1. Introduction
Obstructive sleep apnoea is the most common sleep-related breathing disorder and, in unattended cases, is a major public health problem due to the background comorbidities [
1]. Its increasing prevalence can be explained by the dynamic increase in obesity, the most crucial risk factor for OSA [
2]. The prevalence of obesity has tripled since 1975. In 2016, 39% of adults were overweight and 13% obese, representing 1.9 billion and 650 million people, respectively [
3]. Obesity is typical for developed countries, and explained by increased calorie intake, physical inactivity, and changes in the gut microbiome [
4]. In addition to OSA, obesity is also a risk factor for other conditions, such as insulin resistance, diabetes mellitus, hypertension, atherosclerosis, stroke, or myocardial infarction [
5]. Obesity can be classified into visceral and general types, of which visceral obesity is critical due to its decreasing effects on lung volumes and pharyngeal wall tension [
6]. Although the pathophysiology of OSA is complex and multifactorial, impaired dilator muscle functions, ineffective loop gain, and low arousal threshold are its essential background [
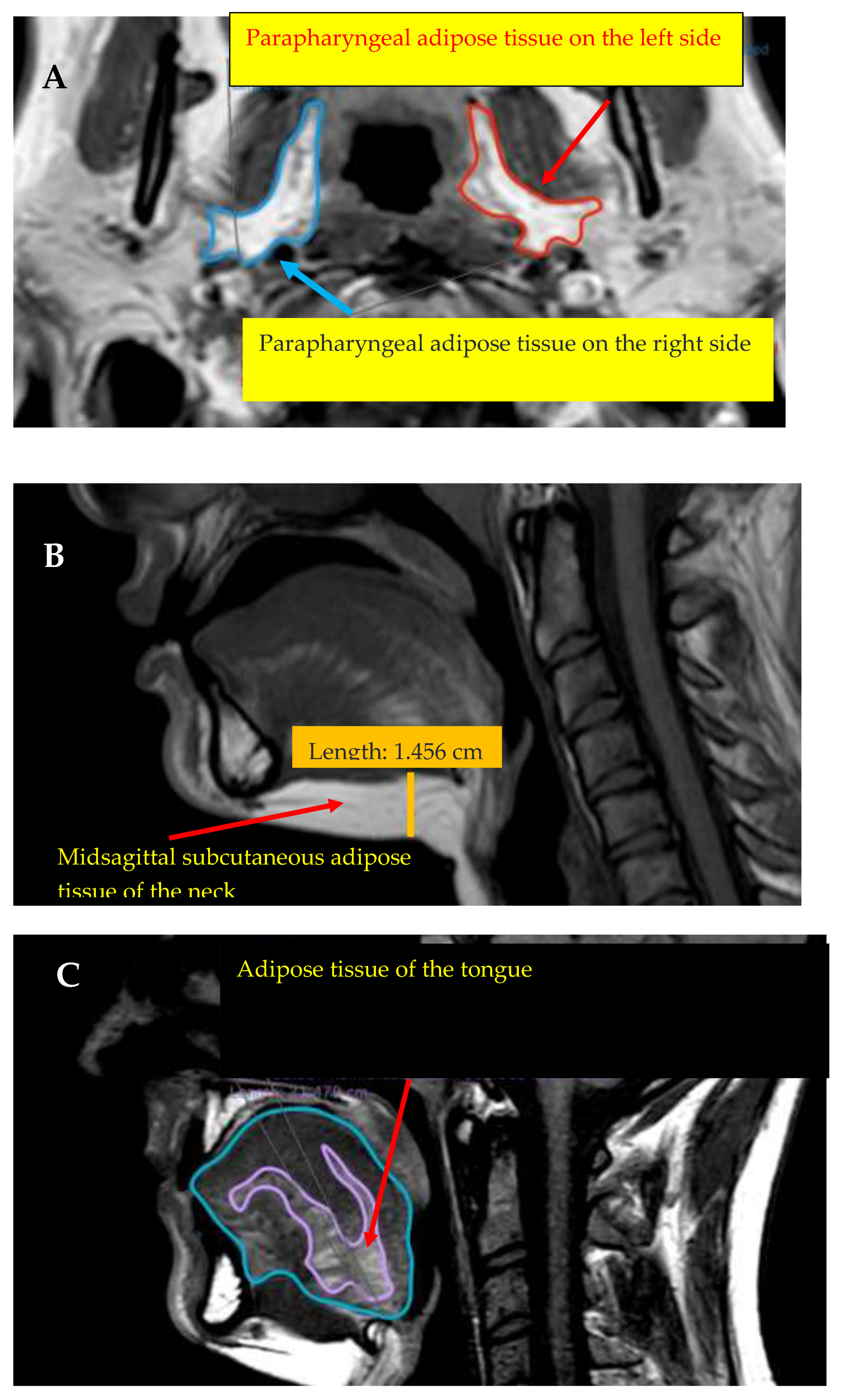
7]. Upper-airway obstruction can be the result of deposits of adipose tissue near the upper airways, which can be examined by CT or MRI. The significance of the parapharyngeal adipose corpus was first mentioned by Wlofram-Gabel et al., in 1996 [
8]. The parapharyngeal region, the tongue, and subcutaneous adipose tissue of the neck lead to upper-airway obstruction in different ways. The correlations between OSA, obesity, and anthropometric parameters have been particularly investigated in several studies to analyse the pathophysiology of OSA in a more detailed manner and to predict OSA. Of the anthropometric parameters, BMI, neck, abdominal, and hip circumferences and waist–hip ratio are mainly investigated. In the recent ELSA-Brasil study, which included 2059 patients, all parameters mentioned above were found to be significantly higher in the OSA group than in the non-OSA group [
9]. The Sleep Heart study, which included 6167 patients, observed significantly higher BMI and neck and hip circumferences in the case of severe OSA. However, it was also concluded that the BMI cut-off does not precisely represent the severity of obesity in different races and sexes [
