Type of ANCA May Be Indispensable in Distinguishing Subphenotypes of Different Clinical Entities in ANCA-Associated Vasculitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology
2.1. Age and Gender
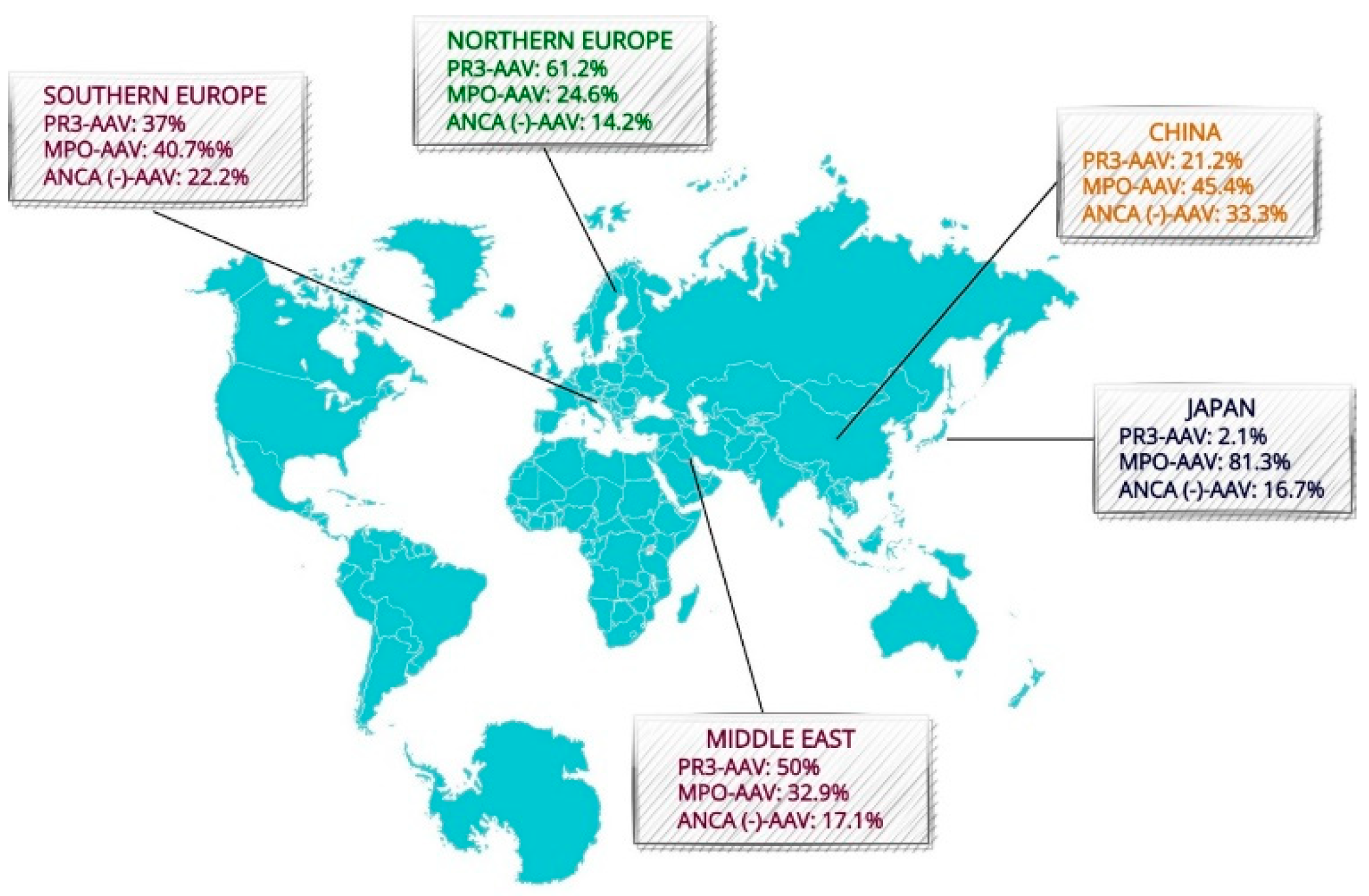
2.2. Geographic Variation
3. Pathogenesis
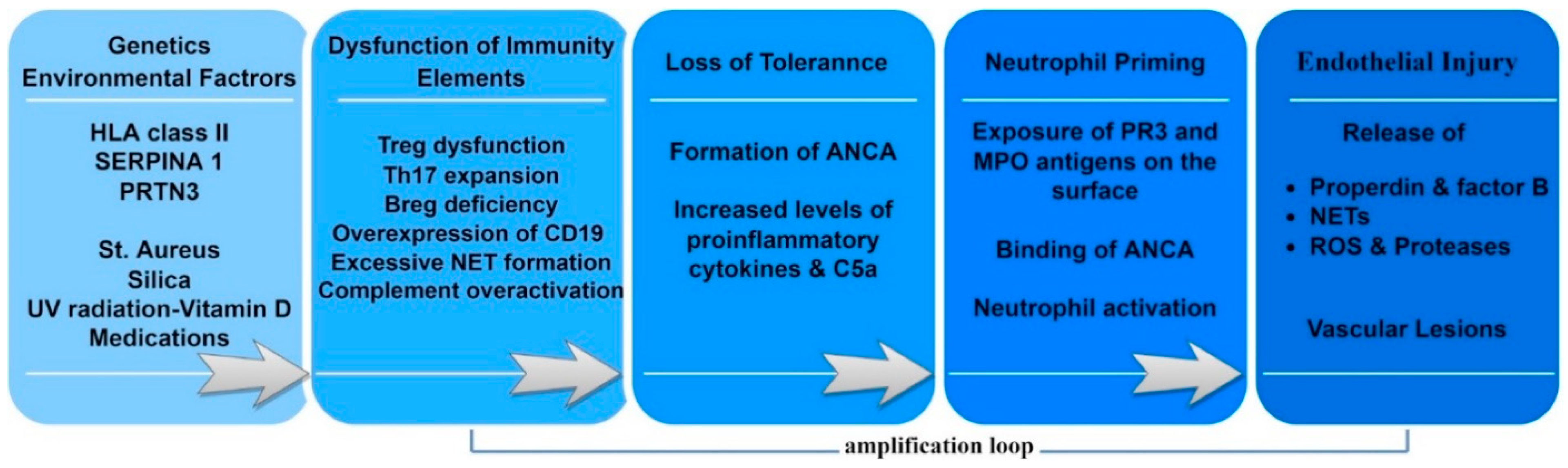
3.1. Predisposing Factors
3.2. Genetic Associations
3.3. Environmental Factors
3.3.1. Staphylococcus Aureus
3.3.2. Latitude and Ultraviolet (UV) Radiation
3.3.3. Drug-Induced Vasculitis
3.4. Immune System Dysfunction
3.4.1. T Cells
3.4.2. B Cells
3.4.3. Complement
3.4.4. NETs
3.4.5. Cytokines
4. Classification
5. ANCA Biology
6. ANCA as a Prognostic and Follow-Up Biomarker
7. Renal Involvement—Histopathology
- Focal class, if >50% of glomeruli are healthy;
- Crescentic class, if >50% of glomeruli present crescents;
- Sclerotic class, if >50% of glomeruli are sclerotic;
- Mixed class, with no predominance of a lesion phenotype (less than 50% normal, less than 50% crescentic, less than 50% sclerotic glomeruli).
8. Extrarenal Manifestations—ENT, Lung and Ocular Involvement
9. Treatment
9.1. Induction of Remission
9.2. Maintenance of Remission
9.3. Considerations before Treatment Selection
10. Suggestions for Novel Classification of AAV
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Morgan, M.D.; Harper, L.; Williams, J.; Savage, C. Anti-neutrophil cytoplasm-associated glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 1224–1234. [Google Scholar] [CrossRef]
- Flores-Suárez, L.F.; Alba, M.A.; Mateos-Toledo, H.; Ruiz, N. Pulmonary Involvement in Systemic Vasculitis. Curr. Rheumatol. Rep. 2017, 19, 56. [Google Scholar] [CrossRef]
- Binda, V.; Moroni, G.; Messa, P. ANCA-associated vasculitis with renal involvement. J. Nephrol. 2018, 31, 197–208. [Google Scholar] [CrossRef]
- Weiner, M.; Bjørneklett, R.; Hrusková, Z.; Mackinnon, B.; Poulton, C.J.; Sindelar, L.; Mohammad, A.J.; Eriksson, P.; Gesualdo, L.; Geetha, D.; et al. Proteinase-3 and myeloperoxidase serotype in relation to demographic factors and geographic distribution in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Nephrol. Dial. Transplant. 2019, 34, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Niiro, H.; Ueda, A.; Sawabe, T.; Nishizaka, H.; Furugo, I.; Yoshizawa, S.; Yoshizawa, S.; Tsukamoto, H.; Kiyohara, C.; et al. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: A retrospective multi-center study in Japan. Rheumatol. Int. 2015, 35, 555–559. [Google Scholar] [CrossRef]
- Wójcik, K.; Masiak, A.; Jeleniewicz, R.; Jakuszko, K.; Brzosko, I.; Storoniak, H.; Kur-Zalewska, J.; Wisłowska, M.; Madej, M.; Hawrot-Kawecka, A.; et al. Association of antineutrophil cytoplasmic antibody (ANCA) specificity with the demographic and clinical characteristics of patients with ANCA-associated vasculitides. Pol. Arch. Intern. Med. 2022, 132, 16187. [Google Scholar] [CrossRef]
- Scott, J.; Hartnett, J.; Mockler, D.; Little, M.A. Environmental risk factors associated with ANCA associated vasculitis: A systematic mapping review. Autoimmun. Rev. 2020, 19, 102660. [Google Scholar] [CrossRef]
- Pearce, F.A.; Craven, A.; Merkel, P.A.; Luqmani, R.A.; Watts, R.A. Global ethnic and geographic differences in the clinical presentations of anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology 2017, 56, 1962–1969. [Google Scholar] [CrossRef]
