Algal Biomass Utilization toward Circular Economy
Abstract
1. Introduction
2. CO2 Capture by Algae
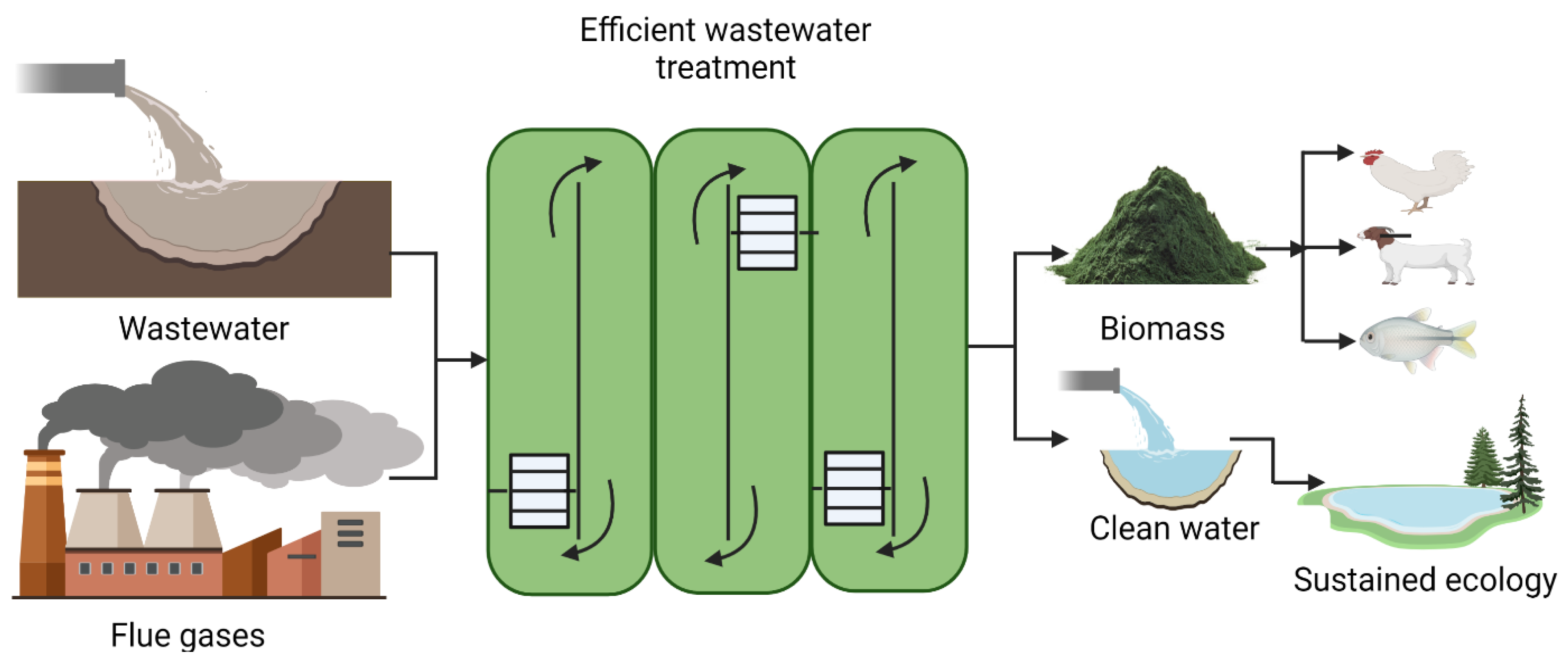
3. Wastewater and Water Treatment
| Strain | Reactor Type | CO2 Source | CO2 Comp. | Growth Rate | CO2 Fix. Rate | Ref. |
|---|---|---|---|---|---|---|
| % | g L−1 d−1 | g L−1 d−1 | ||||
| Anabaena sp. | Circular PBR | commercial | 10 | - | 1.01 | [29] |
| S. dimorphus | flat-panel PBR | comp. CO2 | - | - | 0.60 | [30] |
| Chlorella sp. | Fabricated PBR | boiler gas | 8 | 1.296 | 2.33 | [31] |
| C. minutissma | cylindrical | - | 5 | 0.293 | 51.51 | [22] |
| Coelastrella sp. | Flask | commercial | 1 | 0.80 b | 0.395 | [32] |
| C. sorokiniana | flask | commercial | 1 | 1.06 b | 0.567 | [32] |
| Scenedesmus | flask | commercial | 10 | 0.06 b | 0.446 | [33] |
| Strain | Wastewater | Working | Light | Time | Growth Rate Removal Rate | RE | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Volume | μmol/m2/s | Days | g L−1 d−1 | (mg L−1 d−1) | (%) | ||||
| L | N | P | N | P | Reference | |||||
| N. aquatica | swine | 0.2 | 150 | 7 | 0.82 | 53 a | 58.1 a | 96.2 | 46.3 | [51] |
| Coelastrum sp. | dairy | 0.04 | 42.55 b | 10 | 0.266 | 2.55 | 2.31 | 84.7 | 100 | [52] |
| A. oryzae and | starch | - | 30 | 3 | - | 170.1 c | 15.7 c | 83.56 | 96.58 | [53] |
| C. pyrenoidosa | ||||||||||
| C. sorokiniana | acid prod. | 0.5 | NL | 7 | 0.75 | 83.64 | 5.51 | 88.05 | 82.69 | [54] |
| C. pyrenoidosa | dairy | 1 | - | 8 | 0.08 | 13.25 c | 1.80 c | 97.31 d | 90.25 | [55] |