10]. The predictive role of anthropometric parameters in OSA depends on the sexes. In women, waist circumference and waist-to-height ratio were the most crucial parameters in predicting OSA, while in men, neck circumference and waist-to-height ratio were crucial in predicting mild OSA, and BMI in severe OSA [
11]. The Wisconsin Sleep Cohort study, conducted in the USA, including 1520 participants between 30 and 70 years of age, observed a higher prevalence of sleep-related breathing disorders in older men with higher BMI values. Moreover, BMI was also the most strongly correlated with sleep-disordered breathing in younger participants [
12].
The anthropometric parameters and the parameters of the adipose tissue near the upper airways can be analysed using medical imaging methods, resulting in large databases. The complex pathophysiology behind OSA cannot be described using simple statistical methods in all cases. Given the fast improvement in sciences, using artificial intelligence is advantageous in diagnostics, prediction, and therapy. Although many possibilities regarding OSA diagnostics are accessible (e.g., self-administered questionnaires, home sleep tests, or polysomnography), the ratio of undiagnosed cases is still high.
In the last two decades, the improvement in bioinformatics and artificial intelligence has allowed easy and rapid detection of OSA. At first, machine learning-based models included essential risk factors for OSA (i.e., age, sex, BMI, or neck circumference) [
13,
14], while others performed a prediction using anthropometric and faciocervical measurements [
15]. Regarding the methods, the most vital expectations were simplicity and rapidity; therefore, ECG [
16] and oxyhaemoglobin saturation [
17], despite their effectiveness in prediction, cannot be integrated into daily practice. Other studies investigated the use of artificial intelligence to predict OSA using questionnaires [
18]. The prediction of OSA was also successful by 2D imaging [
19] and 3D face reconstruction using artificial intelligence [
20].
In the present study, in addition to anthropometric parameters, the upper-airway adipose tissue was examined using MRI in an OSA population to analyse its effects on OSA pathogenesis. Furthermore, the prediction of OSA and upper-airway obstruction, including the parameters mentioned above, was also investigated using artificial intelligence (the Flexible Discriminance analysis and the Multivariate Adaptive Regression Splines).