- Hong, Y.; Shi, P.; Liu, X.; Yang, L.; Li, K.; Xu, F.; Liang, S.; Liu, Z.; Zhang, H.; Chen, Y.; et al. Distinction between MPO-ANCA and PR3-ANCA-associated glomerulonephritis in Chinese patients: A retrospective single-center study. Clin. Rheumatol. 2019, 38, 1665–1673. [Google Scholar] [CrossRef]
- Lyons, P.A.; Rayner, T.F.; Trivedi, S.; Holle, J.U.; Watts, R.A.; Jayne, D.R.; Baslund, B.; Brenchley, P.; Bruchfeld, A.; Chaudhry, A.N.; et al. Genetically Distinct Subsets within ANCA-Associated Vasculitis. N. Engl. J. Med. 2012, 367, 214–223. [Google Scholar] [CrossRef]
- Xie, G.; Roshandel, D.; Sherva, R.; Monach, P.A.; Lu, E.Y.; Kung, T.; Carrington, K.; Zhang, S.S.; Pulit, S.L.; Ripke, S.; et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: Evidence grom genome-wide analysis. Arthritis Rheum. 2013, 65, 2457–2468. [Google Scholar] [CrossRef]
- Kain, R.; Exner, M.; Brandes, R.; Ziebermayr, R.; Cunningham, D.; Alderson, C.A.; Davidovits, A.; Raab, I.; Jahn, R.; Ashour, O.; et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat. Med. 2008, 14, 1088–1096. [Google Scholar] [CrossRef]
- Geetha, D.; Jefferson, J.A. ANCA-Associated Vasculitis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 124–137. [Google Scholar] [CrossRef]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, S267–S276. [Google Scholar] [CrossRef]
- Stegeman, C.A.; Cohen Tervaert, J.W.; Sluiter, W.J.; Manson, W.L.; de Jong, P.E.; Kallenberg, C.G.M. Association of Chronic Nasal Carriage of Staphylococcus aureus and Higher Relapse Rates in Wegener Granulomatosis. Ann. Intern. Med. 1994, 120, 12–17. [Google Scholar] [CrossRef]
- Salmela, A.; Rasmussen, N.; Tervaert, J.W.C.; Jayne, D.R.W.; Ekstrand, A. Chronic nasal Staphylococcus aureus carriage identifies a subset of newly diagnosed granulomatosis with polyangiitis patients with high relapse rate. Rheumatology 2017, 56, 965–972. [Google Scholar] [CrossRef]
- Glasner, C.; de Goffau, M.C.; van Timmeren, M.M.; Schulze, M.L.; Jansen, B.; Tavakol, M.; van Wamel, W.J.B.; Stegeman, C.A.; Kallenberg, C.G.M.; Arends, J.P.; et al. Genetic loci of Staphylococcus aureus associated with anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides. Sci. Rep. 2017, 7, 12211. [Google Scholar] [CrossRef]
- Gómez-Puerta, J.A.; Gedmintas, L.; Costenbader, K.H. The association between silica exposure and development of ANCA-associated vasculitis: Systematic review and meta-analysis. Autoimmun. Rev. 2013, 12, 1129–1135. [Google Scholar] [CrossRef]
- Hogan, S.L.; Cooper, G.S.; Savitz, D.A.; Nylander-French, L.A.; Parks, C.G.; Chin, H.; Jennette, C.E.; Lionaki, S.; Jennette, J.C.; Falk, R.J. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: A population-based, case-control study. Clin. J. Am. Soc. Nephrol. 2007, 2, 290–299. [Google Scholar] [CrossRef]
- Gatenby, P.A.; Lucas, R.M.; Engelsen, O.; Ponsonby, A.L.; Clements, M. Antineutrophil cytoplasmic antibody-associated vasculitides: Could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Care Res. 2009, 61, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Hutton, H.L.; Holdsworth, S.R.; Kitching, A.R. ANCA-Associated Vasculitis: Pathogenesis, Models, and Preclinical Testing. Semin. Nephrol. 2017, 37, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Batal, I.; Jim, B.; Mendez, B.; Anis, K. Unusual case of levamisole-induced dual-positive ANCA vasculitis and crescentic glomerulonephritis. BMJ Case Rep. 2018, 2018, bcr-2018-225913. [Google Scholar] [CrossRef]
