Disturbance in Some Fertility Biomarkers Induced and Changes in Testis Architecture by Chronic Exposure to Various Dosages of Each of Nonylphenol or Bisphenol A and Their Mix
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
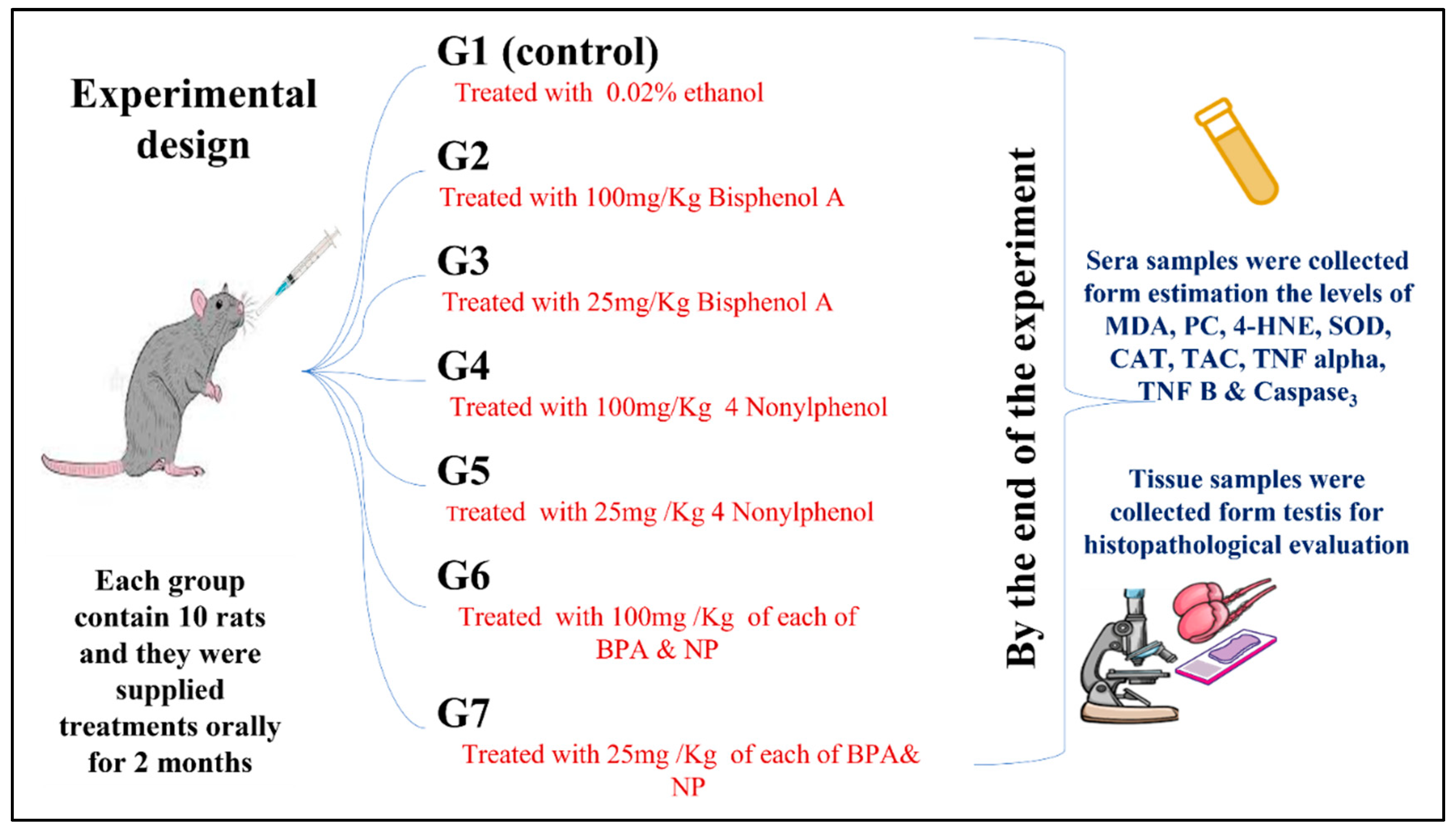
2.2. Experimental Design
2.3. Samples
2.4. Blood Biochemical Markers
2.4.1. Oxidative Stress Markers
2.4.2. Antioxidant Biomarkers
2.4.3. Testis Biomarkers
2.4.4. Inflammatory Apoptic and Markers
2.5. Histopathology
2.6. Statistical Evaluation
3. Results
3.1. Oxidative Stress Biomarkers in Serum
3.2. Antioxidant Biomarkers in Serum
3.3. Serum Testis Biomarkers
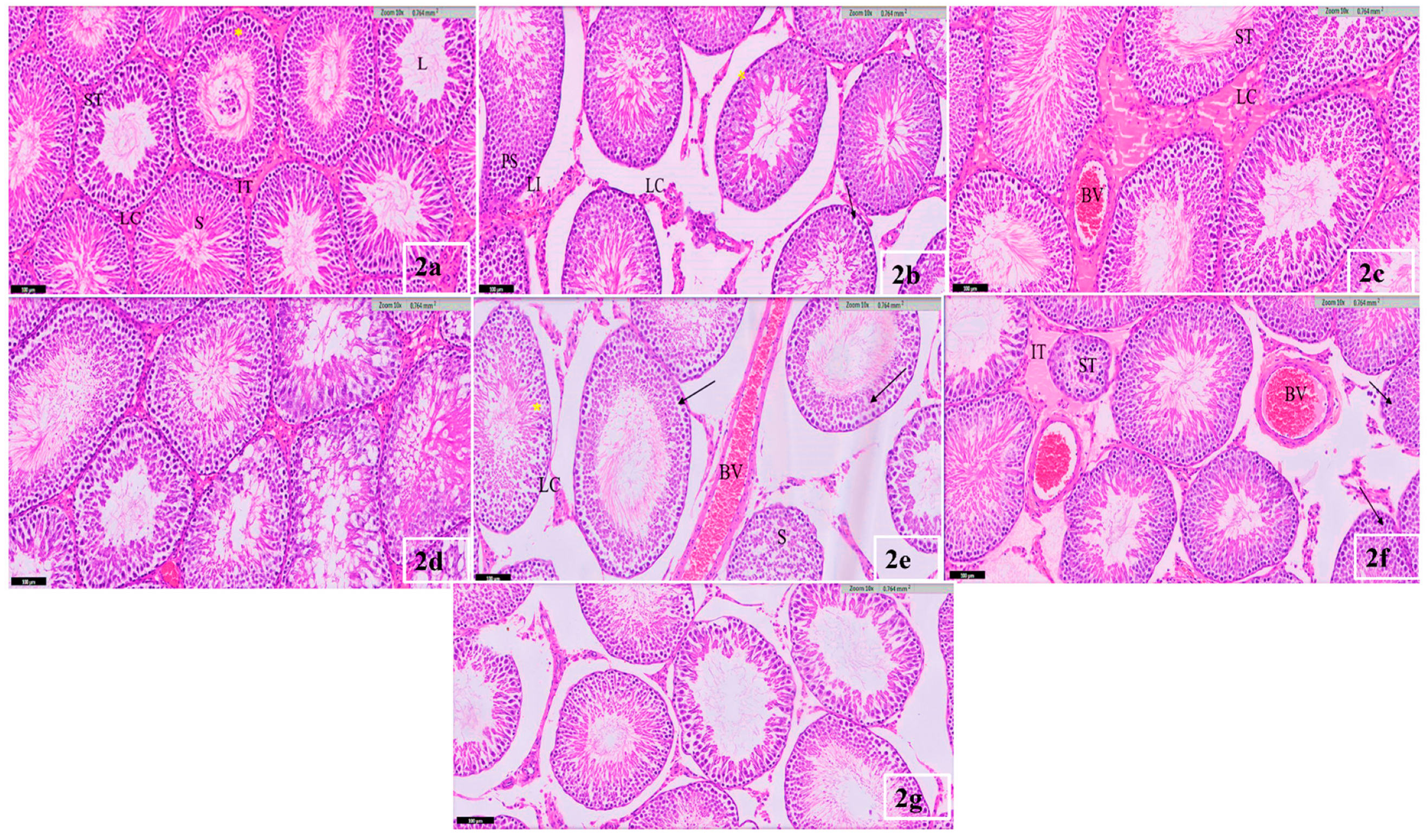
3.4. Testis Histological Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Sati, P.C.; Kaushik, R.; Kumar, V.; Khaliq, F.; Vaney, N. Oxidative status in workers engaged in recycling of plastic: Occupational hazard. Indian J. Physiol. Pharmacol. 2012, 56, 234–238. [Google Scholar] [PubMed]
- Aydoğan, M.; Korkmaz, A.; Barlas, N.; Kolankaya, D. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology 2008, 249, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, J.; Ni, C.; Yu, D.; Wang, H.; Wang, P.; Luo, M.; Yu, J. Effects of subchronic exposure of nonylphenol on the expression of immune-related factors and estrogen receptors in the spleen of rats. Environ. Sci. Eur. 2022, 34, 30. [Google Scholar] [CrossRef]
- Helal, E.G.; Zaki, D.A.; Abdelaziz, M.A.; Zakaria, A. Effect of both bisphenol-A and liquorice on some sexual hormones in male albino rats and illustration of the effect of stem cell enhancer on their actions. Egypt. J. Hosp. Med. 2019, 77, 5922–5929. [Google Scholar] [CrossRef]
- Morgan, A.M.; El-Ballal, S.S.; El-Bialy, B.E.; El-Borai, N.B. Studies on the potential protective effect of cinnamon against bisphenol A-and octylphenol-induced oxidative stress in male albino rats. Toxicol. Rep. 2014, 1, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noorimotlagh, Z.; Haghighi, N.J.; Ahmadimoghadam, M.; Rahim, F. An updated systematic review on the possible effect of nonylphenol on male fertility. Environ. Sci. Pollut. Res. 2017, 24, 3298–3314. [Google Scholar] [CrossRef]
- Chen, M.-L.; Lee, H.-Y.; Chuang, H.-Y.; Guo, B.-R.; Mao, I.-F. Association between nonylphenol exposure and development of secondary sexual characteristics. Chemosphere 2009, 76, 927–931. [Google Scholar] [CrossRef]
- Laws, S.C.; Carey, S.A.; Ferrell, J.M.; Bodman, G.J.; Cooper, R.L. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000, 54, 154–167. [Google Scholar] [CrossRef] [Green Version]