| Scenedesmus sp. | Domestic | 0.25 | 28 | 10 | - | 5.87 c | 0.091 c | 93.81 | 91.04 | [56] |
| C. vulgaris | sewage | 50 | 555–1850 b | 13 | 0.067 | 4.8 | 1.4 | 92.3 | 77.7 | [57] |
| T. obliquus | dairy | 0.25 | - | 8 | - | 5.48 c | 6.98 c | 78.61 | 87.61 | [50] |
| T. obliquus | dairy | 0.25 | - | 8 | - | 6.97 c | 7.35 c | 100 | 92.2 | [50] |
| V. paradoxus | ||||||||||
| Chlorella sp. | slurry | 0.3 | 46.25 b | 10 | 113 | 17.80 c | 2.11 c | 82.07 | 79.6 | [58] |
| Lysinibacillus sp. | ||||||||||
4. Liquid Biofuels
5. Gaseous Biofuels
6. Food
7. Pharmaceuticals and Cosmetics
8. Animal Breeding
9. Fertilizers
10. Pros and Cos of Algae Production
11. Conclusions
- Algae contain many micro- and macroelements that can be used in various areas of life.
- Algal biomass could be utilized toward the circular economy and bring benefits to the environment, economy and society.
- Their breeding process allows for the reduction in CO2 pollution by the binding of this gas from exhaust gases through algae cells in photosynthesis.
- The culture can be carried out using wastewater purified by algae from biogenic compounds, heavy metals, etc.
- It may be applied, for instance, in modern eco-construction, where algae can be used for household wastewater treatment, and the biomass will definitely be applied, e.g., as biofuel for buildings’ heating.
- Algae can produce biofuels and can also be utilized in biogas plants and the production of biohydrogen.
- They can be used as food, cosmetics, pharmaceuticals and also as feed for farm animals and fertilizers.