- Al-Hussain, T.; Hussein, M.H.; Conca, W.; al Mana, H.; Akhtar, M. Pathophysiology of ANCA-associated Vasculitis. Adv. Anat. Pathol. 2017, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Thewissen, M.; Damoiseaux, J.; van Paassen, P.; Witzke, O.; Cohen Tervaert, J.W. T cells in ANCA-associated vasculitis: What can we learn from lesional versus circulating T cells? Arthritis Res. Ther. 2010, 12, 1. [Google Scholar] [CrossRef]
- Wilde, B.; Thewissen, M.; Damoiseaux, J.; Knippenberg, S.; Hilhorst, M.; van Paassen, P.; Witzke, O.; Cohen Tervaert, J.W. Regulatory B cells in ANCA-associated vasculitis. Ann. Rheum. Dis. 2013, 72, 1416–1419. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Daniel, S.; NoËl, L.H.; Mouthon, L. Neutrophils and B lymphocytes in ANCA-associated vasculitis. APMIS 2009, 117, 27–31. [Google Scholar] [CrossRef]
- Ballanti, E.; Chimenti, M.S.; Perricone, R. Small-Medium Vessel Vasculitides: Is the Complement System a Potential Forgotten Target? Isr. Med. Assoc. J. IMAJ 2015, 17, 2. [Google Scholar]
- Chen, S.F.; Wang, F.M.; Li, Z.Y.; Yu, F.; Zhao, M.H.; Chen, M. Plasma complement factor H is associated with disease activity of patients with ANCA-associated vasculitis. Arthritis Res. Ther. 2015, 17, 129. [Google Scholar] [CrossRef]
- Bantis, K.; Stangou, M.; Kalpakidis, S.; Nikolaidou, C.; Lioulios, G.; Mitsoglou, Z.; Iatridi, F.; Fylaktou, A.; Papagianni, A. Systemic complement activation in anti-neutrophil cytoplasmic antibody-associated vasculitis and necrotizing glomerulonephritis. Nephrology 2021, 26, 30–37. [Google Scholar] [CrossRef]
- Frangou, E.; Vassilopoulos, D.; Boletis, J.; Boumpas, D.T. An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment. Autoimmun. Rev. 2019, 18, 751–760. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019, 15, 2. [Google Scholar] [CrossRef]
- Lee, K.H.; Kronbichler, A.; Park, D.D.Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Stangou, M.; Papagianni, A.; Bantis, C.; Liakou, H.; Pliakos, K.; Giamalis, P.; Gionanlis, L.; Pantzaki, A.; Efstratiadis, G.; Memmos, D. Detection of multiple cytokines in the urine of patients with focal necrotising glomerulonephritis may predict short and long term outcome of renal function. Cytokine 2012, 57, 120–126. [Google Scholar] [CrossRef]
- Berti, A.; Warner, R.; Johnson, K.; Cornec, D.; Schroeder, D.; Kabat, B.; Langford, C.A.; Hoffman, G.S.; Fervenza, F.C.; Kallenberg, C.G.M.; et al. Brief Report: Circulating Cytokine Profiles and Antineutrophil Cytoplasmic Antibody Specificity in Patients with Antineutrophil Cytoplasmic Antibody–Associated Vasculitis. Arthritis Rheumatol. 2018, 70, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Watts, R.A. Classification of ANCA-associated vasculitis. Curr. Rheumatol. Rep. 2013, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Luqmani, R.A.; Suppiah, R.; Grayson, P.C.; Merkel, P.A.; Watts, R. Nomenclature and classification of vasculitis—Update on the ACR/EULAR Diagnosis and Classification of Vasculitis Study (DCVAS). Clin. Exp. Immunol. 2011, 164 (Suppl. S1), 11–13. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Haris, Á.; Polner, K.; Arányi, J.; Braunitzer, H.; Kaszás, I.; Rosivall, L.; Kökény, G.; Mucsi, I. Simple, readily available clinical indices predict early and late mortality among patients with ANCA-associated vasculitis. BMC Nephrol. 2017, 18, 76. [Google Scholar] [CrossRef][Green Version]