4. Discussion
OSA affects a significant proportion of society and the ratio of undiagnosed cases is high; therefore, its diagnosis must be improved. Although many possibilities regarding the diagnosis of OSA are accessible, the earliest diagnosis is essential, due to the appearance of comorbidities. The primary purpose of diagnostic methods is to easily and quickly screen for OSA or diagnose the disorder with high specificity and sensitivity. Efficient screening is possible using self-administered questionnaires, of which the STOP-BANG (i.e., snoring, tiredness, observed apnoea, high blood pressure, BMI, age, neck circumference, and male gender) is generally used with reliable results. This questionnaire contains eight questions, and patients can answer with ‘yes’ or ‘no’ [
34]. In addition, screening is also possible using the Berlin, Epworth, or STOP questionnaires. A meta-analysis that included 108 investigations with 47,989 participants determined a significantly higher sensitivity of the STOP-BANG questionnaire, although its specificity was lower than that of the Epworth questionnaire [
35]. An alternative diagnostic approach is a home-sleep test (HST, Types III or IV), which can be effectively used when there is a high risk of moderate or severe OSA. The one-night polysomnography, in which sleep specialists interpret the results, is essential in the follow-up of the effectiveness of therapy and the diagnosis of OSA [
36]. Notwithstanding the relatively low specificity of the questionnaires and their time requirement, and the necessity for qualified staff for home sleep tests, alternative methods, e.g., using artificial intelligence, are necessary. Previous results indicated that anthropometric parameters, the Epworth questionnaire, and expired gas analysis using machine learning could effectively predict OSA; only in 5.7% was a false mild instead of severe classification found [
13]. The prediction of OSA based on machine learning was improved when the model was completed with physical examination parameters [
14]. The prediction based on anthropometric and craniofacial parameters and the STOP-BANG questionnaire was more efficient in cases of moderate to severe OSA with no daytime symptoms [
15]. Based on the correspondences mentioned above, the examination of vital OSA risk factors is not only essential regarding OSA pathophysiology, but using modern statistical methods (e.g., artificial intelligence), their role in OSA prediction can also be analysed.
The correlation between OSA and obesity is highly complex and has been particularly investigated; however, there are still some questions remaining. The present study aimed to investigate the role of anthropometric and AT MRI parameters of the neck, tongue, and parapharyngeal regions in the pathogenesis and prediction of OSA, plus the obstruction and location of the upper airways. Determining the correlation between BMI and AT is relatively easy; however, the correspondence between OSA and AT is more complex, as these correlations are not intuitive and cannot be described using simple functions. Therefore, other methods must be applied to analyse the correspondence between AT and anthropometric parameters (i.e., independent variables) and OSA (i.e., dependent variable) and predictive values. Consequently, artificial intelligence (i.e., Flexible Discriminance analysis and Multivariate Adaptive Regression Splines) was applied in our analyses.
The significance of the present investigation is that the use of artificial intelligence in OSA diagnostics on a relatively large sample was analysed.
Based on the fact that obesity is one of the most critical risk factors for OSA and is also correlated depending on age and sex with the severity of OSA, including anthropometric and AT MRI parameters of the upper airways, the severity categories of OSA could be correctly determined in 99% of cases. In the prediction, gender and hip circumference showed the most vital role. Carlisle et al. also observed the effect of age on pharyngeal morphology. In the case of older males, a higher retropharyngeal and retroglossal length was observed, along with the cross-sectional area of the soft palate and the diameter and cross-sectional area of the parapharyngeal fat pad, in that study [
37,
38]. The effect of age is also presented in increased genioglossus muscle activity in older awake males [
39], which decreases during sleep, leading to vulnerability and collapsibility of the upper airways [
40]. In the OSA prediction, age was a key factor based on the results of the current investigation, although no significant correlation with AHI and BMI was observed. Neck circumference was defined as the second essential parameter in the OSA prediction of anthropometric parameters. The literature contains conflicting data on neck circumference in OSA; some investigations have concluded a strong correlation between neck circumference and OSA severity [
41,
42], while others have not [
43,
44]. A meta-analysis regarding the correlation between neck circumference and obesity stated a sensitivity of neck circumference in the prediction of obesity of 80% and a specificity of 85% [
45]. Neck circumference was defined to correlate with age, BMI, and hip and waist circumference, in both men and women [
46].
