The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Population
2.2. Rutherford Classification
2.3. Short-Chain Fatty Acids (SCFA) Analysis
- SCFA Extraction Process from Feces Sample
- SCFA Analysis Process with GC-MS
- Measurement of Lipid and Glucose Profile
2.4. Estimation of Sample Measurement
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlation of Short-Chain Fatty Acid with Lipid and Glucose Profiles of Diabetes Mellitus Patients with Peripheral Arterial Disease
3.3. Correlation of Short-Chain Fatty Acid with Foot Arterial Diameter, Peak Systolic Velocity (PSV), and Volume Flow (VF) in Diabetes Mellitus Patients with Peripheral Arterial Disease
3.4. Comparative Analysis of Short-Chain Fatty Acid with Foot Arterial Plaque in Diabetes Mellitus Patients with Peripheral Arterial Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Association, A.D. Introduction: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Thiruvoipati, T. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J. Diabetes 2015, 6, 961. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part i. Eur. Heart J. 2013, 34, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef]
- ESVS Guidelines Committee. Editor’s Choice–2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef]
- American Diabetes Association. Peripheral Arterial Disease in People With Diabetes. Diabetes Care 2003, 26, 3333–3341. [Google Scholar] [CrossRef]
- Nativel, M.; Potier, L.; Alexandre, L.; Baillet-Blanco, L.; Ducasse, E.; Velho, G.; Marre, M.; Roussel, R.; Rigalleau, V.; Mohammedi, K. Lower extremity arterial disease in patients with diabetes: A contemporary narrative review 11 Medical and Health Sciences 1103 Clinical Sciences 11 Medical and Health Sciences 1102 Cardiorespiratory Medicine and Haematology. Cardiovasc Diabetol. 2018, 17, 1–14. [Google Scholar]
- Seth, A.; Kumar Attri, A.; Kataria, H.; Kochhar, S.; Kaur, N. Pattern of Vascular Insufficiency on Ultrasound Colour Doppler and Computed Tomographic Angiography in Patients with Diabetic Foot and Its Clinical Outcome. Omi J. Radiol. 2017, 6, 2. [Google Scholar] [CrossRef]
- Soyoye, D.O.; Abiodun, O.O.; Ikem, R.T.; Kolawole, B.A.; Akintomide, A.O. Diabetes and peripheral artery disease: A review. World J. Diabetes 2021, 12, 827. [Google Scholar] [CrossRef]
- Bevan, G.H.; White Solaru, K.T. Evidence-Based Medical Management of Peripheral Artery Disease. Arter. Thromb Vasc Biol. 2020, 40, 541–553. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Serino, M.; Blasco-Baque, V.; Nicolas, S.; Burcelin, R. Far from the Eyes, Close to the Heart: Dysbiosis of Gut Microbiota and Cardiovascular Consequences. Curr. Cardiol. Rep. 2014, 16, 540. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Stumpff, F. A look at the smelly side of physiology: Transport of short chain fatty acids. Pflug. Arch. Eur. J. Physiol. 2018, 470, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.B.; Li, M.; van Esch, B.C.A.M.; Garssen, J.; Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 2016, 4, 68–111. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of classification systems in peripheral artery disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef]
- Scortichini, S.; Boarelli, M.C.; Silvi, S.; Fiorini, D. Development and validation of a GC-FID method for the analysis of short chain fatty acids in rat and human faeces and in fermentation fluids. J. Chromatogr B Anal. Technol. Biomed. Life Sci. 2020, 1143, 121972. [Google Scholar] [CrossRef]
- Wirawati, I.A. Pemeriksaan Profil Lipid. Pemeriksaan Profil Lipid. 2018, p. 26. Available online: http://erepo.unud.ac.id/id/eprint/19189/1/733b43ae6572ac9d48c639dda5c0b5df.pdf (accessed on 10 May 2021).