- The increasing interest in using algal biomass for further new applications toward the circular economy may be forecasted in the coming years.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leite, G.B.; Abdelaziz, A.E.M.; Hallenbeck, P.C. Algal biofuels: Challenges and opportunities. Bioresour. Technol. 2013, 145, 134–141. [Google Scholar] [CrossRef]
- Ghosh, A.; Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Saini, S.; Bhowmick, T.K.; Gayen, K. Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Convers. Manag. 2016, 113, 104–118. [Google Scholar] [CrossRef]
- Sahoo, D.; Baweja, P. General Characteristics of Algae, the Algae World, Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2015; Volume 26, pp. 16–18. [Google Scholar]
- Zhu, L.; Nugroho, Y.K.; Shakeel, S.R.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using microalgae to produce liquid transportation biodiesel: What is next? Renew. Sustain. Energy Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M.; Kamizela, T.; Kowalczyk, M.; Kalaji, H.M.; Bąba, W. Inexpensive and universal growth media for biomass production of microalgae. Glob. Nest J. 2019, 21, 82–89. [Google Scholar]
- Tran, N.A.; Padula, T.M.P.; Evenhuis, C.R.; Comault, A.S.; Ralph, P.J.; Tamburic, B. Proteomic and biophysical analyses reveal a metabolic shift in nitrogen deprived Nannochloropsis oculate. Algal Res. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Shu, Q.; Takala, J.; Hiltunen, E.; Feng, P.; Yuan, Z. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Gupta, P.L.; Choi, H.J.; Pawar, R.R.; Jung, S.P.; Lee, S.M. Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manag. 2016, 184, 585–595. [Google Scholar] [CrossRef]
- Subhash, G.V.; Rajvanshi, M.; Kumar, B.N.; Govindachary, S.; Prasad, V.; Dasgupta, S. Carbon streaming in microalgae: Extraction and analysis methods for high value compounds. Bioresour. Technol. 2017, 244, 1304–1316. [Google Scholar] [CrossRef]
- Gendy, T.S.; El-Temtamy, S.A. Commercialization potential aspects of microalgae for biofuel production: An overview. Egypt. J. Pet. 2013, 22, 43–51. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in microalgae engineering and synthetic biology applications for biofuel production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Ahmad, M.S.; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S.; Anyanwu, C.N. Algal biomass as a global source of transport fuels: Overview and development perspectives. Prog. Nat. Sci. Mater. Int. 2014, 24, 329–339. [Google Scholar] [CrossRef]
- Bayro-Kaiser, V.; Nelson, N. Microalgal hydrogen production: Prospects of essential technology for a clean and sustainable energy economy. Photosynth. Res. 2017, 133, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.W.; Johnson, M.D.; Zhang, X.; Zemke, P.; Chen, W.; Hu, Q. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Razzak, S.A.; Ali, M.; Hossain, M.; DeLasa, H. Biological CO2 fixation with production of microalgae in wastewater—A review. Renew. Sustain. Energy Rev. 2017, 76, 379–390. [Google Scholar]
- Kuo, C.M.; Lin, T.H.; Yang, Y.C.; Zhang, W.X.; Lai, J.T.; Wu, H.T.; Chang, J.S.; Lin, C.S. Ability of an alkali-tolerant mutant strain of the microalga Chlorella sp. AT1 to capture carbon dioxide for increasing carbon dioxide utilization efficiency. Bioresour. Technol. 2017, 244, 243–251. [Google Scholar] [CrossRef]
- Tebbiche, I.; Mocellin, J.; Huong, L.T.; Pasquier, L.-C. Circular Economy and carbon capture, utilization, and storage. In Biomass, Biofuels, Biochemicals; Springer: Berlin/Heidelberg, Germany, 2021; pp. 813–851. [Google Scholar]