- Jie, X.; Yang, W.; Jie, Y.; Hashim, J.H.; Liu, X.Y.; Fan, Q.Y.; Yan, L. Toxic effect of gestational exposure to nonylphenol on F1 male rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 418–428. [Google Scholar] [CrossRef]
- Jubendradass, R.; D’Cruz, S.C.; Rani, S.J.A.; Mathur, P. Nonylphenol induces apoptosis via mitochondria-and Fas-L-mediated pathways in the liver of adult male rat. Regul. Toxicol. Pharmacol. 2012, 62, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kawada, K.; Shimada, T.; Mori, M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am. J. Obstet. Gynecol. 1979, 135, 372–376. [Google Scholar] [CrossRef]
- Cadenas, E.; Boveris, A.; Ragan, C.I.; Stoppani, A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977, 180, 248–257. [Google Scholar] [CrossRef]
- Wakeyama, H.; Takeshige, K.; Takayanagi, R.; Minakami, S. Superoxide-forming NADPH oxidase preparation of pig polymorphonuclear leucocyte. Biochem. J. 1982, 205, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Masayasu, M.; Hiroshi, Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Perkins, N.D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Carleton, H.M.; Drury, R.A.B.; Wallington, E.A. Carleton’s Histological Technique; Oxford University Press: Oxford, UK, 1980. [Google Scholar]
- Sabour, A.N. Effect of Bisphenol A on Some of Antioxidants in White Male Rats. Sci. J. Med. Res. 2019, 3, 83–86. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [Green Version]
- Chitra, K.; Latchoumycandane, C.; Mathur, P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 2003, 185, 119–127. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Hou, Y.; Yao, G.; Wang, Y. Effect of nonylphenol on apoptosis of Sertoli cells in vitro. Bull. Environ. Contam. Toxicol. 2003, 70, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Abnosi, M.H.; Masoomi, S. Para-nonylphenol toxicity induces oxidative stress and arrests the cell cycle in mesenchymal stem cells of bone marrow. Iran J. Toxicol. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Kalb, A.C.; Kalb, A.L.; Cardoso, T.F.; Fernandes, C.G.; Corcini, C.D.; Junior, A.S.V.; Martínez, P.E. Maternal transfer of bisphenol A during nursing causes sperm impairment in male offspring. Arch. Environ. Contam. Toxicol. 2016, 70, 793–801. [Google Scholar] [CrossRef]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Khene, L.; Berrebbah, H.; Yahyaoui, A.; Bouarroudj, T.; Zouainia, S.; Kahli, H.; Bourayou, C. Biomarkers of oxidative stress, lipid peroxidation and ROS production induced by TiO2 microparticles on snails Helix aspersa. In Studia Universitatis “Vasile Goldiş”, Seria Ştiinţele Vieţi; Vasile Goldis University Press: Arad, Romania, 2017; Volume 27, pp. 127–133. [Google Scholar]