- Falk, R.J.; Jennette, J.C. ANCA disease: Where is this field heading? J. Am. Soc. Nephrol. 2010, 21, 745–752. [Google Scholar] [CrossRef]
- Xiao, H.; Heeringa, P.; Hu, P.; Liu, Z.; Zhao, M.; Aratani, Y.; Maeda, N.; Falk, R.J.; Jennette, J.C. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Investig. 2002, 110, 955–963. [Google Scholar] [CrossRef]
- Tervaert, J.W.C.; Damoiseaux, J. Antineutrophil cytoplasmic autoantibodies: How are they detected and what is their use for diagnosis, classification and follow-up? Clin. Rev. Allergy Immunol. 2012, 43, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kain, R.; Tadema, H.; McKinney, E.F.; Benharkou, A.; Brandes, R.; Peschel, A.; Hubert, V.; Feenstra, T.; Sengölge, G.; Stegeman, C.; et al. High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis. J. Am. Soc. Nephrol. 2012, 23, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Basu, N.; Benharkou, A.; Brandes, R.; Brown, M.; Rees, A.J.; Kain, R. Autoantibodies to hLAMP-2 in ANCA-negative pauci-immune focal necrotizing GN. J. Am. Soc. Nephrol. 2014, 25, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.D.; Pusey, C.D. Shining a LAMP on pauci-immune focal segmental glomerulonephritis. Kidney Int. 2009, 76, 15–17. [Google Scholar] [CrossRef]
- Thompson, G.E.; Fussner, L.A.; Hummel, A.M.; Schroeder, D.R.; Silva, F.; Snyder, M.R.; Langford, C.A.; Merkel, P.A.; Monach, P.A.; Seo, P.; et al. Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3 in ANCA-Associated Vasculitis. Front. Immunol. 2020, 11, 2053. [Google Scholar] [CrossRef]
- Fussner, L.A.; Hummel, A.M.; Schroeder, D.R.; Silva, F.; Cartin-Ceba, R.; Snyder, M.R.; Hoffman, G.S.; Kallenberg, C.G.; Langford, C.A.; Merkel, P.A.; et al. Factors Determining the Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3. Arthritis Rheumatol. 2016, 68, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Tian, Y.C.; Wu, H.H.; Tu, K.H.; Liu, S.H.; Lee, C.C.; Fang, J.T.; Yang, C.W.; Li, Y.J. High anti-neutrophil cytoplasmic antibody titers are associated with the requirement of permanent dialysis in patients with myeloperoxidase-ANCA-associated vasculitis. J. Formos. Med. Assoc. 2019, 118, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Haas, M.; Markowitz, G.S.; D’Agati, V.D.; Rennke, H.G.; Jennette, J.C.; Bajema, I.M.; Alpers, C.E.; Chang, A.; Cornell, L.D.; et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J. Am. Soc. Nephrol. 2016, 27, 1278–1287. [Google Scholar] [CrossRef]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic classification of ANCA-associated glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Van Daalen, E.E.; Wester Trejo, M.A.C.; Göçeroglu, A.; Ferrario, F.; Joh, K.; Noel, L.; Olgawa, Y.; Wilhemus, S.; Ball, M.J.; Honsova, E.; et al. Developments in the histopathological classification of ANCA-associated glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2020, 15, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Bjørneklett, R.; Sriskandarajah, S.; Bostad, L. Prognostic value of histologic classification of ANCA-associated glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2159–2167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hakroush, S.; Kluge, I.A.; Ströbel, P.; Korsten, P.; Tampe, D.; Tampe, B. Systematic histological scoring reveals more prominent interstitial inflammation in myeloperoxidase-anca compared to proteinase 3-anca glomerulonephritis. J. Clin. Med. 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hauer, H.A.; Bajema, I.M.; van Houwelingen, H.C.; Ferrario, F.; Noel, L.; Waldherr, R.; Jayn, D.; Rasmussen, N.; Bruijn, J.; Hagen, E.; et al. Renal Histology in ANCA-Associated Vasculitis: Differences between Diagnostic and Serologic Subgroups. Kidney Int. 2002, 61, 80–89. [Google Scholar] [CrossRef]