- Ghiat, I.; Mahmood, F.; Govindan, R.; Al-Ansari, T. CO2 utilisation in agricultural greenhouses: A novel ‘plant to plant’ approach driven by bioenergy with carbon capture systems within the energy, water and food Nexus. Energy Convers. Manag. 2021, 228, 113668. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Se, R.; Sarmah, A.K. Consolidated bioprocessing of wastewater cocktail in an algal biorefinery for enhanced biomass, lipid and lutein production coupled with efficient CO2 capture: An advanced optimization approach. J. Environ. Manag. 2019, 252, 109696. [Google Scholar]
- Raslavičius, L.; Striūgas, N.; Felneris, N. New insights into algae factories of the future. Renew. Sustain. Energy Rev. 2018, 81, 643–654. [Google Scholar]
- Doshi, A.; Pascoe, S.; Coglan, L.; Rainey, T.J. Economic and policy issues in the production of algae-based biofuels: A review. Renew. Sustain. Energy Rev. 2016, 64, 329–337. [Google Scholar] [CrossRef]
- Naraharisetti, P.K.; Das, P.; Sharratt, P.N. Critical factors in energy generation from microalgae. Energy 2017, 120, 138–152. [Google Scholar]
- Yadav, G.; Shanmugam, S.; Sivaramakrishnan, R.; Kumar, D.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A.; Rajendran, K. Mechanism and challenges behind algae as a wastewater treatment choice for bioenergy production and beyond. Fuel 2021, 285, 119093. [Google Scholar]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 42168. [Google Scholar]
- Bień, J.; Zabochnicka-Świątek, M.; Sławik, L. Możliwości wykorzystania glonów z biomasy zeutrofizowanych zbiorników wodnych jako surowca do produkcji biopaliw. Inżynieria I Ochr. Sr. 2010, 13, 197–209. [Google Scholar]
- Chiang, C.L.; Lee, C.M.; Chen, P.C. Utilization of the cyanobacteria Anabaena sp. CH1 in biological carbon dioxide mitigation processes. Bioresour. Technol. 2011, 102, 5400–5405. [Google Scholar] [CrossRef]
- Kang, J.; Wen, Z. Use of microalgae for mitigating ammonia and CO2 emissions from animal production operations—Evaluation of gas removal efficiency and algal biomass composition. Algal Res. 2015, 11, 204–210. [Google Scholar]
- Kuo, C.; Jian, J.F.; Chang, Y.B.; Wan, X.H.; Lai, J.; Chang, J.S.; Lin, C.S. Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour. Technol. 2016, 221, 241–250. [Google Scholar] [CrossRef]
- Ding, G.T.; Mohd Yasin, N.H.; Takriff, M.S.; Kamarudin, K.F.; Salihon, J.; Yaakob, Z.; Mohd Hakimi, N.I.N. Phycoremediation of palm oil mill effluent (POME) and CO2 fixation by locally isolated microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J. Water Process Eng. 2020, 35, 101202. [Google Scholar]
- López-Pacheco, I.Y.; Rodas-Zuluaga, L.I.; Fuentes-Tristan, F.; Castillo-Zacarías, C.; Sosa-Hernandez, J.E.; Barcelo, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Phycocapture of CO2 as an option to reduce greenhouse gases in cities: Carbon sinks in urban spaces. J. CO2 Util. 2021, 53, 101704. [Google Scholar] [CrossRef]
- Rajkumar, R.; Yaakob, Z.; Mohd, S.T. Algal biofuel production. Bioresour. Technol. 2014, 9, 1606–1633. [Google Scholar]
- Aitken, D.B.; Antizar, L. Achieving a Green Solution: Limitations and Focus Points for Sustainable Algal Fuels. Energies 2012, 5, 1613–1647. [Google Scholar] [CrossRef]
- Eloka-Eboka, A.C.; Inambao, F.L. Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Appl. Energy 2017, 195, 1100–1111. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Demirbas, A. Biodiesel: Hopes and dreads. Biofuel Res. J. 2016, 10, 379. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M.; Malińska, K.; Krzywonos, M. Removal of biogens from synthetic wastewater by microalgae. Environ. Prot. Eng. 2014, 40, 87–104. [Google Scholar] [CrossRef]
- Dębowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass Production and Nutrient Removal by Chlorella vulgaris from Anaerobic Digestion Effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Krzemieniewski, M.; Dudek, M.; Grala, A. Możliwość namnażania biomasy glonów na bazie odcieku pochodzącego z odwadniania osadów pofermentacyjnych. Rocz. Ochr. Srodowiska 2013, 15, 1612–1622. [Google Scholar]
- Mehrabadi, A.; Farid, M.M.; Craggs, R. Variation of biomass energy yield in wastewater treatment high rate algal ponds. Algal Res. 2016, 15, 143–151. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M. Utilization of Chlorella vulgaris and sediments after N-NH4 removal containing clinoptilolite for sorption of heavy metals from wastewater. Rocz. Ochr. Srodowiska 2013, 15, 324–347. [Google Scholar]
- Chojnacka, K.; Górecki, H.; Zielińska, A.; Michalak, I. Technologia wytwarzania biologicznych dodatków paszowych z mikroelementami na bazie alg. Przemysł Chem. 2009, 88, 634–639. [Google Scholar]
- Zabochnicka-Świątek, M.; Krzywonos, M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Pol. J. Environ. Stud. 2014, 23, 551–561. [Google Scholar]
- Zabochnicka-Świątek, M.; Rygał, A. The Effect of biomass (Chlorella vulgaris, Scenedesmus armatus) Concentrations on Zn2+, Pb2+ and Cd2+ biosorption from zinc smelting wastewater. Inżynieria I Ochr. Sr. 2017, 20, 211–220. [Google Scholar]
- Almomani, F.; Bohsale, R.R. Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: Application of isotherm, kinetic models, and process optimization. Sci. Total Environ. 2021, 755, 142654. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Panneerselvan, L.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Co-culturing of microalgae and bacteria in real wastewaters alters indigenous bacterial communities enhancing effluent bioremediation. Algal Res. 2022, 64, 102705. [Google Scholar] [CrossRef]
- Mousavi, S.; Najafpour, G.D.; Mohammadi, M.; Seifi, M.H. Cultivation of newly isolated microalgae Coelastrum sp. In wastewater for simultaneous CO2 fixation, lipid production and wastewater treatment. Bioprocess Biosyst. Eng. 2018, 41, 519–530. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.H.; Cheng, C.L.; Nagarajan, D.; Guo, W.Q.; Lin, C.; Li, S.; Ren, N.; Chang, J.S. Nutrients and COD removal of swine wastewater with an isolated microalgal strain Neochloris aquatica CL-M1 accumulating high carbohydrate content used for biobutanol production. Bioresour. Technol. 2017, 242, 7–14. [Google Scholar] [CrossRef]
- Wang, S.K.; Yang, K.X.; Zhu, Y.R.; Zhu, X.Y.; Nie, D.F.; Jiao, N.; Angelidaki, I. One-step co-cultivation and flocculation of microalgae with filamentous fungi to valorize starch wastewater into high-value biomass. Bioresour. Technol. 2022, 361, 127625. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Li, X.; Lu, T.; Mou, Y.; Liu, N.; Song, M.; Yu, Z. Screening of the heterotrophic microalgae strain for the reclamation of acid producing wastewater. Chemosphere 2022, 307, 136047. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kundu, P.; Adhikari, S. Two stage treatability and biokinetic study of dairy wastewater using bacterial consortium and microalgae. Biocatal. Agric. Biotechnol. 2022, 43, 102387. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Hong, Y.; Liu, X.; Zhao, G.; Zhang, H.; Zhai, Q. Microalgae cultivation in domestic wastewater for wastewater treatment and high value-added production: Species selection and comparison. Biochem. Eng. J. 2022, 185, 108493. [Google Scholar] [CrossRef]
- Pooja, K.; Priyanka, V.; Rao, B.C.S.; Raghavender, V. Cost-effective treatment of sewage wastewater using microalgae Chlorella vulgaris and its application as bio-fertilizer. Energy Nexus 2022, 7, 100122. [Google Scholar] [CrossRef]