- Wang, Y.-X.; Liu, C.; Shen, Y.; Wang, Q.; Pan, A.; Yang, P.; Chen, Y.-J.; Deng, Y.-L.; Lu, Q.; Cheng, L.-M.; et al. Urinary levels of bisphenol A, F and S and markers of oxidative stress among healthy adult men: Variability and association analysis. Environ. Int. 2019, 123, 301–309. [Google Scholar] [CrossRef]
- Dutta, M.; Paul, G. Bisphenol a dose-and time-dependently induces oxidative stress in rat liver mitochondria ex vivo. Asian J. Pharm. Clin. Res. 2018, 11, 98–105. [Google Scholar] [CrossRef]
- Lv, Y.; Lu, S.; Dai, Y.; Rui, C.; Wang, Y.; Zhou, Y.; Li, Y.; Pang, Q.; Fan, R. Higher dermal exposure of cashiers to BPA and its association with DNA oxidative damage. Environ. Int. 2017, 98, 69–74. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, J.; Gao, C.-Z.; Qiu, R.-l.; Li, Y.-X.; Li, X.; Huang, M.-Z.; Kannan, K. Urinary concentrations of bisphenols and their association with biomarkers of oxidative stress in people living near e-waste recycling facilities in China. Environ. Sci. Technol. 2016, 50, 4045–4053. [Google Scholar]
- Avci, B.; Bahadir, A.; Tuncel, O.K.; Bilgici, B. Influence of α-tocopherol and α-lipoic acid on bisphenol-A-induced oxidative damage in liver and ovarian tissue of rats. Toxicol. Ind. Health 2016, 32, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Karafakioglu, Y.S.; Aslan, R. Taurine prevents nonylphenol-induced oxidative stress in rats. J. Anim. Vet. Adv. 2010, 9, 37–43. [Google Scholar]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular mechanisms of action of BPA. Dose-Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, L.-C.; Chen, J.-F.; Song, L.; Wang, X.-R. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem. Toxicol. 2006, 44, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Norazit, J.M.A.; Razak, S.A.; Abdulla, M.A. Effects of soya bean extract, bisphenol A and 17β-estradiol on the testis and circulating levels of testosterone and estradiol among peripubertal juvenile male sprague-dawley rats. Sains Malays. 2012, 41, 63–69. [Google Scholar]
- Han, X.D.; Tu, Z.G.; Gong, Y.; Shen, S.N.; Wang, X.Y.; Kang, L.N.; Hou, Y.Y.; Chen, J.X. The toxic effects of nonylphenol on the reproductive system of male rats. Reprod. Toxicol. 2004, 19, 215–221. [Google Scholar] [CrossRef]
- Omran, B.; Abdallah, E.; Abdelwahab, M. Study of Probable Toxic Effects of Bisphenol A & the Protective Role of Vitamin E on Testes and Prostate of Adult Male Albino Rats. Ain Shams J. Forensic Med. Clin. Toxicol. 2017, 29, 7–18. [Google Scholar]
- Qiu, L.-L.; Wang, X.; Zhang, X.-h.; Zhang, Z.; Gu, J.; Liu, L.; Wang, Y.; Wang, X.; Wang, S.-L. Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A. Toxicol. Lett. 2013, 219, 116–124. [Google Scholar] [CrossRef]