- Endiyasa, E.; Ariami, P.; Urip, U. Perbedaan Kadar Glukosa Darah Metode Poin of Care Test (Poct) Dengan Photometer Pada Sampel Serum Di Wilayah Kerja Puskesmas Jereweh. J. Anal. Med. Biosains 2019, 5, 40. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; WHO: Geneva, Switzerland, 2011; pp. 1–6. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?sequence=22&isAllowed=y (accessed on 10 May 2021).
- Tao, R.; De Zoeten, E.F.; Özkaynak, E.; Chen, C.; Wang, L.; Porrett, P.M.; Li, B.; Turka, L.A.; Olson, E.N.; Greene, M.I.; et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007, 13, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Suastika, K.; Semadi, I.M.S.; Dwipayana, I.M.P.; Saraswati, M.R.; Gotera, W.; Budhiarta, A.A.G.; Matsumoto, K.; Kajiwara, N.; Taniguchi, H. Dyslipidemia in diabetes: A population-based study in Bali. Int. J. Gen. Med. 2019, 12, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Cecchini, A.L.; Landolfi, R.; Flex, A. The role of the microbiota in the diabetic peripheral artery disease. Mediat. Inflamm. 2019, 2019, 4128682. [Google Scholar] [CrossRef]
- Zwartjes, M.S.Z.; Gerdes, V.E.A.; Nieuwdorp, M. The role of gut microbiota and its produced metabolites in obesity, dyslipidemia, adipocyte dysfunction, and its interventions. Metabolites 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Vourakis, M.; Mayer, G.; Rousseau, G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef]
- Jude, E.B.; Oyibo, S.O.; Chalmers, N.; Boulton, A.J. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care 2001, 24, 1433–1437. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. 1), 5–67. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2019, 11, 51. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J.; et al. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep. 2020, 10, 6436. [Google Scholar] [CrossRef] [Green Version]
- Huart, J.; Leenders, J.; Taminiau, B.; Descy, J.; Saint-Remy, A.; Daube, G.; Krzesinski, J.M.; Melin, P.; De Tullio, P.; Jouret, F. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension 2019, 74, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, K.; Jiang, Y.; Zhu, C.; Suo, C.; Cui, M.; Wang, Y.; Yuan, Z.; Xue, J.; Wang, J.; et al. The gut microbiome in subclinical atherosclerosis: A population-based multiphenotype analysis. Rheumatology 2021, 61, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; An, S.; Wan, Y.; Yang, F.; Liu, Q. How to best use acetic acid for the prevention of heart disease and cancer. Eur. J. Prev. Cardiol. 