- Yan, H.; Lu, R.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Ruan, R.; Zhang, Q. Development of microalgae-bacteria symbiosis system for enhanced treatment of biogas slurry. Bioresour. Technol. 2022, 354, 127187. [Google Scholar] [CrossRef]
- Zhu, L.D.; Hiltunen, E.; Antila, E.; Zhong, J.J.; Yuan, Z.H.; Wang, Z.M. Microalgal biofuels: Flexible bioenergies for sustainable development. Renew. Sustain. Energy Rev. 2014, 30, 1035–1046. [Google Scholar] [CrossRef]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef]
- Krzywonos, M.; Borowski, P.F.; Kupczyk, A.; Zabochnicka-Świątek, M. Ograniczenie emisji CO2 poprzez stosowanie biopaliw motorowych. Przemysł Chem. 2014, 93, 1124–1127. [Google Scholar]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. A review on the extraction of lipid from microalgae for biodiesel production. Algal Res. 2015, 7, 117–123. [Google Scholar] [CrossRef]
- Del Río, E.; García-Gómez, E.; Moreno, J.; Guerrero, M.G.; García-González, M. Microalgae for oil. Assessment of fatty acid productivity in continuous culture by two high-yield strains, Chlorococcum oleofaciens and Pseudokirchneriella subcapitata. Algal Res. 2017, 23, 37–42. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, R.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sustain. Energy Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Sanchez Rizza, L.; Sanz Smachetti, M.E.; Do Nascimento, M.; Salerno, G.L.; Curatti, L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Bekirogullari, M.; Fragkopoulos, I.S.; Pittman, J.K.; Theodoropoulos, C. Production of lipid-based fuels and chemicals from microalgae: An integrated experimental and model-based optimization study. Algal Res. 2017, 23, 78–87. [Google Scholar] [CrossRef]
- Yen, H.W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, V.B.; Maneein, S.; Harvey, P.J. A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and alueadded products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Ramaraj, R.; Tsai, D.D.-W.; Chen, P.H. Freshwater microalgae niche of air carbon dioxide mitigation. Ecol. Eng. 2014, 68, 47–52. [Google Scholar] [CrossRef]
- Dasgupta, C.N.; Gilbert, J.; Lindblad, P.; Heidorn, T.; Borgvang, S.A.; Skjanes, K.; Das, D. Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int. J. Hydrog. Energy 2010, 35, 10218–10238. [Google Scholar] [CrossRef]
- Zhang, Q.; Dhir, A.; Kaur, P. Circular economy and the food sector: A systematic literature review. Sustain. Prod. Consum. 2022, 32, 655–668. [Google Scholar] [CrossRef]
- Van Schoubroeck, S.; Vermeyen, V.; Alaerts, L.; Van Acker, K.; Van Passel, S. How to monitor the progress towards a circular food economy: A Delphi study. Sustain. Prod. Consum. 2022, 32, 457–467. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Nazzal, M.A.; Darras, B.M.; Deiab, I.M. A comprehensive multi-level circular economy assessment framework. Sustain. Prod. Consum. 2022, 32, 700–717. [Google Scholar] [CrossRef]
- Dłużewska, E.; Krygier, K. Hydrokoloidy we współczesnej produkcji żywności. Przemysł Spożywczy 2007, 5, 12–16. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 1–37. [Google Scholar] [CrossRef]
- Borowski, J.; Borowska, E.J. Hydrokoloidy roślinne i mikrobiologiczne-technologiczne i żywieniowe aspekty ich stosowania. Przemysł Ferment. I Owocowo-Warzywny 2005, 1, 23–26. [Google Scholar]
- Tang, G.; Suter, P.M. Vitamin A, Nutrition, and Health Values of Algae: Spirulina, Chlorella, and Dunaliella. J. Pharm. Nutr. Sci. 2011, 1, 111–118. [Google Scholar]
- Lu, H.K.; Hsieh, C.C.; Hsu, J.J.; Yang, Y.K.; Chou, H.N. Preventive effects of Spirulina platensis on skeletal muscle damage under exercise-induced oxidative stress. Eur. J. Appl. Physiol. 2006, 98, 220–226. [Google Scholar] [CrossRef]