- Kazemi, S.; Feizi, F.; Aghapour, F.; Joorsaraee, G.A.; Moghadamnia, A.A. Histopathology and histomorphometric investigation of bisphenol A and nonylphenol on the male rat reproductive system. N. Am. J. Med. Sci. 2016, 8, 215. [Google Scholar]
- Nagao, T.; Wada, K.; Marumo, H.; Yoshimura, S.; Ono, H. Reproductive effects of nonylphenol in rats after gavage administration: A two-generation study. Reprod. Toxicol. 2001, 15, 293–315. [Google Scholar] [CrossRef]
- Cardinali, M.; Maradonna, F.; Olivotto, I.; Bortoluzzi, G.; Mosconi, G.; Polzonetti-Magni, A.M.; Carnevali, O. Temporary impairment of reproduction in freshwater teleost exposed to nonylphenol. Reprod. Toxicol. 2004, 18, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Kassim, N.M.; Mohd, M.A. Assessment of pubertal development in juvenile male rats after sub-acute exposure to bisphenol A and nonylphenol. Toxicol. Lett. 2003, 143, 261–270. [Google Scholar] [CrossRef]
- Adoamnei, E.; Mendiola, J.; Vela-Soria, F.; Fernández, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018, 161, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Quan, C.; Li, X.; Shi, Y.; Duan, P.; Yang, K. Mutual promotion of apoptosis and autophagy in prepubertal rat testes induced by joint exposure of bisphenol A and nonylphenol. Environ. Pollut. 2018, 243, 693–702. [Google Scholar] [CrossRef]
- Jung, J.-C.; Lee, Y.-H.; Kim, S.H.; Kim, K.-J.; Kim, K.-M.; Oh, S.; Jung, Y.-S. Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. BMC Complement. Altern. Med. 2015, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Abdelzaher, W.; Ali, D.; Khalil, W. Could licorice prevent bisphenol A-induced biochemical, histopathological and genetic effects in the adult male albino rats? Ain-Shams J. Forensic Med. Clin. Toxicol. 2018, 30, 73–87. [Google Scholar] [CrossRef]
- Li, Y.C.; Kuan, Y.H.; Huang, F.M.; Chang, Y.C. The role of DNA damage and caspase activation in cytotoxicity and genotoxicity of macrophages induced by bisphenol-A-glycidyldimethacrylate. Int. Endod. J. 2012, 45, 499–507. [Google Scholar] [CrossRef]
- Yao, G.; Ling, L.; Luan, J.; Ye, D.; Zhu, P. Nonylphenol induces apoptosis of Jurkat cells by a caspase-8 dependent mechanism. Int. Immunopharmacol. 2007, 7, 444–453. [Google Scholar] [CrossRef]
- Liu, X.L.; Chen, X.Y.; Wang, Z.C.; Shen, T.; Zhao, H. Effects of exposure to bisphenol A during pregnancy and lactation on the testicular morphology and caspase-3 protein expression of ICR pups. Biomed. Rep. 2013, 1, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Fas, S.C.; Fritzsching, B.; Suri-Payer, E.; Krammer, P.H. Death receptor signaling and its function in the immune system. Apoptosis Relev. Autoimmun. 2006, 9, 1–17. [Google Scholar]