- Eisenberger, U.; Fakhouri, F.; Vanhille, P.; Beafulis, H.; Mahr, A.; Guilevin, L.; Lesarve, P.; Noel, L. ANCA-negative pauci-immune renal vasculitis: Histology and outcome. Nephrol. Dial. Transplant. 2005, 20, 1392–1399. [Google Scholar] [CrossRef]
- Bantis, K.; Stangou, M.J.; Kalpakidis, S.; Nikolaidou, C.; Lioulios, G.; Mitsoglou, Z.; Iatridi, F.; Fylaktou, A.; Papagianni, A. Different Types of ANCA Determine Different Clinical Phenotypes and Outcome in ANCA-Associated Vasculitis (AAV). Front. Med. 2022, 8, 783757. [Google Scholar] [CrossRef]
- Paulsen, J.I.; Rudert, H. Manifestations of Primary Vasculitis in the ENT Region. Z. Fur Rheumatol. 2001, 60, 219–225. [Google Scholar] [CrossRef]
- Padoan, R.; Campaniello, D.; Felicetti, M.; Cazzador, D.; Schiavon, F. Ear, nose, and throat in ANCA-associated vasculitis: A comprehensive review. Vessel. Plus 2021, 5, 41. [Google Scholar] [CrossRef]
- Rahmattulla, C.; de Lind Van Wijngaarden, R.A.F.; Berden, A.E.; Hauer, H.A.; Floßmann, O.; Jayne, D.R.; Gaskin, G.; Rasmussen, N.; Noël, L.H.; Ferrario, F.; et al. Renal function and ear, nose, throat involvement in anti-neutrophil cytoplasmic antibody-associated vasculitis: Prospective data from the European Vasculitis Society clinical trials. Rheumatology 2014, 54, 899–907. [Google Scholar] [CrossRef]
- Solans-Laqué, R.; Fraile, G.; Rodriguez-Carballeira, M.; Caminal, L.; Castillo, M.J.; Martínez-Valle, F.; Sáez, L.; Rios, J.J.; Solanich, X.; Oristrell, J.; et al. Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides Impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine 2017, 96, e6083. [Google Scholar] [CrossRef]
- Mohammad, A.J.; Mortensen, K.H.; Babar, J.; Smith, R.; Jones, R.; Nakagomi, D.; Sivasothy, P.; Jayne, D. Pulmonary involvement in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: The influence of ANCA subtype. J. Rheumatol. 2017, 44, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lakshman, A.; Nampoothiri, R.V.; Verma, R.; Rathi, M.; Naidu, G.S.; Pinto, B.; Sharma, K.; Dhir, V.; Nada, R.; et al. Pulmonary and Ear, Nose and Throat (ENT) Involvement in ANCA-Associated Vasculitis at Diagnosis-Experience from a Tertiary Care Centre in North India. J. Assoc. Physicians India 2017, 65, 40–47. [Google Scholar]
- Ungprasert, P.; Crowson, C.S.; Cartin-Ceba, R.; Garrity, J.A.; Smith, W.M.; Specks, U.; Matteson, E.L.; Makol, A. Clinical characteristics of inflammatory ocular disease in anti-neutrophil cytoplasmic antibody associated vasculitis: A retrospective cohort study. Rheumatology 2017, 56, 1763–1770. [Google Scholar] [CrossRef]
- Pagnoux, C. Updates in ANCA-associated vasculitis. Eur. J. Rheumatol. 2016, 3, 122–133. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W.; Stegeman, C.A.; Kallenberg, C.G.M. Novel Therapies for Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis. Curr. Opin. Nephrol. Hypertens. 2001, 10, 211–217. [Google Scholar] [CrossRef]
- Jayne, D. Treating vasculitis with conventional immunosuppressive agents. Clevel. Clin. J. Med. 2012, 79 (Suppl. S3), 46–49. [Google Scholar] [CrossRef]