- Bartek, L.; Strid, I.; Henryson, K.; Junne, S.; Rasi, S.; Eriksson, M. Life cycle assessment of fish oil substitute produced by microalgae using food waste. Sustain. Prod. Consum. 2021, 27, 2002–2021. [Google Scholar] [CrossRef]
- Rzymski, P.; Jaśkiewicz, M. Microalgal food supplements from the perspective of Polish consumers: Patterns of use, adverse events, and beneficial effects. J. Appl. Phycol. 2017, 29, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Czerpak, R.; Jabłońska-Trypuć, A.; Pietryczuk, A. Znaczenie terapeutyczne, kosmetyczne i dietetyczne niektórych glonów. Postępy Fitoter. 2009, 3, 168–174. [Google Scholar]
- Morris, H.J.; Carrillo, O.V.; Almarales, Á.; Bermúdez, R.C.; Alonso, M.E.; Borges, L. Protein hydrolysates from the alga Chlorella vulgaris 87/1 with potentialities in immunonutrition. Biotecnol. Appl. 2009, 26, 162–165. [Google Scholar]
- Adams, M. Superfoods for Optimum Health Chlorella and Spirulina; The Truth Publishing International Ltd.: Elkhart, IN, USA, 2009. [Google Scholar]
- Walsh, M.J.; Gerber Van Doren, L.; Sills, D.L.; Archibald, I.; Beal, C.M.; Lei, X.G.; Huntley, M.E.; Johnson, Z.; Greene, C.H. Algal food and fuel coproduction can mitigate greenhouse gas emissions while improving land and water-use efficiency. Environ. Res. Lett. 2016, 11, 11. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2017, 26, 709–722. [Google Scholar] [CrossRef]
- Skrede, A.; Mydland, L.T.; Ahlstrøm, Ø.; Reitan, K.I.; Gislerød, H.R.; Øverland, M. Evaluation of microalgae as sources of digestible nutrients for monogastric animals. J. Anim. Feed. Sci. 2011, 20, 131–142. [Google Scholar] [CrossRef]
- Burr, G.S.; Barrows, F.T.; Gaylord, G.; Wolters, W.R. Apparent digestibility of macroınutrients and phosphorus in plantıderived ingredients for Atlantic salmon, Salmo salar and Arctic charr, Salvelinus alpinus. Aquac. Nutr. 2011, 5, 570–577. [Google Scholar] [CrossRef]
- Gbadamosi, O.; Lupatsch, I. Effects of dietary Nannochloropsis salina on the nutritional performance and fatty acid profile of Nile tilapia, Oreochromis niloticus. Algal Res. 2018, 33, 48–54. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.M.; Giorgi, G.; Chini-Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 485, 173–182. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Mørkøre, T.; Nengas, I.; Berge, R.K.; Sweetman, J. Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.). Aquaculture 2016, 451, 47–57. [Google Scholar] [CrossRef]
- Sprague, M.; Walton, J.; Campbell, P.J.; Strachan, F.; Dick, J.R.; Bell, J.G. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 2015, 185, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Nobre, B.; Marcelo, F.; Mrejen, S.; Tavares Cardoso, M.A.; Palavra, A.; Mendes, R.L. Functional food oil coloured by pigments extracted from microalgae with supercritical CO2. Food Chem. 2007, 101, 717–723. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. Thessalon. 2014, 21, 6. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, K.; Kaczmarek, S.; Kierzek, R.; Kardasz, P. Effect of seaweeds extracts and humic and fulvic acids on the germination and early growth of winter oilseed rape (Brassica napus L.). J. Res. Appl. Agric. Eng. 2010, 55, 28–32. [Google Scholar]
- Bai, N.R.; Banu, N.R.L.; Prakash, J.W.; Goldi, S.J. Effects of Asparagopsis taxiformis extract on the growth and yield of Phaseolus aureus. J. Basic Appl. Biol. 2007, 1, 6–11. [Google Scholar]
- Reitz, S.R.; Trumble, J.T. Effects of cytokinin-containing seaweed extract on Phaseolus lunatus L.: Influence of nutrient availability and apex removal. Bot. Mar. 1996, 36, 33–38. [Google Scholar] [CrossRef]