- Padanilam, B.J. Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am. J. Physiol. Ren. Physiol. 2003, 284, F608–F627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saied, N.; Darwish, S. A possible ameliorating effects of Tribulus terrestris on testicular dysfunction induced by xenoestrogens exposure in adult rats. Curr. Sci. Int. 2015, 4, 73–89. [Google Scholar]
- Yousaf, B.; Amina; Liu, G.; Wang, R.; Qadir, A.; Ali, M.U.; Kanwal, Q.; Munir, B.; Asmatullah; Abbas, Z. Bisphenol A exposure and healing effects of Adiantum capillus-veneris L. plant extract (APE) in bisphenol A-induced reproductive toxicity in albino rats. Environ. Sci. Pollut. Res. 2016, 23, 11645–11657. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.-Y. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gurmeet, K.; Rosnah, I.; Normadiah, M.; Das, S.; Mustafa, A. Detrimental effects of bisphenol A on development and functions of the male reproductive system in experimental rats. EXCLI J. 2014, 13, 151. [Google Scholar]
- Munir, B.; Qadir, A.; Tahir, M. Negative effects of bisphenol A on testicular functions in albino rats and their abolitions with Tribulus terristeris L. Braz. J. Pharm. Sci. 2017, 53, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hutanu, D. Experimental Investigations reagarding the effects of bisphenol a in adult mice spermatogenesis. Ann. Rom. Soc. Cell Biol. 2011, 16, 74–78. [Google Scholar]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; ElAmin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med. Cell. Longev. 2012, 2012, 194829. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Lee, C.; Yeung, W.; Giesy, J.P.; Wong, M.H.; Zhang, X.; Hecker, M.; Wong, C.K. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod. Toxicol. 2011, 31, 409–417. [Google Scholar] [CrossRef]
- Tohei, A.; Suda, S.; Taya, K.; Hashimoto, T.; Kogo, H. Bisphenol A inhibits testicular functions and increases luteinizing hormone secretion in adult male rats. Exp. Biol. Med. 2001, 226, 216–221. [Google Scholar] [CrossRef]
- Karumari, J.; Balasubramanian, E. Evaluation of antifertility potential of the Aqueous Extract of Ocimum sanctum (Linnaeus, 1767) leaves on the testicular histology of Rattus norvegicus Berkenhout (1769). Asian J. Biochem. Pharm. Res. 2014, 4, 20–29. [Google Scholar]