2019, 26, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, M.; Xiao, H.; Liu, X.; Wang, K.; Zhang, Y. Acetic Acid Influences BRL-3A Cell Lipid Metabolism via the AMPK Signalling Pathway. Cell Physiol. Biochem. 2018, 45, 2021–2030. [Google Scholar] [CrossRef]
- AlDallal, S.M.; Jena, N. Prevalence of Anemia in Type 2 Diabetic Patients. J. Hematol. 2018, 7, 57–61. [Google Scholar] [CrossRef]
| Characteristics | Mean/N | % |
|---|---|---|
| Age (year) | 59.15 ± 10.29 | |
| Sex | ||
| Men | 30 | 56.6 |
| Women | 23 | 43.4 |
| Sore Feet | ||
| Right | 21 | 39.6 |
| Left | 32 | 60.4 |
| LDL (mg/dL) * | 108 (35–183) | |
| HDL (mg/dL) * | 39 (5–83) | |
| Triglycerides (mg/dL) * | 130 (60–361) | |
| RBG (mg/dL) | 228.77 ± 73.11 | |
| Ankle Pressure (mmHg) * | 90 (0–140) | |
| Toe Pressure (mmHg) * | 60 (30–130) | |
| Brachial Pressure (mmHg) * | 120 (100–160) | |
| ABI * | 0.75 (0–1.17) | |
| PAD Degree | ||
| Rutherford 0–3 | 22 | 41.5 |
| Rutherford 4–6 | 31 | 58.5 |
| Short-Chain Fatty Acids | ||
| Acetate (%) | 57.45 ± 19.69 | |
| Absolute Acetate (mg/mL) * | 3.08 (0.28–19.35) | |
| Propionate (%) | 16.83 ± 10.04 | |
| Absolute Propionate (mg/mL) * | 1.25 (0.04–12.16) | |
| Butyrate (%) | 14.28 ± 10.13 | |
| Absolute Butyrate (mg/mL) * | 1 (0.01–13.6) | |
| Valerate (%) * | 2 (0.01–9.6) | |
| Absolute Valerate (mg/mL) * | 0.23 (0.01–5.03) | |
| Total SCFA (mg/mL) * | 7 (1–50) | |
| SCFA | Triglycerides | RBG | ||
|---|---|---|---|---|
| r | p | r | p | |
| Acetate (%) | 0.03 | 0.29 | 0.043 | 0.22 |
| Absolut Acetate (mg/mL) * | 0.05 | 0.21 | 0.06 | 0.15 |
| Propionate (%) | 0.01 | 0.54 | 0.06 | 0.13 |
| Absolut Propionate (mg/mL) * | 0.07 | 0.104 | 0.12 | 0.04 |
| Butyrate (%) | 0.08 | 0.09 | 0.01 | 0.55 |
| Absolute Butyrate (mg/mL) * | 0.07 | 0.11 | 0.13 | 0.03 |
| Valerate (%) * | 0.02 | 0.37 | 0.05 | 0.17 |
| Absolute Valerate (mg/mL) * | 0.12 | 0.04 | 0.06 | 0.16 |
| Total SCFA (mg/mL) * | −0.101 | 0.044 | −0.115 | 0.026 |
| Artery | SCFA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate (%) | Absolut Acetate (mg/mL) | Propionate (%) | Absolut Propionate (mg/mL) | Butyrate (%) | Absolut Butyrate (mg/mL) | Valerate (%) | Absolut Valerat (mg/mL) | Total SCFA | ||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| CFA * | ||||||||||||||||||