- Geetha, D.; Shah, S. Place in therapy of rituximab in the treatment of granulomatosis with polyangiitis and microscopic polyangiitis. ImmunoTargets Ther. 2015, 4, 173–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langford, C.A. Complications of Cyclophosphamide Therapy. Eur. Arch. Oto-Rhino-Laryngol. 1997, 254, 65–72. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Harper, L.; Jayne, D.R.; Flores Suarez, L.F.; Gregorini, G.; Gross, W.L.; Luqmani, R.; Pusey, C.D.; Rasmussen, N.; Sinico, R.A.; et al. Pulse Versus Daily Oral Cyclophosphamide for Induction of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis A Randomized Trial. Ann. Intern. Med. 2009, 150, 670–680. [Google Scholar] [CrossRef]
- Harper, L.; Morgan, M.D.; Walsh, M.; Hoglund, P.; Westman, K.; Flossmann, O.; Tesar, V.; Vanhille, P.; de Groot, K.; Luqmani, R.; et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: Long-term follow-up. Ann. Rheum. Dis. 2012, 71, 955–960. [Google Scholar] [CrossRef]
- Geetha, D.; Specks, U.; Stone, J.H.; Merkel, P.A.; Seo, P.; Spiera, R.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J. Am. Soc. Nephrol. 2015, 26, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Unizony, S.; Villarreal, M.; Miloslavsky, E.M.; Lu, N.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.M.; et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann. Rheum. Dis. 2016, 75, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Cohen Tervaert, J.W.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; van Paassen, P.; et al. Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis. N. Engl. J. Med. 2010, 363, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Furuta, S.; Tervaert, J.W.C.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann. Rheum. Dis. 2015, 74, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Faurschou, M.; Berden, A.; Flossmann, O.; Bajema, I.; Hoglund, P.; Smith, R.; Szpirt, W.; Westman, K.; Pusey, C.D.; et al. Long-term follow-up of cyclophosphamide compared with azathioprine for initial maintenance therapy in anca-associated vasculitis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1571–1576. [Google Scholar] [CrossRef]
- Karras, A.; Pagnoux, C.; Haubitz, M.; De Groot, K.; Puechal, X.; Tervaert, J.W.; Segelmark, M.; Guillevin, L.; Jayne, D. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann. Rheum. Dis. 2017, 76, 1662–1668. [Google Scholar] [CrossRef]
- De Joode, A.A.E.; Sanders, J.S.F.; Puéchal, X.; Groot, K.; Puechal, X.; Tervaert, J.W.C.; Segelmark, M.; Guillevin, L.; Jayne, D.; European Vasculitis Society. Long term azathioprine maintenance therapy in ANCA-associated vasculitis: Combined results of long-term follow-up data. Rheumatology 2017, 56, 1894–1901. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Karras, A.; Khouatra, C.; Aumaître, O.; Cohen, P.; Maurier, F.; Decaux, O.; Ninet, J.; Gobert, P.; et al. Rituximab versus Azathioprine for Maintenance in ANCA-Associated Vasculitis. N. Engl. J. Med. 2014, 371, 1771–1780. [Google Scholar] [CrossRef]