- Lee, I.-K.; Rhee, S.-K. Inhibitory effect of bisphenol A on gap junctional intercellular communication in an epithelial cell line of rat mammary tissue. Arch. Pharmacal. Res. 2007, 30, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.; Peter, R.; Sloley, B. Testosterone and estradiol potentiate the serum gonadotropin response to gonadotropin-releasing hormone in goldfish. Biol. Reprod. 1991, 44, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, H.; Parvez, S.; Raisuddin, S. Melatonin abrogates nonylphenol-induced testicular dysfunction in Wistar rats. Andrologia 2017, 49, e12648. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Xiang, Z.; Qian, W.; Han, X.; Li, D. Antagonistic effects of a mixture of low-dose nonylphenol and di-n-butyl phthalate (monobutyl phthalate) on the Sertoli cells and serum reproductive hormones in prepubertal male rats in vitro and in vivo. PLoS ONE 2014, 9, e93425. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Hu, C.; Butler, H.J.; Quan, C.; Chen, W.; Huang, W.; Tang, S.; Zhou, W.; Yuan, M.; Shi, Y. Effects of 4-nonylphenol on spermatogenesis and induction of testicular apoptosis through oxidative stress-related pathways. Reprod. Toxicol. 2016, 62, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-r.; Wang, K.; Yu, J.; Yu, Z.-x.; Yu, X.-b.; Zhang, Z.-z. Distribution and fate modeling of 4-nonylphenol, 4-t-octylphenol, and bisphenol A in the Yong River of China. Chemosphere 2018, 195, 594–605. [Google Scholar] [CrossRef]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Huang, W.; Chen, W.; Tang, S.; Shi, Y.; Martin, F.L.; Yang, K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating AMPK activation in Sertoli cells. Toxicol. Lett. 2017, 267, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.E.-S.; Abdel-Aziz, S.; El-Sayed, A.-F.; Zeid, S. Structural and functional effects of early exposure to 4-nonylphenol on gonadal development of Nile tilapia (Oreochromis niloticus): B-histological alterations in testes. Fish Physiol. Biochem. 2014, 40, 1495–1507. [Google Scholar]
- Dalgaard, M.; Pilegaard, K.; Ladefoged, O. In utero exposure to diethylstilboestrol or 4-n-nonylphenol in rats: Number of sertoli cells, diameter and length of seminiferous tubules estimated by stereological methods. Pharmacol. Toxicol. 2002, 90, 59–65. [Google Scholar] [CrossRef]
- Kinnberg, K.; Toft, G. Effects of estrogenic and antiandrogenic compounds on the testis structure of the adult guppy (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2003, 54, 16–24. [Google Scholar] [CrossRef]
- Jobling, S.; Sumpter, J.P.; Sheahan, D.; Osborne, J.A.; Matthiessen, P. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ. Toxicol. Chem. Int. J. 1996, 15, 194–202. [Google Scholar] [CrossRef]
- Miles-Richardson, S.; Pierens, S.; Nichols, K.; Kramer, V.; Snyder, E.; Snyder, S.; Render, J.; Fitzgerald, S.; Giesy, J. Effects of waterborne exposure to 4-nonylphenol and nonylphenol ethoxylate on secondary sex characteristics and gonads of fathead minnows (Pimephales promelas). Environ. Res. 1999, 80, S122–S137. [Google Scholar] [CrossRef] [PubMed]