| PSV | −0.22 | 0.188 | −0.4 | 0.016 | 0.273 | 0.107 | −0.113 | 0.51 | −0.016 | 0.928 | −0.19 | 0.278 | 0.193 | 0.259 | −0.018 | 0.92 | 0.002 | 0.656 |
| VF | −0.001 | 0.994 | −0.165 | 0.336 | −0.118 | 0.493 | −0.293 | 0.083 | −0.327 | 0.051 | −0.327 | 0.05 | 0.202 | 0.238 | −0.092 | 0.595 | 0.122 | 0.04 |
| SFA | ||||||||||||||||||
| PSV | 0.118 | 0.03 | 0.113 | 0.51 | 0.125 | 0.467 | 0.206 | 0.229 | 0.11 | 0.52 | 0.125 | 0.469 | −0.02 | 0.92 | 0.194 | 0.256 | 0.065 | 0.675 |
| VF | 0.09 | 0.01 | 0.238 | 0.162 | −0.05 | 0.774 | −0.112 | 0.514 | −0.192 | 0.26 | −0.19 | 0.26 | 0.214 | 0.002 | 0.025 | 0.886 | −0.21 | 0.215 |
| POPA | ||||||||||||||||||
| PSV | −0.185 | 0.31 | 0.089 | 0.626 | 0.105 | 0.566 | 0.123 | 0.086 | 0.349 | 0.05 | 0.281 | 0.119 | −0.11 | 0.565 | 0.207 | 0.255 | −0.101 | 0.04 |
| VF | 0.03 | 0.01 | 0.159 | 0.384 | −0.022 | 0.906 | −0.07 | 0.705 | −0.115 | 0.53 | −0.12 | 0.53 | 0.076 | 0.679 | 0.081 | 0.66 | −0.13 | 0.466 |
| PTA | ||||||||||||||||||
| PSV | 0.143 | 0.45 | −0.01 | 0.94 | −0.07 | 0.73 | 0.049 | 0.591 | −0.21 | 0.275 | −0.148 | 0.435 | −0.05 | 0.803 | −0.14 | 0.467 | −0.115 | 0.03 |
| VF | −0.296 | 0.112 | 0.26 | 0.166 | −0.007 | 0.97 | −0.014 | 0.943 | −0.056 | 0.769 | −0.06 | 0.769 | 0.156 | 0.411 | 0.66 | 0.01 | −0.07 | 0.7 |
| DPA | ||||||||||||||||||
| PSV | 0.381 | 0.038 | 0.126 | 0.507 | −0.376 | 0.04 | −0.086 | 0.652 | −0.114 | 0.55 | −0.06 | 0.767 | −0.14 | 0.457 | −0.08 | 0.67 | - | - |
| VF | −0.199 | 0.291 | 0.17 | 0.368 | 0.269 | 0.15 | 0.018 | 0.924 | 0.943 | 0.018 | 0.769 | 0.153 | 0.016 | 0.932 | 0.957 | 0.303 | 0.224 | 0.234 |
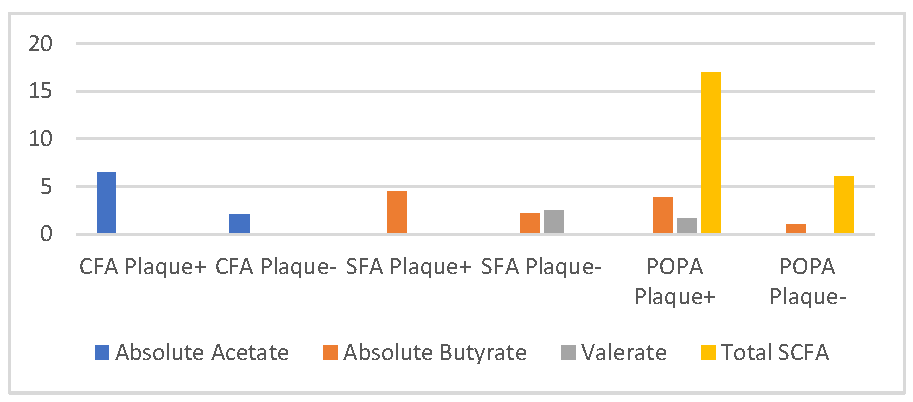
| SCFA | CFA | SFA | POPA | PTA | DPA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plaque+ (n = 16) | Plaque- (n = 20) | p | Plaque+ (n = 11) | Plaque- (n = 25) | p | Plaque+ (n = 11) | Plaque- (n = 21) | p | Plaque+ (n = 17) | Plaque- (n = 13) | p | Plaque+ (n = 15) | Plaque- (n = 15) | p | |
| Acetate (%) | 58 ± 13.92 | 53.45 ± 25.16 | 0.497 | 62.4 ± 12.45 | 52.8 ± 22.85 | 0.22 | 60.63 ± 16.28 | 51.85 ± 23.99 | 0.286 | 55.47 ± 21.65 | 57.38 ± 20.44 | 0.808 | 55.2 ± 22.96 | 57.4 ± 19.13 | 0.778 |
| Absolute acetate (mg/mL) * | 6.505 (1.04,16.73) | 2.08 (0.28,19.35) | 0.028 | 3.27 (1.04,16.73) | 4.455 (0.28,19.35) | 0.52 | 8.5 (0.48,15.73) | 1.86 (0.28,19.35) | 0.16 | 5.43 (0.48,15.73) | 2.5 (0.28,19.35) | 0.385 | 3.5 (0.48,15.73) | 3.49 (0.28,19.35) | 0.967 |