- Charles, P.; Terrier, B.; Perrodeau, É.; Cohen, P.; Faguer, S.; Huart, A.; Hamidou, M.; Agard, C.; Bonnotte, B.; Samson, M.; et al. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: Results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann. Rheum. Dis. 2018, 77, 1144–1150. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Golbin, J.M.; Keogh, K.A.; Peikert, T.; Sánchez-Menéndez, M.; Ytterberg, S.R.; Fervenza, F.C.; Specks, U. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): Ten-year experience at a single center. Arthritis Rheum. 2012, 64, 3770–3778. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.; Jones, R.B. Treatment of relapses in ANCA-associated vasculitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Tesar, V.; Hruskova, Z. Treatment of granulomatosis with polyangiitis and microscopic polyangiitis: Should type of ANCA guide the treatment? Clin. J. Am. Soc. Nephrol. 2020, 15, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Mahr, A.; Katsahian, S.; Varet, H.; Guillevin, L.; Hagen, E.C.; Höglund, P.; Merkel, P.A.; Pagnoux, C.; Rasmussen, N.; Westman, K.; et al. French Vasculitis Study Group (FVSG) and the European Vasculitis Society (EUVAS). Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: A cluster analysis. Ann. Rheum. Dis. 2013, 72, 1003–1010. [Google Scholar] [CrossRef]
- Wójcik, K.; Biedroń, G.; Wawrzycka-Adamczyk, K.; Bazan-Socha, S.; Ćmiel, A.; Zdrojewski, Z.; Masiak, A.; Czuszyńska, Z.; Majdan, M.; Jeleniewicz, R.; et al. Subphenotypes of ANCA-associated vasculitis identified by latent class analysis. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S129), 62–68. [Google Scholar] [CrossRef]
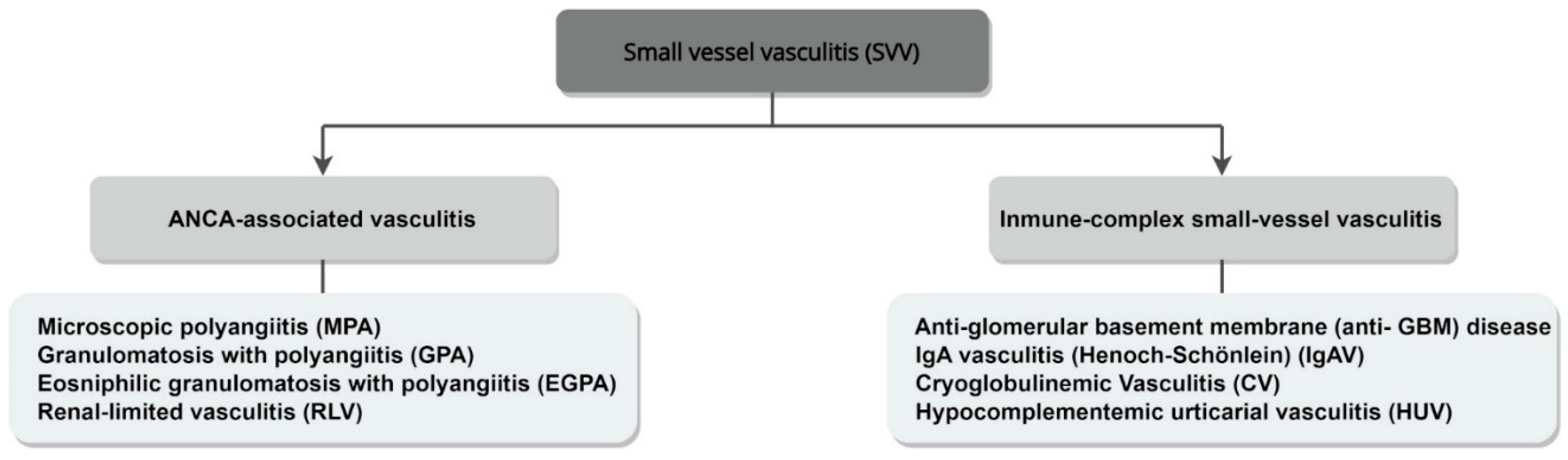

| CHCC 2012 Name | CHCC 2012 Definition |
|---|---|
| Granulomatosis with polyangiitis (GPA) | Necrotizing granulomatous inflammation, usually involving the upper and lower respiratory tract, and necrotizing vasculitis, predominantly affecting small-to-medium vessels. Necrotizing glomerulonephritis is common. |
| Microscopic polyangiitis (MPA) | Necrotizing vasculitis, predominantly affecting small vessels. Necrotizing glomerulonephritis is very common. Pulmonary capillaritis often occurs. Granulomatous inflammation is absent. |
| Eosinophilic granulomatosis with polyangiitis (EGPA) | Eosinophil-rich and necrotizing granulomatous inflammation, often involving the respiratory tract, and necrotizing vasculitis, predominantly affecting small-to-medium vessels. Associated with asthma and eosinophilia. |
| Single-organ AAV | For example, renal-limited vasculitis (RLV). Vasculitis in arteries or veins of a single organ, without any features indicating that it is a limited expression of a systemic vasculitis. |
| Feature | PR3-AAV | MPO-AAV |
|---|---|---|
| Male-to-female ratio | Higher | Lower |
| Age of onset | Younger | Older |
| Geographic distribution | Predominant in Northern Europe | Predominant in Japan and China |
| Genetics | SNPs at the HLA-DP locus HLA-DPB1*04 allele SERPINA 1 PRTN3 | SNPs at the HLA-DQ locus |
| Environmental factors | St. Aureus Low exposure to UV radiation Vitamin D deficiency | Silica exposure |
| Cytokines | IL-6, GM-CSF, IL-15, IL-18, CXCL8/IL-8, CCL-17/TARC, IL-18BP, sIL-2Ra, NGFb, BAFF | sIL6R, sTNFRII, NGAL, sICAM-1 |
| Ocular involvement | More common | Less common |
| ENT involvement | Very common | Less common |
| Pulmonary involvement | Central airway disease Nodular lesions | Interstitial fibrosis Bronchiectasis Alveolar hemorrhage |
| Renal histology | Higher fraction of healthy glomeruli Frequently focal and crescentic class | Smaller fraction of healthy glomeruli Frequently sclerotic class Interstitial fibrosis Tubular atrophy |
| Relapses | Higher risk | Lower risk |
| Renal survival | Better | Worse |
| Clinical Trial | Objective | Results |
|---|---|---|