- Panter, G.; Thompson, R.; Sumpter, J. Adverse reproductive effects in male fathead minnows (Pimephales promelas) exposed to environmentally relevant concentrations of the natural oestrogens, oestradiol and oestrone. Aquat. Toxicol. 1998, 42, 243–253. [Google Scholar] [CrossRef]
- Desbrow, C.; Routledge, E.; Brighty, G.; Sumpter, J.; Waldock, M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 1998, 32, 1549–1558. [Google Scholar] [CrossRef]
- Jobling, S.; Beresford, N.; Nolan, M.; Rodgers-Gray, T.; Brighty, G.; Sumpter, J.; Tyler, C. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol. Reprod. 2002, 66, 272–281. [Google Scholar] [CrossRef]
- Chen, L.W.; Qing, W.A.; Ling, C.X.; Guo, Y.; Yao, L.; Ou, C.Y.; Jiao, H.C.; Lin, T.H. 90d exposure to nonylphenol has adverse effects on the spermatogenesis and sperm maturation of adult male rats. Biomed. Environ. Sci. 2014, 27, 907–911. [Google Scholar]
- Chitra, K.; Latchoumycandane, C.; Mathur, P. Effect of nonylphenol on the antioxidant system in epididymal sperm of rats. Arch. Toxicol. 2002, 76, 545–551. [Google Scholar]
- Ahn, J.H.; Lee, T.w.; Kim, K.H.; Byun, H.; Ryu, B.; Lee, K.T.; Jang, D.S.; Choi, J.H. 6-Acetoxy cyperene, a patchoulane-type sesquiterpene isolated from cyperus rotundus rhizomes induces caspase-dependent apoptosis in human ovarian cancer cells. Phytother. Res. 2015, 29, 1330–1338. [Google Scholar] [CrossRef]
- Leonard, I.S.; Passino-Reader, D.R.; Peter, G.M.; Geneva, M.O. Xenobiotic-induced apoptosis: Significance and potential application as a general biomarker of response. Biomarkers 1999, 4, 237–253. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef] [PubMed]
| Parameters | MDA nmol/mL | PC μmol/mg | 4-HNE ng/mL | |
|---|---|---|---|---|
| Groups | ||||
| Control | 1.14 ± 0.06 | 8.90 ± 0.29 | 194.40 ± 5.71 | |
| Bisphenol A high dose (BH) | 1.16 ± 0.04 | 12.68 ± 0.49 a | 218.00 ± 8.69 a | |
| Bisphenol A low dose (BL) | 1.01 ± 0.02 | 10.52 ± 0.21 b | 145.20 ± 7.99 ab | |
| Nonylphenol high dose (NH) | 1.22 ± 0.03 c | 11.06 ± 0.83 a | 206.60 ± 6.54 c | |
| Nonylphenol low dose (NL) | 1.11 ± 0.05 | 9.88 ± 0.38 b | 183.60 ± 5.82 bcd | |
| Mix (BH & NH) | 1.97 ± 0.05 abcde | 17.80 ± 1.33 abcde | 306.60 ± 7.59 abcde | |
| Mix (BL & NL) | 1.40 ± 0.06 abcdef | 12.78 ± 0.65 acef | 237.60 ± 10.08 acdef | |
| Parameters | SOD u/mL | CAT Mu/L | TAC μmol/mg | |
|---|---|---|---|---|
| Groups | ||||
| Control | 303.60 ± 4.75 | 92.00 ± 3.00 | 38.00 ± 2.24 | |
| Bisphenol A high dose (BH) | 219.80 ± 7.41 a | 83.70 ± 4.62 a | 30.16 ± 4.17 a | |