| Propionate (%) | 18.43 ± 9.99 | 17.9 ± 12.45 | 0.889 | 16.9 ± 9.99 | 18.61 ± 11.87 | 0.689 | 15.09 ± 8.82 | 18.47 ± 11.66 | 0.406 | 17.94 ± 10.22 | 16.92 ± 12.14 | 0.805 | 18.67 ± 10.42 | 16.33 ± 11.61 | 0.567 |
| Absolute propionate (mg/mL) * | 1.74 (0.1,12.16) | 1.17 (0.04,7.82) | 0.132 | 1.74 (0.1,5.6) | 1.455 (0.04,12.16) | 0.931 | 1.83 (0.1,12.16) | 1.37 (0.04,7.82) | 0.254 | 1.6 (0.1,12.16) | 1.37 (0.04,7.82) | 0.341 | 1.6 (0.1,12.16) | 1.4 (0.04,7.82) | 0.683 |
| Butyrate (%) | 18 (2.3) | 10.5 (0.49) | 0.2 | 11 (2.28) | 13.5 (0.49) | 0.958 | 21 (2.29) | 11 (0.49) | 0.223 | 19 (0.33) | 10 (1.28) | 0.145 | 18 (0.33) | 11 (1.29) | 0.595 |
| Absolute Butyrate (mg/mL) * | 2.5 (0.1,13.6) | 0.9 (0.01,7.4) | 0.124 | 4.5 (0.1,11.2) | 2.17 (0.01,13.6) | 0.000 | 3.8 (0.1,13.6) | 1 (0.01,7.4) | 0.046 | 2.4 (0.01,13.6) | 0.8 (0.01,9.6) | 0.263 | 2.1 (0.01,13.6) | 1 (0.01,9.6) | 0.902 |
| Valerate (%) * | 1.35 (0.2,4.8) | 1.55 (0.01,6.7) | 0.741 | 2.35 (0.01,8.7) | 1.15 (0.7,5.8) | 0.033 | 2.44 (0.01,6.7) | 1.67 (0.01,4.5) | 0.03 | 2.4 (0.01,6.4) | 1.4 (0.01,6.7) | 0.711 | 2.4 (0.01,6.4) | 1.4 (0.01,6.7) | 0.775 |
| Absolute valerate (mg/mL) * | 0.28 (0.01,1.9) | 0.22 (0.01,5.03) | 0.962 | 0.1 (0.01,1.9) | 0.325 (0.01,5.03) | 0.59 | 0.3 (0.01,1.9) | 0.3 (0.01,5.03) | 0.611 | 0.3 (0.01,1.9) | 0.09 (0.01,5.03) | 0.341 | 0.3 (0.01,1.57) | 0.09 (0.01,5.03) | 0.624 |
| Total SCFA (mg/mL) * | 13.5 (1.46) | 6.5 (1.50) | 0.116 | 7 (1.29) | 10 (1.50) | 0.903 | 17 (1.46) | 6 (1.50) | 0.046 | 13 (1.46) | 6 (1.50) | 0.3 | 11 (1.46) | 6 (1.50) | 0.935 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muradi, A.; Jasirwan, C.O.M.; Simanjuntak, C.D.; Pratama, D.; Suhartono, R.; Darwis, P.; Kekalih, A. The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients. Life 2022, 12, 1464. https://doi.org/10.3390/life12101464
Muradi A, Jasirwan COM, Simanjuntak CD, Pratama D, Suhartono R, Darwis P, Kekalih A. The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients. Life. 2022; 12(10):1464. https://doi.org/10.3390/life12101464
Chicago/Turabian StyleMuradi, Akhmadu, Chyntia Olivia Maurine Jasirwan, Charley D. Simanjuntak, Dedy Pratama, Raden Suhartono, Patrianef Darwis, and Aria Kekalih. 2022. "The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients" Life 12, no. 10: 1464. https://doi.org/10.3390/life12101464






