; Hubacher, D.; Stuart, G.; Van Vliet, H.A.A.M. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database Syst. Rev. 2015, 6, CD003036. [Google Scholar] [CrossRef]
- Tatum, H.J.; Beltran, R.S.; Ramos, R.; Van Kets, H.; Sivin, I.M.; Schmidt, F.H. Immediate postplacental insertion of GYNE-T 380 and GYNE-T 380 postpartum intrauterine contraceptive devices: Randomized study. Am. J. Obstet. Gynecol. 1996, 175, 1231–1235. Available online: https://pubmed.ncbi.nlm.nih.gov/8942493/ (accessed on 3 January 2022). [CrossRef]
- Goldthwaite, L.M.; Sheeder, J.; Hyer, J.; Tocce, K.; Teal, S.B. Postplacental intrauterine device expulsion by 12 weeks: A prospective cohort study. Am. J. Obstet. Gynecol. 2017, 217, 674.e1–674.e8. [Google Scholar] [CrossRef]
- Wildemeersch, D.; Hasskamp, T.; Nolte, K.; Jandi, S.; Pett, A.; Linden, S.; Van Santen, M.; Julen, O. A multicenter study assessing uterine cavity width in over 400 nulliparous women seeking IUD insertion using 2D and 3-D sonography. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Wildemeersch, D.; Rowe, P.J. Assessment of menstrual blood loss in Belgian users of the frameless copper-releasing IUD with copper surface area of 200 mm2 and users of a copper-levonorgestrel-releasing intrauterine system. Contraception 2004, 70, 169–172. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, G.; Zhou, C. Study on GyneFix PP IUD insertion during cesarean section. Chin. J. Fam. Plan. 2004, 12, 481–482. Available online: https://www.semanticscholar.org/paper/Study-on-GyneFix-PP-IUD-insertion-during-cesarean-Zhang-Fang/16faec7f803c5392fb8e391d7c5d3a4a81f1f61e (accessed on 3 January 2022).
- Kutlucan, H.; Karabacak, R.O.; Wildemeersch, D. Considerations on a new, frameless copper-releasing intrauterine system for intracesarean insertion and its future clinical significance: A review. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Unal, C.; Eser, A.; Tozkir, E.; Wildemeersch, D. Comparison of expulsions following intracesarean placement of an innovative frameless copper-releasing IUD (Gyn-CS®) versus the TCu380A: A randomized trial. Contraception 2018, 98, 135–140. [Google Scholar] [CrossRef]
- Brito, M.B.; Ferriani, R.A.; Quintana, S.M.; Yazlle, M.E.; Silva de Sá, M.F.; Vieira, C.S. Safety of the etonogestrel-releasing implant during the immediate postpartum period: A pilot study. Contraception 2009, 80, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Chaovisitsaree, S.; Noi-um, S.; Kietpeerakool, C. Review of postpartum contraceptive practices at Chiang Mai University Hospital: Implications for improving quality of service. Med. Princ. Pract. 2012, 21, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, J.A.; Espey, E.; Stonehocker, J. Barriers to intrauterine device insertion in postpartum women. Contraception 2005, 72, 426–429. [Google Scholar] [CrossRef]
- Baldwin, M.K.; Edelman, A.B. The effect of long-acting reversible contraception on rapid repeat pregnancy in adolescents: A review. J. Adolesc. Health 2013, 52, S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.R.A.; Aiken, C.E.M.; Trussell, J.; Guthrie, K.A. Immediate postpartum provision of highly effective reversible contraception. Curr. Opin. Obstet. Gynecol. 2015, 122, 1050–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyi, E.G.Y.; Mollamahmutoglu, L. An analysis of the high cesarean section rates in Turkey by Robson classification. J. Matern.-Fetal Neonatal Med. 2019, 34, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Azzam, M.E.; Wildemeersch, D.; Mustafa, M.H.; El-Kady, E.M. Intra Caesarean fixation of frameless copper IUD (Gynefix CS 300). Nat. Sci. 2019, 17, 4. Available online: http://www.sciencepub.net/nature/nsj170419/13_34580nsj170419_139_147.pdf (accessed on 3 January 2022).
- Eser, A.; Unal, C.; Albayrak, B.; Wildemeersch, D. Clinical experience with a novel anchored, frameless copper-releasing contraceptive device for intra-caesarean insertion. Eur. J. Contracept. Reprod. Health Care 2018, 23, 255–259. [Google Scholar] [CrossRef]



| Count | Column Valid N% | ||
|---|---|---|---|
| Gravidity/Parity | 1-1 | 2 | 9.5% |
| 2-2 | 6 | 28.6% | |
| 3-2 | 4 | 19.0% | |
| 3-3 | 5 | 23.8% | |
| 4-2 | 2 | 9.5% | |
| 4-3 | 1 | 4.8% | |
| 5-4 | 1 | 4.8% | |
| Number of previous CS | 0 | 5 | 23.8% |
| 1 | 12 | 57.1% | |
| 2 | 4 | 19.0% | |
| Reason for CS | Previous caserean section (CS) | 16 | 76.2% |
| Maternal disease (history of VSD operation) | 1 | 4.8% | |
| Maternal disease (history of rectocele operation) | 1 | 4.8% | |
| Maternal request | 3 | 14.3% |
| Event | Number of Participants | ||
|---|---|---|---|
| Insertion | 21 | ||
| Discharge | 20 | ||
| 6 weeks | 10 | ||
| Exit visit (months) | 3 | 12 (57.12%) | 21 |
| 4 | 1 (4.76%) | ||
| 5 | 2 (9.52%) | ||
| 6 | 3 (14.28%) | ||
| 8 | 1 (4.76%) | ||
| 9 | 1 (4.76%) | ||
| 14 | 1 (4.76%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutlucan, H.; Karabacak, R.O.; De Buyser, S.; Erdem, A.; Bozkurt, N.; Demirdağ, E.; Wildemeersch, D. Assessment of a Novel Fixation Method of a Frameless Intrauterine Contraceptive Device Inserted during Cesarean Delivery as a Means of Preventing Displacements and Expulsions: A Prospective Observational Study. Life 2022, 12, 83. https://doi.org/10.3390/life12010083
Kutlucan H, Karabacak RO, De Buyser S, Erdem A, Bozkurt N, Demirdağ E, Wildemeersch D. Assessment of a Novel Fixation Method of a Frameless Intrauterine Contraceptive Device Inserted during Cesarean Delivery as a Means of Preventing Displacements and Expulsions: A Prospective Observational Study. Life. 2022; 12(1):83. https://doi.org/10.3390/life12010083
Chicago/Turabian StyleKutlucan, Hazal, Recep Onur Karabacak, Stefanie De Buyser, Ahmet Erdem, Nuray Bozkurt, Erhan Demirdağ, and Dirk Wildemeersch. 2022. "Assessment of a Novel Fixation Method of a Frameless Intrauterine Contraceptive Device Inserted during Cesarean Delivery as a Means of Preventing Displacements and Expulsions: A Prospective Observational Study" Life 12, no. 1: 83. https://doi.org/10.3390/life12010083
APA StyleKutlucan, H., Karabacak, R. O., De Buyser, S., Erdem, A., Bozkurt, N., Demirdağ, E., & Wildemeersch, D. (2022). Assessment of a Novel Fixation Method of a Frameless Intrauterine Contraceptive Device Inserted during Cesarean Delivery as a Means of Preventing Displacements and Expulsions: A Prospective Observational Study. Life, 12(1), 83. https://doi.org/10.3390/life12010083