| Bisphenol A low dose (BL) | 274.00 ± 14.15 b | 88.66 ± 1.85 ab | 31.26 ± 1.99 ab | |
| Nonylphenol high dose (NH) | 226.80 ± 6.92 abc | 88.80 ± 3.71 c | 26.16 ± 1.58 | |
| Nonylphenol low dose (NL) | 207.80 ± 7.48 abc | 98.60 ± 1.63 abd | 32.70 ± 1.67 | |
| Mix (BH & NH) | 220.60 ± 10.99 abcde | 48.80 ± 3.43 abcde | 21.40 ± 1.39 abcde | |
| Mix (BL & NL) | 274.60 ± 17.53 bdef | 82.60 ± 5.27 bcef | 30.32 ± 1.91 abcde | |
| Parameters | Testosterone ng/mL | LH mIU/mL | |
|---|---|---|---|
| Groups | |||
| Control | 181.62 ± 10.09 | 9.69 ± 0.23 | |
| Bisphenol A high dose (BH) | 137.60 ± 5.69 a | 11.38 ± 0.57 | |
| Bisphenol A low dose (BL) | 201.00 ± 7.09 b | 11.24 ± 0.97 | |
| Nonylphenol high dose (NH) | 118.20 ± 4.02 ac | 13.26 ± 0.36 abc | |
| Nonylphenol low dose (NL) | 179.60 ± 12.78 bd | 11.88 ± 0.39 a | |
| Mix (BH & NH) | 100.20 ± 3.95 abce | 17.76 ± 1.05 abcde | |
| Mix (BL & NL) | 160.80 ± 10.06 cdf | 13.60 ± 0.29 abcf | |
| Parameters | NF α pg/mL | NF-κB pg/mL | Caspase ng/mL | |
|---|---|---|---|---|
| Groups | ||||
| Control | 23.34 ± 2.07 | 48.08 ± 1.29 | 0.81 ± 0.06 | |
| Bisphenol A high dose (BH) | 31.80 ± 5.75 | 56.21 ± 1.19 a | 0.84 ± 0.11 | |
| Bisphenol A low dose (BL) | 19.66 ± 1.12 b | 40.72 ± 0.64 ab | 0.19 ± 0.03 ab | |
| Nonylphenol high dose (NH) | 24.48 ± 2.20 | 55.06 ± 1.96 ac | 0.89 ± 0.07 c | |
| Nonylphenol low dose (NL) | 19.68 ± 0.81 b | 44.90 ± 2.50 bd | 0.17 ± 0.04 abd | |
| Mix (BH & NH) | 75.20 ± 3.11 abcde | 76.10 ± 3.63 abcde | 1.19 ± 0.10 abcde | |
| Mix (BL & NL) | 45.00 ± 3.03 abcdef | 54.60 ± 2.04 acef | 0.52 ± 0.03 abcdef | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melebary, S.J.; AlGhamdi, M.S.; Elhalwagy, M.E.A.; Alsolmy, S.A.; Bin Dohaish, A.J.A. Disturbance in Some Fertility Biomarkers Induced and Changes in Testis Architecture by Chronic Exposure to Various Dosages of Each of Nonylphenol or Bisphenol A and Their Mix. Life 2022, 12, 1555. https://doi.org/10.3390/life12101555
Melebary SJ, AlGhamdi MS, Elhalwagy MEA, Alsolmy SA, Bin Dohaish AJA. Disturbance in Some Fertility Biomarkers Induced and Changes in Testis Architecture by Chronic Exposure to Various Dosages of Each of Nonylphenol or Bisphenol A and Their Mix. Life. 2022; 12(10):1555. https://doi.org/10.3390/life12101555
Chicago/Turabian StyleMelebary, Sahar J., Mariam S. AlGhamdi, Manal E. A. Elhalwagy, Soha A. Alsolmy, and Al Jawaher A. Bin Dohaish. 2022. "Disturbance in Some Fertility Biomarkers Induced and Changes in Testis Architecture by Chronic Exposure to Various Dosages of Each of Nonylphenol or Bisphenol A and Their Mix" Life 12, no. 10: 1555. https://doi.org/10.3390/life12101555