In the OSA prediction, the algorithm indicated the anthropometric and left-sided parapharyngeal and tongue fat midsagittal MRI parameters as being the most crucial, showing a strong correlation with obesity. Accumulation of AT near the upper airways (i.e., tongue, parapharyngeal space, and central region) in obesity leads to increased collapsibility of the pharynx by mechanical effects and based on neuromuscular regulations in the central nervous system [
47]. Compared to other somatic muscles, AT accumulation in the tongue showed a higher correlation with BMI and therefore, with obesity severity, which is strongly correlated with OSA severity [
48]. Jugé et al. found similar results, and observed a significant positive correlation between tongue AT and BMI and older ages [
49]. Our results showed that tongue fat% did not significantly differ between OSA categories and the control group, but the midsagittal region fat% parameter did. The tongue fat% neither correlated with AHI nor BMI; however, tongue midsagittal region fat% significantly correlated with BMI. Kim et al. observed a significant positive correlation between tongue fat volumes and AHI and BMI. Furthermore, a higher percentage of tongue fat% in the OSA group was detected; however, there was no significant difference compared to the control group [
50]. Parapharyngeal AT is strongly correlated with obesity, highlighted by the correlation between parapharyngeal AT and BMI. However, no correlation with AHI was detected. Consequently, parapharyngeal AT parameters contained essential information in the algorithm; however, they did not significantly correlate with OSA severity. Chen et al. detected a significant correlation between AHI and the subglosso-supraglottic-level parapharyngeal fat pad, independently of BMI and neck circumference parameters [
51]. According to Gao et al., in patients with a BMI over 28 kg/m
2, a significant positive effect of age on parapharyngeal AT volumes was detected [
52].
In predicting velopharyngeal obstruction, the algorithm determined parapharyngeal AT as the most vital parameter, followed by neck circumference and age. Using these parameters, by artificial intelligence, the velopharyngeal obstruction could be correctly detected in 80% of cases. Jang et al. detected a higher percentage of retropalatal concentric obstruction in patients with OSA with higher parapharyngeal AT volumes [
53]. This is in agreement with our results, referring to the significant role of parapharyngeal AT in velopharyngeal obstruction.
The importance of parapharyngeal AT in predicting oropharyngeal obstruction was not found, in contrast to the prediction of velopharyngeal obstruction, since BMI was indicated as the most crucial parameter, followed by abdominal circumference and parapharyngeal AT, with the latter two showing the same importance. Applying these three parameters, using artificial intelligence, the oropharyngeal obstruction could be predicted in 77% of cases. However, interestingly, the algorithm did not determine the other anthropometric and MRI parameters that are essential for prediction. Pahkala et al. highlighted the importance of increased lateral pharyngeal collapsibility associated with accumulation of parapharyngeal adipose tissue in obese patients, explained by impaired mechanisms controlling passive collapse of the pharyngeal wall [
54]. However, Li et al. determined the increased mechanical loading of parapharyngeal AT on the lateral pharyngeal wall as a possible background [
22]. Chen et al. observed a strong correlation between subglosso-supraglottic-level AT and lateral pharyngeal obstruction at the same level [
51].
Regarding tongue-based obstruction, the higher tongue volume, the adipose deposits accumulated in the tongue, and the decreased muscle activity during sleep can be defined, and are also negatively influenced by the accumulated intramuscular AT [
55]. To predict tongue-based obstruction, BMI was defined as the most vital parameter; thus, tongue-based obstruction could be predicted in 79% of cases. The correspondence between BMI and tongue volumes is highly complex; some researchers have indicated a strong correlation between them [
56], while others have not [
50]. According to our investigation results, tongue volumes significantly correlated with AHI and BMI in both sexes, while tongue fat significantly correlated with BMI.
Finally, it can be concluded that an MRI of the adipose tissue surrounding the upper airways can be an alternative examination of OSA when an MRI in the neck region is used with another indication other than OSA. Both OSA and velopharyngeal obstruction can be predicted using artificial intelligence. Compared to self-administered questionnaires, an essential advantage of our algorithm is that the location of obstruction can be identified with high precision and the examination is relatively fast compared to the home sleep test. Our results are especially crucial in cases where MRI was performed and was previously not diagnosed.
The present investigation had some limitations. First, the relatively low number of participants did not allow for the division of OSA into categories based on its severity. Moreover, the magnetic resonance examinations were performed on awake subjects and, therefore, did not present the situations during physiological sleep.