| CYCLOPS | Pulse CTX versus daily oral CTX for remission induction | Pulse CTX maintains the efficacy of oral CTX at a smaller cumulative dose |
| CYCLOPS long-term follow up | Long-term outcomes of the CYCLOPS study | MPO-ANCA patients respond equally to both regimes PR3-ANCA patients are at a higher risk of relapse when treated with CTX in pulses |
| RAVE | RTX versus oral CTX for remission induction | RTX matches the efficacy of oral CTX |
| RAVE post hoc analysis | Effect of ANCA specificity on treatment response | PR3-ANCA patients respond better to RTX than to oral CTX Comparable efficacy in MPO-ANCA patients |
| RITUXVAS | RTX versus CTX in pulses for remission induction | RTX matches the efficacy of CTX in pulses |
| Two-year follow up of the RITUXVAS study | Long-term outcomes of the RITUXVAS study | Outcome of death, ESRD and relapse did not differ significantly between the two regimen groups |
| CYCAZAREM | Optimal time interval to switch from CTX to AZT for remission maintenance | Early CTX replacement leads to more relapses, but the difference is not statistically important. PR3-ANCA patients relapse more often than MPO-ANCA patients after CTX suspension |
| REMAIN | Efficacy of two different durations of an AZT-based maintenance therapy | Prolonged maintenance therapy, beyond 24 months, reduces relapse risk and improves survival |
| IMPROVE | MMF versus AZT for remission maintenance | MMF is less effective than AZT for remission maintenance |
| MAINRITSAN | RTX versus AZT for remission maintenance | RTX is superior to AZT for maintenance of remission |
| MAINRITSAN2 | Tailored, based on trimestral biological parameters versus fixed-schedule RTX infusions | Relapse rates were similar between the two administration regimens |
| MAINRITSAN2 post hoc analysis | Effect of omitting RTX administration at day 14 | Relapse-free survival rates did not differ significantly |
| Rituximab for remission induction and maintenance in refractory GPA | Efficacy of RTX for maintenance of long-term remission in refractory GPA | RTX is effective for long-term maintenance of remission in PR3-ANCA patients. Monitoring B-cell levels can help to predict and prevent a relapse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantouli, A.M.; Lioulios, G.; Stai, S.; Moysidou, E.; Fylaktou, A.; Papagianni, A.; Stangou, M. Type of ANCA May Be Indispensable in Distinguishing Subphenotypes of Different Clinical Entities in ANCA-Associated Vasculitis. Life 2022, 12, 1467. https://doi.org/10.3390/life12101467
Konstantouli AM, Lioulios G, Stai S, Moysidou E, Fylaktou A, Papagianni A, Stangou M. Type of ANCA May Be Indispensable in Distinguishing Subphenotypes of Different Clinical Entities in ANCA-Associated Vasculitis. Life. 2022; 12(10):1467. https://doi.org/10.3390/life12101467
Chicago/Turabian StyleKonstantouli, Afroditi Maria, Georgios Lioulios, Stamatia Stai, Eleni Moysidou, Asimina Fylaktou, Aikaterini Papagianni, and Maria Stangou. 2022. "Type of ANCA May Be Indispensable in Distinguishing Subphenotypes of Different Clinical Entities in ANCA-Associated Vasculitis" Life 12, no. 10: 1467. https://doi.org/10.3390/life12101467
APA StyleKonstantouli, A. M., Lioulios, G., Stai, S., Moysidou, E., Fylaktou, A., Papagianni, A., & Stangou, M. (2022). Type of ANCA May Be Indispensable in Distinguishing Subphenotypes of Different Clinical Entities in ANCA-Associated Vasculitis. Life, 12(10), 1467. https://doi.org/10.3390/life12101467