- Safi, C.; Bachar, Z.; Othmane, M.; Pierre-Yves, P.; Carlos, V.C. Morphology, composition, production, processing and applications of Chlorella vulgaris. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Piwowar, A.; Harasym, J. The Importance and Prospects of the Use of Algae in Agribusiness. Sustainability 2020, 12, 5669. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.; Kalaji, H.M. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 2017, 55, 510–521. [Google Scholar] [CrossRef]
- Uysal, O.; Ekinci, K.; Ozdemir, F.O. Evaluation of Microalgae as Microbial Fertilizer. Eur. J. Sustain. Dev. 2015, 4, 77–82. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Collas, E.; Damiano, D.K.; Tagg, K.; Graham, N.S.; Coates, J.C. Effects of green seaweed extract on Arabidopsis early development suggest roles for hormone signalling in plant responses to algal fertilisers. Sci. Rep. 2019, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, J.; Cwalina-Ambroziak, B.; Głosek-Sobieraj, M.; Sienkiewicz, S. Effect of biostimulators on yield and selected chemical properties of potato tubers. J. Elem. 2015, 20, 757–768. [Google Scholar] [CrossRef][Green Version]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Prakash, P.; Medhi, S.; Saikia, S.; Narendrakumar, G.; Thirugnanasambandam, T.; Abraham, L.S. Production, formulation and application of seaweed liquid fertilizer using humic acid on growth of arachis hypogaea. Biosci. Biotechnol. Res. Asia 2014, 11, 1515–1519. [Google Scholar] [CrossRef]
- Głosek-Sobieraj, M.; Cwalina-Ambroziak, B.; Hamouz, K. The Effect of Growth Regulators and a Biostimulator on the Health Status, Yield and Yield Components of Potatoes (Solanum tuberosum L.). Gesunde Pflanz. 2017, 71, 45–60. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Heimann, K. Novel approaches to microalgal and cyanobacterial cultivation for bioenergy and biofuel production. Curr. Opin. Biotechnol. 2016, 38, 183–189. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef]
- Kargbo, H.; Harris, J.S.; Phan, A.N. “Drop-in” fuel production from biomass: Critical review on techno-economic feasibility and sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Pragya, N.; Pandey, K.K.; Sahoo, P.K. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
| Microalgae | Oil Content (% d.m.) |
|---|---|
| Botryococcus braunii | 25–75 (Chisti 2007) |
| Chlorella sp. | 2–32 (Chisti 2007) [64,65] |
| Crypthecodinium cohnii | 20 (Chisti 2007) |
| Cylindrotheca sp. | 16–37 (Chisti 2007) |
| Dunaliella sp. | 6–42 (Chisti 2007) [64] |
| Isochrysis sp. | 7–33 (Chisti 2007) [64] |
| Monallanthus salina | >20 (Chisti 2007) |
| Nannochloris sp. | 20–35 (Chisti 2007) |
| Neochloris oleoabundans | 35–54 (Chisti 2007) |
| Nitzschia sp. | 45–47 (Chisti 2007) |
| Phaeodactylum tricornutum | 20–30 (Chisti 2007) |
| Schizochytrium sp. | 50–77 (Chisti 2007) |
| Scenedesmus sp. | 1.9–40 [64,65] |
| Spirulina sp. | 2–9 [64,65] |
| Tetraselmis suecica | 15–23 (Chisti 2007) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabochnicka, M.; Krzywonos, M.; Romanowska-Duda, Z.; Szufa, S.; Darkalt, A.; Mubashar, M. Algal Biomass Utilization toward Circular Economy. Life 2022, 12, 1480. https://doi.org/10.3390/life12101480
Zabochnicka M, Krzywonos M, Romanowska-Duda Z, Szufa S, Darkalt A, Mubashar M. Algal Biomass Utilization toward Circular Economy. Life. 2022; 12(10):1480. https://doi.org/10.3390/life12101480
Chicago/Turabian StyleZabochnicka, Magdalena, Małgorzata Krzywonos, Zdzisława Romanowska-Duda, Szymon Szufa, Ahmad Darkalt, and Muhammad Mubashar. 2022. "Algal Biomass Utilization toward Circular Economy" Life 12, no. 10: 1480. https://doi.org/10.3390/life12101480
APA StyleZabochnicka, M., Krzywonos, M., Romanowska-Duda, Z., Szufa, S., Darkalt, A., & Mubashar, M. (2022). Algal Biomass Utilization toward Circular Economy. Life, 12(10), 1480. https://doi.org/10.3390/life12101480










